Comment on Gray et al, page 3777
The relative radioresistance of thymic epithelium has led to the assumption that this population is static and exhibits little turnover during postnatal life.
Recent studies demonstrating significant proliferation by thymic epithelial cells in the postnatal thymus have challenged the notion of a static population.1,2 In this issue of Blood, Gray and colleagues present data that underscore the dynamic nature of thymic epithelium in the postnatal thymus and that provide new insight into several fundamental questions of thymic epithelial cell (TEC) biology. Here, they demonstrate that the rate of thymic epithelial turnover approaches that of the epidermis, with a replacement time of 10 to 14 days. This means that the composition and organization of thymic epithelial compartment represents a “snapshot” of the dynamic relationship between progenitor cells, transit-amplifying cells, and loss of cells from terminally differentiated epithelial compartments. From this perspective, thymic involution becomes less mysterious and can now be viewed as a failure of progenitor and/or transit-amplifying TECs to maintain the thymic epithelial compartment at steady-state levels. The reversal of thymic involution following castration can be considered as another perturbation of the dynamics of thymic epithelial (TE) differentiation, where the populations of progenitor and/or transit-amplifying epithelial cells are expanded in response to castration-induced alterations of the hormonal milieu.FIG1
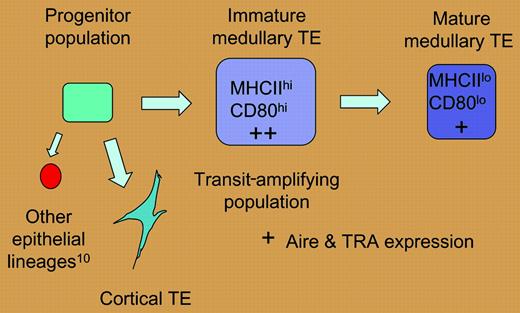
A hypothetical model of thymic epithelial differentiation. If the high level of mitotic activity described by Gray et al for the MHC IIhi, CD80hi medullary epithelial population is indicative of a transit-amplifying population, these cells may be less mature than and give rise to the MHC IIlo, CD80lo population. According to this scenario, levels of tissue-restricted antigens (TRAs) and aire expression would be higher in the immature medullary epithelial compartment.
A hypothetical model of thymic epithelial differentiation. If the high level of mitotic activity described by Gray et al for the MHC IIhi, CD80hi medullary epithelial population is indicative of a transit-amplifying population, these cells may be less mature than and give rise to the MHC IIlo, CD80lo population. According to this scenario, levels of tissue-restricted antigens (TRAs) and aire expression would be higher in the immature medullary epithelial compartment.
The mitotic activity of TE subsets defined in this report helps to model the developmental relationship between subsets of thymic epithelium. Gray and colleagues have demonstrated that the MHC IIhi (CD80hi) medullary TEC (mTEC) subset is heterogeneous and exhibits greater mitotic activity than the MHC IIlo subset, and have suggested that the former population may represent a transit-amplifying population. Furthermore, the temporal relationship observed between the appearance of MHC IIhi and MHClo mTECs may reflect a precursor-product relationship. The developmental relationship suggested by these data is presented in the figure.
As levels of tissue-restricted antigen (TRA) expression are highest in the MHC IIhi (CD80hi) subset of mTECs,3 this model of TE differentiation associates these properties with immature mTECs, rather than terminally differentiated mTECs as has been proposed.3 However, the program of mTEC differentiation suggested by these data is consistent with the notion that TRA expression reflects the activity of immature mTECs.1,4 It follows that the death of thymic epithelial cells that would accompany this dynamic epithelial turnover may facilitate the cross presentation of mTEC-derived antigens by bone marrow–derived antigen-presenting cells within the medullary compartment5 and thus enhance the efficiency of this negative selection process. This scenario would reconcile the efficient negative selection of TRA-specific thymocytes that has been previously observed6,7 with the established rarity and spatial distribution of the epithelial cells that express them.1,7,8
This report also presents provocative data regarding the antigen-presenting capacity of different thymic epithelial subsets. The prevalent view of cortical TEC (cTEC) and mTEC function proposes a division of labor, where cTECs mediate positive selection and function poorly as conventional antigen-presenting cells, while mTECs can serve as antigen-presenting cells and mediate negative selection but do not mediate positive selection.9 In demonstrating that isolated cTECs can effectively present peptide antigens to TCR transgenic T cells, Gray and colleagues challenge this widely held view of cTEC properties. If validated, this observation will require a re-evaluation of the role of cortical epithelial cells in shaping the T-cell antigen receptor specificity repertoire. However, it will be important to demonstrate that the level of dendritic cell contamination present in the cTEC populations does not contribute to the T-cell responses observed. In sum, this study contributes significantly to a dynamic view of thymic epithelium that will influence the design of new models and experimental approaches to understand the differentiation and function of thymic epithelium.
The authors declare no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal