Abstract
In persons with HIV/AIDS (PWHAs), Hodgkin lymphoma (HL) risk is increased. However, HL incidence in PWHAs has unexpectedly increased since highly active antiretroviral therapy (HAART) was introduced. We linked nationwide HIV/AIDS and cancer registry data from 1980 through 2002. Immunity was assessed by CD4 T-lymphocyte counts at AIDS onset. Annual HL incidence rates were calculated for 4 through 27 months after AIDS onset. During 477 368 person years (py's) of follow-up in 317 428 persons with AIDS (PWAs), 173 HL cases occurred (36.2 per 105 py's). Incidence was significantly higher in 1996 to 2002 than earlier. Incidence in PWAs with 150 to 199 CD4 cells/μL was 53.7 per 105 py's, whereas in PWAs with fewer than 50 CD4 cells/μL, it was 20.7 per 105 py's (Ptrend = .002). For each HL subtype, incidence decreased with declining CD4 counts, but nodular sclerosing decreased more precipitously than mixed cellularity, thereby increasing the proportion of mixed cellularity HL seen in PWAs. We conclude that HL incidence is lower with severe immunosuppression than with moderate immunosuppression, and HAART-related improvements in CD4 counts likely explain the increasing HL incidence in PWHAS observed since 1996. With more severe immunosuppression, nodular sclerosing HL becomes infrequent, explaining the higher proportion of mixed cellularity HL found in PWAs. Pathogenesis implications are discussed.
Introduction
Hodgkin lymphoma (HL) risk is significantly increased in persons with HIV/AIDS (PWHAs) of all ages.1-11 Risks relative to the general population are increased from 5- to 15-fold. Additionally, most HL cases in PWHAs are mixed cellularity (MC) and lymphocyte-depleted (LD) subtypes, whereas the nodular sclerosing (NS) subtype predominates in young adults without HIV/AIDS.2,12,13 That HIV/AIDS-related immunosuppression would affect HL risk and subtype distribution is not surprising. Persons with genetic conditions associated with T-lymphocyte immune dysfunction have a higher risk of HL, and patients with HL may have underlying abnormalities of T-cell immunity.14
Few studies of HL in PWHAs have had sufficient data to examine risks in the era of highly active antiretroviral therapy (HAART). By controlling HIV replication with combination therapies, HAART improves immune function, and its use is associated with reduced incidence of Kaposi sarcoma and non-Hodgkin lymphoma (NHL), the 2 most common AIDS-associated cancers.15-18 Unexpectedly, however, HL risk in PWHAs increased after HAART became available in 1996.15,18 Consistent with this temporal increase, HL incidence was reported to be higher in HIV-infected persons using HAART than in those not using it.16
To address the role of immunity in HL etiology, we examined HL risk in a large multicenter study linking HIV/AIDS and cancer registry data. Since 1990, CD4 T-lymphocyte counts have been increasingly recorded by HIV/AIDS registries, permitting prospective studies of HL incidence using this marker of immune status to stratify subjects. We also examined the relationship between CD4 count and the incidence of specific HL subtypes provided by the cancer registries.
Patients, materials, and methods
Since 1991, we have periodically linked HIV/AIDS and cancer registry data in the United States. The current linkage included the states of Connecticut, Georgia, Florida, Massachusetts, Michigan, and New Jersey, and the metropolitan areas of Los Angeles, San Diego, and San Francisco, CA; New York City, NY; and Seattle, WA. We included HIV/AIDS cases registered through 2002 and linked them to cancer diagnoses during periods when cancer registry data were considered complete. Details of the matching approach have been presented elsewhere.6,9,11,19 Briefly, a probabilistic algorithm identified record linkages using the social security number, name, sex, race, and birth and death dates. Possible linkages were reviewed by authorized registry personnel to ensure their validity, and questionable linkages were rejected. All personal identifying data were deleted by the registries before the analysis data set was sent to the National Cancer Institute. All linkage activities were approved by an institutional review board at each HIV/AIDS and cancer registry. The study was exempted from review by the National Cancer Institute because the analysis file contained only anonymized data.
Diagnoses of lymphoma were obtained from cancer registries and were classified according to the International Classification of Diseases for Oncology, Third Edition (ICDO-3) codes,20 using cancer registry data without further review. We have previously reported that HL diagnoses in cancer registry data appear to be reliable.21 HL tumors were categorized into 4 groups: NS (ICDO-3 9663-9667); MC (ICDO-3 9652); LD (ICDO-3 9653-9655) types; and not otherwise specified (NOS) types (ICDO-3 9650). Other histologies were too few to analyze separately but were included, when appropriate, in analyses pertaining to all HL.
The incidence of HL in adult PWAs (≥ 15 years old) was examined according to the latest CD4 count within the AIDS onset period (within 6 months before to 3 months after AIDS onset). The HL incidence evaluation period started at 4 months after AIDS onset to minimize ascertainment bias from medical evaluations occurring at AIDS onset. Follow-up for a person ended at the time of HL diagnosis, death, or the end of complete cancer registration, whichever came first. Follow-up was also censored at 28 months after AIDS onset in order to minimize the possibility that PWAs might have migrated from the registry area or died without being linked to registry data. Incidence was calculated as the rate of HL events in the follow-up period, that is, the number of HL events divided by the follow-up time in person years [py's]). Standardized incidence ratios (SIRs) were calculated as the ratio of observed to the expected number of HL cases, using population rates specific for sex, age group, race/ethnicity, calendar-time period, and cancer registry. The calendar-time periods evaluated correspond to 3 time periods of AIDS onset: when antiretroviral therapy was generally unavailable or limited to single drugs (prior to 1990); when more effective mono- and dual-therapies became standard (1990-1995); and when HAART was available (1996-2002). For comparison, we also examined NHL risks using the same approach. In using this approach, all incidence data and evaluation of the impact of CD4 count were derived from PWAs in the 4- to 27-month period after AIDS onset. Data about HL in persons with HIV infection only (not AIDS) could not be used for incidence-based analyses because we had no reference onset date for a prospective study.
We used Poisson regression to calculate relative risks across subgroups, adjusted for sex, age, race/ethnicity, presumed mode of HIV exposure, calendar-time period, and cancer registry. Unless specified as a trend, regression-based P values are tests of heterogeneity. To examine trends in incidence by CD4 count categories (0-24, 25-49,... to ≥ 300 cells/μL), we fitted Poisson regression models that included CD4 categories with a linear term. To allow for a nonlinear relationship between incidence and CD4 count, we added a quadratic term and compared the fit of that model to the fit of the linear model. For analyses of all HL cases combined, there were sufficient data to examine CD4 groups to the highest category of at least 300 CD4 cells/μL, but for HL subtypes, the highest category was at least 200 CD4 cells/μL because data in some specific groups were sparse. Because CD4 data generally were not available before 1990 (only 2 HL cases), we restricted CD4 analyses to 1990 to 2002. Confidence intervals (CIs) were 2-sided, and P values less than .05 were considered significant.
We also undertook a descriptive study of persons in the HIV/AIDS registry who developed lymphoma at any time before, at, or after AIDS onset and had CD4 counts within 0 to 12 months before lymphoma onset. P values comparing CD4 counts across subtypes were calculated using nonparametric Wilcoxon rank sum and Kruskal-Wallis tests for heterogeneity because the variance differed significantly in the subtypes.
Studies were approved by both the National Cancer Institute and by the institutional review boards at the state and regional levels for each HIV/AIDS and cancer registry.
Results
We identified 1140 HL and 14 606 NHL cases occurring in 473 643 persons registered in HIV/AIDS registries (86.1% PWAs; 13.9% HIV-infected without AIDS) between 1980 and 2002. The site of most HL (95.2%) was identified as lymph node, with 1.6% being in bone marrow, and the remainder being in less common extranodal sites. The proportion of NOS lymphomas, overall 32.1% for HL and 40.6% for NHL, remained stable throughout the study period. Of 774 HL cases with known histology, 416 (53.7%) were MC, 285 (36.8%) were NS, and 54 (7.0%) were LD types, the remaining 19 cases being nodular or diffuse lymphocyte predominance or granulomatous HL, as classified by the older Rye classification of HL.
Of all HL cases, 26.8% were diagnosed before the AIDS onset period, 41.1% in the AIDS onset period (–6 through +3 months), 15.2% in the +4 through +27 months after AIDS diagnosis, and 17.0% after +28 months after AIDS diagnosis. Among those with CD4 counts, the average time at risk between 4 through 27 months varied modestly by CD4 count, ranging from 17 months in PWAs with fewer than 50 CD4 cells/μL to 21 months in PWAs with more than 300 CD4 cells/μL at AIDS onset. The proportion of HL cases with CD4 data was slightly higher than for those without HL (8% vs 7% in 1980-1989; 71% vs 66% in 1990-1995; and 89% vs 87% in 1996-2002, respectively). By comparison, for 2475 NHL cases in the incidence evaluation period, CD4 counts were known at AIDS onset in 8%, 63%, and 85%, for the same calendar periods, respectively.
Incidence and standardized incidence ratios
Incidence was evaluated in the 317 428 adult PWAs who contributed person-time to the follow-up period 4 through 27 months after AIDS onset, resulting in a total of 477 368 person years (Table 1). During this period, 173 HL cases were diagnosed in PWAs, corresponding to an incidence of 36.2 per 105 py's. In 1980 to 1989 and 1990 to 1995, respectively, incidence was 30.9 and 30.4 but in 1996 to 2002, it increased to 49.3 (Ptrend = .01). In comparison, NHL incidence declined steadily throughout the HIV/AIDS epidemic (P < .001; data not shown). Nevertheless, in 1996 to 2002, NHL incidence was 401.6 per 105 py's in the 4 through 27 months after AIDS onset, still 8.1-fold higher than for HL incidence in the same period.
Incidence rates and standardized incidence ratios (SIRs) with 95% confidence intervals (CIs) of Hodgkin lymphoma, 4 through 27 months after AIDS onset
Category . | No. of cases . | Person-years at risk . | Incidence per 105 py's . | Adjusted RR (95% CI) . | Adjusted P . | Expected cases* . | SIR (95% CI)* . |
|---|---|---|---|---|---|---|---|
| All | 173 | 477 368 | 36.2 | — | 18.2 | 9.4 (8.0-10.9) | |
| Sex | .03 | ||||||
| Male | 154 | 384 990 | 40.0 | 1.86 (1.07-3.23) | 15.6 | 9.7 (8.3-11.4) | |
| Female | 19 | 92 377 | 20.6 | 1.00 | 2.6 | 7.3 (4.4-11.4) | |
| Age at AIDS onset, y | .02 | ||||||
| 15 to less than 30 | 18 | 77 806 | 23.1 | 1.00 | 3.4 | 5.2 (3.1-8.3) | |
| 30 to less than 40 | 73 | 221 611 | 32.9 | 1.38 (0.82-2.32) | 8.6 | 8.3 (6.5-10.4) | |
| 40 to less than 50 | 63 | 131 391 | 47.9 | 1.96 (1.15-3.33) | 4.5 | 13.9 (10.7-17.8) | |
| 50 or more | 19 | 46 558 | 40.8 | 1.64 (0.86-3.16) | 1.7 | 11.4 (6.8-17.7) | |
| Race/ethnicity | .22 | ||||||
| White | 68 | 191 786 | 35.5 | 1.00 | 8.2 | 8.3 (6.5-10.6) | |
| Black | 55 | 175 978 | 31.3 | 1.10 (0.74-1.64) | 5.7 | 9.7 (7.3-12.6) | |
| Hispanic | 48 | 103 262 | 46.5 | 1.50 (1.02-2.22) | 4.4 | 11.0 (8.1-14.6) | |
| Other/unknown | 2 | 6 341 | 31.5 | 0.92 (0.22-3.75) | Excluded | Excluded | |
| Mode of HIV exposure | .09 | ||||||
| MSM | 95 | 214 017 | 44.4 | 1.00 | 8.7 | 10.6 (8.6-13.0) | |
| IDU | 42 | 126 691 | 33.2 | 0.75 (0.50-1.13) | 4.8 | 8.8 (6.4-11.9) | |
| MSM who are also IDU | 5 | 24 982 | 20.0 | 0.45 (0.18-1.12) | 1.0 | 4.9 (1.6-11.3) | |
| Heterosexual | 17 | 54 582 | 31.1 | 0.88 (0.48-1.63) | 1.7 | 9.9 (5.8-15.8) | |
| Other/unknown | 14 | 57 095 | 24.5 | 0.54 (0.30-0.98) | 2.0 | 7.1 (3.9-11.9) | |
| Calendar year of AIDS onset | .01 | ||||||
| 1980-1989 | 24 | 77 602 | 30.9 | 1.00 | 3.4 | 7.0 (4.5-10.4) | |
| 1990-1995 | 77 | 253 604 | 30.4 | 0.96 (0.57-1.61) | 9.5 | 8.1 (6.4-10.1) | |
| 1996-2002 | 72 | 146 162 | 49.3 | 1.60 (0.91-2.82) | 5.3 | 13.2 (10.3-16.7) | |
| CD4 cells/μL | .002† | ||||||
| 0-49 | 17 | 81 977 | 20.7 | 0.46 (0.24-0.86) | 3.0 | 5.3 (3.0-8.6) | |
| 50-99 | 16 | 49 388 | 32.4 | 0.70 (0.37-1.33) | 1.8 | 8.2 (4.6-13.5) | |
| 100-149 | 24 | 53 652 | 44.7 | 0.97 (0.55-1.73) | 2.0 | 12.0 (7.7-17.9) | |
| 150-199 | 41 | 76 306 | 53.7 | 1.18 (0.71-1.97) | 2.8 | 14.5 (10.4-19.6) | |
| 200 or more | 23 | 48 767 | 47.2 | 1.00 | 1.8 | 12.6 (8.0-18.9) | |
| Missing | 52 | 167 277 | 31.1 | 0.79 (0.46-1.35) | 6.7 | 7.8 (5.8-10.2) |
Category . | No. of cases . | Person-years at risk . | Incidence per 105 py's . | Adjusted RR (95% CI) . | Adjusted P . | Expected cases* . | SIR (95% CI)* . |
|---|---|---|---|---|---|---|---|
| All | 173 | 477 368 | 36.2 | — | 18.2 | 9.4 (8.0-10.9) | |
| Sex | .03 | ||||||
| Male | 154 | 384 990 | 40.0 | 1.86 (1.07-3.23) | 15.6 | 9.7 (8.3-11.4) | |
| Female | 19 | 92 377 | 20.6 | 1.00 | 2.6 | 7.3 (4.4-11.4) | |
| Age at AIDS onset, y | .02 | ||||||
| 15 to less than 30 | 18 | 77 806 | 23.1 | 1.00 | 3.4 | 5.2 (3.1-8.3) | |
| 30 to less than 40 | 73 | 221 611 | 32.9 | 1.38 (0.82-2.32) | 8.6 | 8.3 (6.5-10.4) | |
| 40 to less than 50 | 63 | 131 391 | 47.9 | 1.96 (1.15-3.33) | 4.5 | 13.9 (10.7-17.8) | |
| 50 or more | 19 | 46 558 | 40.8 | 1.64 (0.86-3.16) | 1.7 | 11.4 (6.8-17.7) | |
| Race/ethnicity | .22 | ||||||
| White | 68 | 191 786 | 35.5 | 1.00 | 8.2 | 8.3 (6.5-10.6) | |
| Black | 55 | 175 978 | 31.3 | 1.10 (0.74-1.64) | 5.7 | 9.7 (7.3-12.6) | |
| Hispanic | 48 | 103 262 | 46.5 | 1.50 (1.02-2.22) | 4.4 | 11.0 (8.1-14.6) | |
| Other/unknown | 2 | 6 341 | 31.5 | 0.92 (0.22-3.75) | Excluded | Excluded | |
| Mode of HIV exposure | .09 | ||||||
| MSM | 95 | 214 017 | 44.4 | 1.00 | 8.7 | 10.6 (8.6-13.0) | |
| IDU | 42 | 126 691 | 33.2 | 0.75 (0.50-1.13) | 4.8 | 8.8 (6.4-11.9) | |
| MSM who are also IDU | 5 | 24 982 | 20.0 | 0.45 (0.18-1.12) | 1.0 | 4.9 (1.6-11.3) | |
| Heterosexual | 17 | 54 582 | 31.1 | 0.88 (0.48-1.63) | 1.7 | 9.9 (5.8-15.8) | |
| Other/unknown | 14 | 57 095 | 24.5 | 0.54 (0.30-0.98) | 2.0 | 7.1 (3.9-11.9) | |
| Calendar year of AIDS onset | .01 | ||||||
| 1980-1989 | 24 | 77 602 | 30.9 | 1.00 | 3.4 | 7.0 (4.5-10.4) | |
| 1990-1995 | 77 | 253 604 | 30.4 | 0.96 (0.57-1.61) | 9.5 | 8.1 (6.4-10.1) | |
| 1996-2002 | 72 | 146 162 | 49.3 | 1.60 (0.91-2.82) | 5.3 | 13.2 (10.3-16.7) | |
| CD4 cells/μL | .002† | ||||||
| 0-49 | 17 | 81 977 | 20.7 | 0.46 (0.24-0.86) | 3.0 | 5.3 (3.0-8.6) | |
| 50-99 | 16 | 49 388 | 32.4 | 0.70 (0.37-1.33) | 1.8 | 8.2 (4.6-13.5) | |
| 100-149 | 24 | 53 652 | 44.7 | 0.97 (0.55-1.73) | 2.0 | 12.0 (7.7-17.9) | |
| 150-199 | 41 | 76 306 | 53.7 | 1.18 (0.71-1.97) | 2.8 | 14.5 (10.4-19.6) | |
| 200 or more | 23 | 48 767 | 47.2 | 1.00 | 1.8 | 12.6 (8.0-18.9) | |
| Missing | 52 | 167 277 | 31.1 | 0.79 (0.46-1.35) | 6.7 | 7.8 (5.8-10.2) |
Relative risks (RR) for all variables except CD4 count are presented adjusted for cancer registry and all other listed variables except CD4 count. For CD4 count, the adjusted RR is adjusted for cancer registry and all other listed variables. No RR is provided for “All” because there are no strata in this category. Person years do not sum exactly because of rounding. py indicates person years; MSM, men who have sex with men; IDU, intravenous drug users; —, not applicable.
Expected and SIR based on excluding 2 persons with unknown race (N = 171 total).
Test of trend (for CD4 cells/μL, does not include observations with missing CD4 counts).
Compared with the general population, HL risk was 9.4-fold (95% confidence interval [CI]: 8.0-10.9) higher in PWAs (Table 1). SIRs were similarly elevated, about 10-fold, in most demographic subgroups. However, because there was no young adult age peak in HL incidence among PWAs, the resulting SIR (5.2) was lower for 15- to 29-year-old PWAs. SIRs for HL were similar in 1980 to 1989 (7.0) and 1990 to 1995 (8.1) but increased significantly in 1996 to 2000 (13.2).
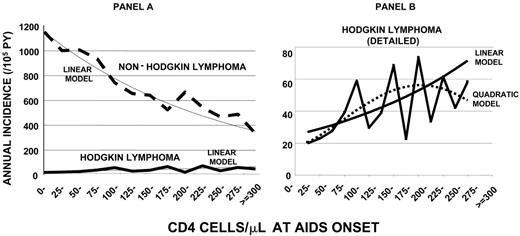
Trends in incidence for NHL and HL differed markedly by CD4 count at AIDS onset (Figure 1A). As expected, NHL incidence increased steadily as CD4 counts declined (Plinear trend < .001). In sharp contrast, HL incidence peaked at 73.4 per 105 py's in PWAs with 225 to 249 CD4 cells/μL and decreased thereafter to 26.7 per 105 py's in PWAs with fewer than 25 CD4 cells/μL (overall Plinear trend = .002; based on 121 cases with CD4 count data). A regression model including a quadratic term fit the data better than a linear model alone (P = .053; Figure 1B), indicating a peak incidence of 55.5 at 225 to 249 CD4 cells/μL and a lower incidence of 19.8 in PWAs with fewer than 25 CD4 cells/μL (Figure 1B). Using categories of 50 CD4 cells/μL for robustness, a similar relationship between HL and immunity was observed in 1990-1995 (Plinear trend = .04) and 1996-2002 (Plinear trend = .08).
For both HL and NHL, patterns for SIRs by CD4 counts reflected incidence patterns. The SIR for NHL increased steadily with declining CD4 counts. For HL, the estimated SIR in the linear model was 18.4 for PWAs with at least 300 CD4 cells/μL, but for PWAs with fewer than 25 CD4 cells/μL, it was 7.0. A model that included a quadratic term again fit better than a linear model alone (P = .049), indicating a peak SIR of 15.0 in PWAs with 225 to 249 CD4 cells/μL and a SIR of 5.1 in PWAs with fewer than 25 CD4 cells/μL.
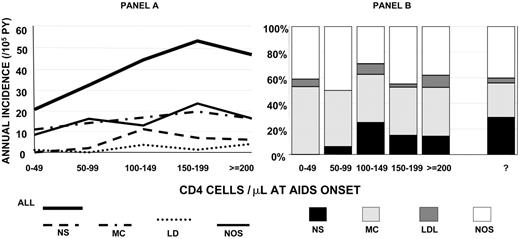
Subtypes were specified for 104 (60%) HL cases in the 4 through 27 months after AIDS onset. The distributions of HL subtypes were similar in sex, age, race/ethnicity, and mode of HIV exposure strata (not presented). For every specified HL subtype and for NOS HL, incidence declined with declining CD4 counts, although the decline was statistically significant only for the NS subtype (Ptrend = .047; Figure 2A). At all CD4 counts in PWAs, the incidence of MC HL was higher than NS HL, but the high proportion of MC was particularly evident in PWAs with very low CD4 counts, among whom no NS cases occurred in PWAs with fewer than 50 CD4 cells/μL at AIDS onset (Figure 2B).
CD4 counts within 12 months preceding lymphoma onset
Of 1140 HL cases found in PWHAs, 311 (27%) had CD4 counts within 12 months before HL diagnosis. In comparison, 4344 (30%) of 14 606 NHL cases had CD4 counts within 12 months before diagnosis. The CD4 count in HL cases was higher than in NHL cases (median 123 vs 62 CD4 cells/μL, respectively; P < .001; Figure 3).
Incidence of HL and NHL by CD4 count at AIDS onset. (A) HL compared to NHL on the same scale. (B) Comparison of linear with quadratic model for HL. Linear models fit the observed data well and showed significant trends with CD4 counts (P = .002 for HL; P < .001 for NHL). A model including a quadratic term fit the HL data better than a linear model alone (P = .053).
Incidence of HL and NHL by CD4 count at AIDS onset. (A) HL compared to NHL on the same scale. (B) Comparison of linear with quadratic model for HL. Linear models fit the observed data well and showed significant trends with CD4 counts (P = .002 for HL; P < .001 for NHL). A model including a quadratic term fit the HL data better than a linear model alone (P = .053).
Median CD4 counts were similar in NS and MC but lower in LD subtypes (median 133 cells/μL, 130 cells/μL, and 75 cells/μL, respectively; Figure 3). In nonparametric tests for heterogeneity, these differences were marginally significant (P = .065). Average CD4 counts were higher in NS than MC or LD HL (mean 229 cells/μL, 159 cells/μL, 102 cells/μL, respectively), but because variances were unequal, parametric comparisons were inappropriate.
Incidence rates and proportional distribution of HL subtypes by CD4 count at onset of AIDS. (A) Incidence rates of all HL and subtypes. (B) Proportion distribution including those not otherwise specified (NOS). NS indicates nodular sclerosing; MC, mixed cellularity; LD, lymphocyte depleted. 3 HLs with old histology classifications were not included in subtype analyses but were included in the All HL analysis because they had CD4 counts in the AIDS onset period (1 with 150-199 and 2 with ≥ 200 cells/μL).
Incidence rates and proportional distribution of HL subtypes by CD4 count at onset of AIDS. (A) Incidence rates of all HL and subtypes. (B) Proportion distribution including those not otherwise specified (NOS). NS indicates nodular sclerosing; MC, mixed cellularity; LD, lymphocyte depleted. 3 HLs with old histology classifications were not included in subtype analyses but were included in the All HL analysis because they had CD4 counts in the AIDS onset period (1 with 150-199 and 2 with ≥ 200 cells/μL).
Discussion
We and others have observed that HL incidence is significantly increased in PWAs.1-11,15-17 Consistent with this observation, we found approximately 10-fold increased risks overall and in most demographic groups, compared with the general population. Excess risks for HL have also been noted in patients with congenital immunodeficiencies and patients with iatrogenic immunosuppression,14 such as those undergoing allogeneic bone marrow transplantation22 and those receiving methotrexate for autoimmune conditions.23,24 Notably, some HL tumors have undergone spontaneous remission when immunosuppressive therapy was discontinued.23 These findings suggest that the increased incidence of HL may be directly related to immunosuppression.
However, our detailed analyses revealed a more complex relationship, in which HL risk in PWAs was highest in moderately immunosuppressed PWAs but decreased significantly in more severely immunosuppressed PWAs. This pattern contrasted with the steady increase in NHL risk seen with increasing immunosuppression. Moreover, decreasing risks with declining CD4 counts were observed in every HL subtype and in NOS HL. Specifically, NS HL was not observed in the most severely immunosuppressed PWAs group. NS HL differs from MC and LP HL in having different demographic and prognostic features and in its relationship to Epstein-Barr virus.13 Indeed, NS HL has not been linked to immunodeficiency, and our data suggest that NS HL is uncommon in PWAs with severe immunodeficiency. Instead, an MC or LD histology predominates at all levels of immunity found in PWAs. Thus, the overall MC/NS HL ratio in PWAs, 53.7% versus 35.8%, was reversed compared with that expected in North America, in which the majority (70%) are NS HL and 20% to 25% are MC HL.13
Medians and interquartile distributions of CD4 counts 0 to 12 months before lymphoma onset for Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) in all PWHAs. CD4 counts are expressed as cells/μL. n = 311 for HL cases; n = 4344 for NHL cases. NS indicates nodular sclerosing (n = 58); MC, mixed cellularity (n = 117); LD, lymphocyte depleted (n = 19); NOS, not otherwise specified (n = 113).
Medians and interquartile distributions of CD4 counts 0 to 12 months before lymphoma onset for Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) in all PWHAs. CD4 counts are expressed as cells/μL. n = 311 for HL cases; n = 4344 for NHL cases. NS indicates nodular sclerosing (n = 58); MC, mixed cellularity (n = 117); LD, lymphocyte depleted (n = 19); NOS, not otherwise specified (n = 113).
These results need to be interpreted carefully. CD4 counts were not available on all PWAs. However, the proportions with CD4 counts were very similar in PWAs with HL, NHL, and AIDS without lymphoma. To accommodate differences in follow-up time in PWAs in different CD4 count categories, we used Poisson models that carefully accounted for censoring events, including death. To obtain unbiased estimates of incidence, the only assumption is that within each CD4 category, censoring is independent from the outcome, which can be ensured by using fine CD4 categorization, as we did.
Antiretroviral therapies control HIV replication and have been especially effective in the HAART era. With HIV control, CD4 levels would likely have been partially restored in the incidence evaluation period.25-28 However, it is difficult to estimate the impact of antiretroviral therapy because some subjects would have already been on such therapies before AIDS developed (ie, failed therapy), whereas others would have been therapy-naive and would, therefore, have had good responses to therapy.29-33 Despite these limitations, we observed a significant decline in HL incidence in PWAs with very low CD4 counts at AIDS onset in both the pre-HAART and HAART eras, whereas for NHL, our control tumor, we observed the expected relationship of a higher risk with lower CD4 counts.
To generalize our findings beyond the 4- through 27-month period after AIDS onset, we examined CD4 counts in the 0 to 12 months before HL onset, thereby using all possible HL cases and ensuring the CD4 count was measured closer to the onset of HL. In these patients, CD4 counts were highest in those with NS HL, consistent with the incidence findings after AIDS onset. Most HL (60%) occurred at AIDS onset, and therefore the CD4 counts were not affected by therapies that were first started at the time of AIDS onset, but we had no data about actual use of antiretroviral or other therapies.
We cannot determine the level at which an abnormal CD4 count becomes associated with an elevated HL risk because our subjects all had low CD4 counts at entry. However, risk cannot continue to increase with improving CD4 counts, as implied by a simple linear model. A regression model with a quadratic term is therefore intuitively appealing, and this model fit our results better than did the linear model. Using the quadratic model, HL risk peaked at 15-fold higher than the general population rate for PWAs with 225 to 249 CD4 cells/μL at AIDS onset, then decreased with increasingly severe immunosuppression. However, risk for PWAs with 0 to 24 CD4 cells/μL still remained 5-fold higher than in the general population.
The relationship with immunity likely explains why HL incidence in PWAs has increased in recent years.15-17 During the 1990s, antiretroviral therapies became widely used and increasingly effective, culminating in the widespread use of HAART after 1996. These therapies resulted in better immunity, lower incidence, and improved survival for PWHAs.25-28 Our data imply that HAART-induced increases in immunity may have placed PWHAs at higher risk of HL. MC HL dominated the mix of HL at all CD4 counts seen in PWHAs and therefore continued to be the majority of HL subtypes even after 1996.
We can speculate about why these relationships are observed. An essential feature of HL is the nonneoplastic milieu of reactive cells in the tumor mass. The neoplastic Hodgkin-Reed Sternberg (H-RS) cells usually constitute only 0.1% to 1% of the cells present in the tumor.13,33,34 The H-RS cells are likely of B-lymphocyte lineage and therefore are unlikely to be directly impacted by HIV, which infects CD4+ T-lymphocytes. However, H-RS cells produce many cytokines and chemokines, resulting in an influx of activated CD4 cells (CD40+, CD26–), histiocytes, and other cells.13,33-37 Moreover, H-RS cells can respond to the inflammatory cells surrounding them.13,33-37 Thus, recruited inflammatory cells may provide essential feedback signals that stimulate proliferation or inhibit apoptosis of H-RS cells. HL incidence may decline in severely immunosuppressed PWAs because the malignant H-RS cells are not able to recruit lymphocytes and histiocytes required for their survival. Alternatively, with advanced immunosuppression, perhaps HL remains occult until the immune system is sufficiently reconstituted to respond to the H-RS cells, or lymphomagenesis could shift toward NHL phenotypes, which are independent of an inflammatory milieu.38-41
In summary, moderate immunosuppression increases HL incidence, but incidence declines in more severely immunosuppressed PWAs. HAART use has improved immunity after AIDS onset, paradoxically increasing HL incidence among PWAs, especially those of MC subtype. We speculate that, with severe immunosuppression, the cellular background surrounding the H-RS cells may be altered. Whether this change affects its categorization as HL or whether it inhibits or delays HL development is unknown.
Authorship
R.J.B. and E.A.E. were primarily responsible for the design, analysis, and writing of this report; E.S.J. provided input on the HL and NHL pathology and pathogenesis; A.C. and R.P. provided statistical support; J.J.G. helped to establish the linkage studies and write the paper; and all authors approved submission.
The authors have no conflict of interest or financial involvement in the findings of this study.
A list of the representative registries of the HIV/AIDS Cancer Match study appears as a supplement to the online version of this article.
Prepublished online as Blood First Edition Paper, August 17, 2006; DOI 10.1182/blood-2006-05-024109.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The HIV/AIDS Cancer Match Study is an NCI-funded multicenter HIV/AIDS and cancer registry–linkage study involving state and regional areas throughout the United States. HIV/AIDS and cancer registries in the following regions participated in the HIV/AIDS Cancer Match Study: the states of Connecticut, Florida, Georgia, Massachusetts, Michigan, and New Jersey; and the metropolitan areas of Los Angeles, San Diego, and San Francisco (CA), New York City (NY), and Seattle (WA). We are grateful for the many staff members who participated in the record linkages. We also thank Norma Kim and Emily Moser (RTI International, Rockville, MD) for study management; Phil Virgo (Computer Sciences Corporation, Rockville, MD) for assistance with data collection (contract HHSN261 200 555 004C); Timothy McNeel (Information Management Services, Silver Spring, MD) for data management (contract N02-CP-31 003); and Leonard Chang (summer student; currently a doctoral student in Biostatistics at the University of Medicine and Dentistry, Piscataway, NJ) for analyses.
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal