Abstract
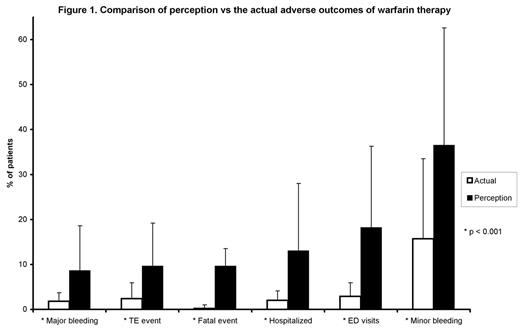
While studies have reported clinical and adverse outcomes of individual anticoagulation clinics (ACC), collective data from ACC has not been reported. The objective of the study was to assess efficacy, safety, monitoring, and resource utilization outcomes associated with warfarin therapy in US ACC. We conducted a postal survey among the members of the Anticoagulation Forum (AF) in January 2006. The response rate was 27.9% (146 of 524 clinics); respondents were spread across 36 states in the US and together were representative of a base of 94,177 patients (pts). The majority of respondents were pharmacists (61%, 89 of 146) affiliated with hospitals (62.3%, 91 of 146). Atrial fibrillation and venous thromboembolisms were the 2 most common indications for anticoagulation therapy. The median time devoted to a new and follow-up visit was 30 and 15 minutes respectively. The median number of visits required for dose adjustment for warfarin naïve and established pts were 4 and 1.5 respectively per patient per month. The median % of pts having difficulty in attaining therapeutic international normalized ratio (INR) in the first 4–6 weeks of therapy was 10, as was the % of pts whose INR was poorly controlled (in range <50% of time). The median % of pts with INR controlled within therapeutic and expanded therapeutic (+/− 0.2) range was 65% and 77.5% respectively, and it took a median of 10 days to reach therapeutic INR in both groups of pts. The median % of pts who visited the emergency room (ER) and those who were hospitalized due to warfarin related adverse drug event was 2% and 1.5% respectively. Warfarin associated adverse drug event fatality rate was 0.2±0.8%. The median % of pts who develop a thromboembolic event (TE), major and minor bleeding at least once during therapy with warfarin was reported to be 1, 1 and 10% respectively. Drug interactions of warfarin and poor patient compliance were reported to be the two most important barriers for maintaining desired anticoagulation levels. We also performed an item-to-item comparison of the actual efficacy and safety data of warfarin from the present survey with a previous perception-based, in-person survey, conducted in May 2005. The response rate of the in-person survey was 56% (329 of 586). Respondent’s demographics were comparable in both surveys. Comparison of the perceived and the actual rates for warfarin related ER visits, hospitalization, TE events, and mortality rate is illustrated in (Figure1). In the perception based survey, respondents underrated the efficacy of warfarin and overrated the adverse outcomes of warfarin. It is possible that this perception contributes to the underutilization of warfarin. This is the first study to report aggregate data on efficacy, safety, monitoring, and resource utilization outcomes associated with warfarin therapy in US ACC. This data can be used as a benchmark by individual ACC to rate their performance, identify opportunities for improvement and as a source of secondary data for pharmacoeconomic and outcomes research studies.
Comparison of perception vs the actual adverse outcomes of warfarin therapy
Disclosures: The study was funded by Astra Zeneca.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal