Abstract
During innate immune responses, natural killer (NK) cells may interact with both plasmacytoid dendritic cells (pDCs) and monocyte-derived dendritic cells (MDDCs). We show that freshly isolated NK cells promote the release by pDCs of IFN-α, in a CpG-dependent manner, whereas they induce IL-6 production in a CpG-independent manner. In turn pDC-derived IFN-α up-regulates NK-mediated killing, whereas IL-6 could promote B-cell differentiation. We also show that exposure to exogenous IL-12 or coculture with maturing MDDCs up-regulates the NK-cell–dependent IFN-α production by pDCs. On the other hand, NK cells cocultured with pDCs acquire the ability to kill immature MDDCs, thus favoring their editing process. Finally, we show that activated NK cells are unable to lyse pDCs because these cells display an intrinsic resistance to lysis. The exposure of pDCs to IL-3 increased their susceptibility to NK-cell cytotoxicity resulting from a de novo expression of ligands for activating NK-cell receptors, such as the DNAM-1 ligand nectin-2. Thus, different cell-to-cell interactions and various cytokines appear to control a multidirectional network between NK cells, MDDCs, and pDCs that is likely to play an important role during the early phase of innate immune responses to viral infections and to tumors.
Introduction
Innate immune responses play a crucial role not only in controlling pathogen invasion, but also in instructing the adaptive immune system to initiate a specific response against infecting pathogens.1 Natural killer (NK) cells and dendritic cells (DCs) represent crucial effector cells of the innate immune system.2,3 Several reports have highlighted the importance of the reciprocal interaction between NK cells and DCs during the early stages of innate immune responses.4-9 The NK/DC cross talk can occur within inflamed peripheral tissues and results in a potent DC-induced activation of NK-cell functions, which, in turn, exert a modulatory role on DC activity. Pathogen clearance mediated by innate effector mechanisms is, at least in part, the result of the DC-induced up-regulation of NK-cell cytotoxicity and of the release of antimicrobial cytokines from various cell types recruited at the inflammatory sites.10,11 The reciprocal interactions between NK and DCs could regulate the quality of DCs undergoing maturation. For example, because NK cells are capable of killing immature DC (iDCs), the existence of an “editing” process has been suggested, by which DCs may or may not acquire the ability to prime Th1 immune responses.12,13 Most reports regarding the cross talk between NK cells and DCs in humans have been focused on the interactions with monocyte-derived dendritic cells (MDDCs), whereas only limited information exists on the interaction with plasmacytoid DCs (pDCs).14,15 pDCs are capable of producing large amounts of IFN-α in response to viral DNA or RNA.16 This suggests that, together with NK cells, they play an important role in sensing viral infections and in promoting antiviral innate responses. We have previously shown that pDCs, on stimulation through TLR9, can activate NK cells to kill various types of tumor cells. Moreover, following TLR9 engagement, pDCs could induce a selective proliferation of the small NK-cell subset characterized by the CD56bright phenotype.15 In addition, IL-2–activated NK cells have been shown to promote pDC maturation and IFN-α release.14 On the other hand, it is not clear whether, in humans, this event can actually occur during the early phases of innate immune responses in which T cells (ie, the only IL-2–producing cells) are not involved.
In the present study we show that the regulatory effect of NK cells on pDC function does not strictly require exposure to IL-2, because also freshly isolated NK cells promoted pDC maturation and cytokine release. Moreover, an abundant IFN-α release by pDCs could be induced when NK cells were exposed to IL-12, thus suggesting that an early release of this cytokine by pathogen-activated MDDCs may represent an important link between NK/DC and NK/pDC interactions. In turn, NK cells activated by pDC acquired the ability to kill immature MDDCs, thus mediating their editing process.10 Finally, we show that blood-derived pDCs are resistant to NK-cell–mediated lysis, because of the low surface expression of ligands for activating NK receptors.
Materials and methods
Antibodies and blocking reagents
The following monoclonal antibodies (mAbs) were used in this study: M5A10 (IgG1, anti-PVRa), ECM17/120 (IgM anti–LFA-1), L14 (IgG2a, anti–nectin-2), BAM195 (IgG1, anti-MICA), M7E22 (IgG1, anti–ICAM-1), and A6/136 (IgM, anti–HLA class I). The various anti-NK receptors were previously described.8 M295 (IgG1, anti-ULBP1), M310 (IgG1, anti-ULBP2), M551 (IgG1, anti-ULBP3), and M475 (IgG1, antiULBP4) were kindly provided by Amgen (Seattle, WA). Anti–CD1a-PE (IgG1), anti–CD14-PE (IgG2a), anti–CD83-PE (IgG2b), anti–CD86-PE (IgG2b), a mixture of anti–CD56-PC5 mAb and anti–CD3-FITC mAb (Immunotech, Marseille, France), anti-CD83 (HB15e), and anti-CD86 (2331; BD Biosciences, Milan, Italy), anti–BDCA2-FITC, anti–CD19-FITC, and anti–BDCA4-PE (Miltenyi Biotec, Bergisch Gladbach, Germany) were also used. The following neutralizing antibodies were used in this study: anti-IFNα (MMH11 or MMHA-2; PBL Biomedical Laboratories, Piscataway, NJ; NYRhIFNα, ProSpec-Tany TechnoGene, Rehovot, Israel), anti-IFNαβ receptor (PBL Biomedical Laboratories), anti–IL-12 (goat polyclonal antihuman; PeproTech, London, United Kingdom). For intracellular detection of cytokines mouse antihuman anti–IL-6 (20F30) and anti-TNF (MAb11) from BD Biosciences were used.
Cell preparations
Peripheral-blood mononuclear cells (PBMCs) were derived from healthy donors by Ficoll-Hypaque gradients (Sigma-Aldrich, St Louis, MO). pDCs were obtained from PBMCs by positive magnetic selection with anti-BDCA4 microbeads (Miltenyi Biotec). Purified cells were more than 97% pDCs, as evaluated with anti–BDCA2-FITC mAb. NK cells were purified from pDC-depleted PBMCs by negative selection, using the NK-cell isolation kit II (Miltenyi Biotec). In some experiments both pDCs and NK cells were subsequently sorted by fluorescence-activated cell sorting (FACS) to obtain highly purified cell populations (DIVA; BD Biosciences). Where indicated, pDCs were supplemented with 10 ng/mL rhIL-3 (PeproTech).
The MDDCs were generated as previously described.8 Briefly, plastic adherent cells obtained from PBMCs were cultured in RPMI 1640 containing 10% FCS, in the presence of IL-4 and GM-CSF (PeproTech) at final concentrations of 20 ng/mL and 50 ng/mL, respectively. After 6 days of culture, cells were characterized by the CD14–CD1a+CD83– phenotype corresponding to immature MDDCs (iMDDCs). To generate CD83+CD86+ mature DCs, iMDDCs were stimulated with LPS (Sigma-Aldrich) at a final concentration of 1 μg/mL.
To obtain polyclonally activated NK cells, freshly isolated NK cells were cultured on irradiated feeder cells in the presence of 100 U/mL rhIL-2 (Proleukin; Chiron, Emeryville, CA) and 1.5 ng/mL phytohemagglutinin (Gibco, Paisley, United Kingdom).
The medium used throughout the experiments was RPMI 1640 supplemented with 2 mM l-glutamine, 1% penicillin-streptomycin-neomycin mixture, and 10% heat-inactivated FCS.
Approval was obtained from the University of Genoa (Italy) Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki.
NK/pDC cocultures
Purified resting NK cells were cultured in 96-well round-bottomed microtiter plates (105 cells/well) in the presence of autologous freshly isolated pDCs, at the NK/pDC ratio of 10:1 or 5:1, in the presence or in the absence of CpG-A ODN2216 or CpG-B ODN2006. CpG-ODNs have been synthesized and purified by TIB MOLBIOL (CBA, Genoa, Italy) and used at a final concentration of 5 μg/mL or 0.5 μg/mL. As control pDCs were cultured alone (ie, without NK cells) in the presence or in the absence of CpG-ODN.
Where indicated, purified resting NK cells were treated overnight with rhIL-12 (PeproTech) or rhIL-2 or rhIL-4 at the final concentrations of 2 ng/mL, 100 U/mL, and 25 ng/mL, respectively. Cytokine-exposed NK cells were washed before culture with pDCs, whereas pDCs were kept overnight at 4°C before coculture with NK cells. After 24 to 72 hours of culture supernatants from NK/pDC cocultures were collected for subsequent analysis of IFN-α and IL-6 content.
NK/MDDC cocultures
Purified resting NK cells were cultured overnight in the presence of allogeneic iMDDCs stimulated with 1 μg/mL LPS. MDDC-cultured NK cells were then separated from MDDCs by immunomagnetic depletion. Negatively separated cells were more than 98% NK cells as indicated by CD56+ and CD1a– staining. Separated NK cells were then cocultured with autologous pDCs as indicated in “NK/pDC cocultures.”
Transwell and blocking experiments
Transwell experiments were performed in 24-well transwell plates (0.4-μm pore size; Corning Costar, Corning, NY). NK cells (5 × 105) placed in the bottom chamber were stimulated with 0.5 × 105 pDCs placed in the same or in the top chamber. As control, pDCs alone (ie, without NK cells) were cultured in a 24-well plate or in the top chamber of the transwell. In the blocking experiments, saturating amounts of one or another of the following reagents were added at the onset of the cell cultures: a mixture of anti–IFN-α and anti-IFN receptor; anti–IL-12 antisera; anti-NKp46, anti-NKp30, anti-2B4, anti–LFA-1, anti–DNAM-1 (IgM), and anti-NKG2D (IgG2b).
Cytokine assays
Supernatants collected from NK/pDC cocultures were evaluated for IFN-α content by using enzyme-linked immunosorbent assay (ELISA) kits (Biosource International, Camarillo, CA), whereas IL-6 content was analyzed with Bioplex assay (Bio-Rad, Hercules, CA). To detect pDC intracellular production of IL-6 and TNF, pDCs were cultured together with CFSE-labeled NK cells as described.15 After 18 hours of coculture, cells were stimulated or not with IL-3 or IL-3 and CpG, and after 2 hours brefeldin A (5 μg/mL; Sigma-Aldrich) was added for an additional 4 hours. After 6 hours of stimulation, cells were washed, fixed with 2% formaldehyde in PBS for 15 minutes at room temperature, washed, and then permeabilized with 0.5% saponin (Sigma-Aldrich) in PBS/BSA/azide and stained with mouse antihuman anti–IL-6 and anti-TNF mAb for 15 minutes at room temperature. NK cells were excluded from the cytofluorimetric analysis on the basis of CFSE staining.
Cytolytic assays and flow cytofluorimetric analysis
NK cells that had been cultured for 24 hours with autologous pDCs in the presence or in the absence of CpG-A, with the addition or not of neutralizing mAbs specific for IFN-α and for its receptor, were tested for cytolytic activity against allogeneic iMDDCs or allogeneic freshly blood-derived pDCs in a 4-hour 51Cr-release assay as previously described.8 In other experiments polyclonally activated NK cells were tested for their ability to lyse allogeneic iMDDCs or LPS-matured MDDCs or allogeneic pDCs, activated or not with rhIL-3 for 48 hours, in 4-hour 51Cr-release assays. For masking experiments mAbs of IgM isotype were added at the concentration of 10 μg/mL. One-, 2- or 3-color cytofluorimetric analysis (FACSCalibur; Becton Dickinson, Mountain View, CA), was performed as described.8
Results
Freshly isolated peripheral-blood NK cells modulate pDC functions
As shown in previous reports, human pDCs stimulated via TLR9 could enhance NK-cell function by promoting cell activation, antitumor cytotoxicity, and cell proliferation.14,15 In the present study we further investigated whether, in turn, NK cells could exert any effect on pDC functions.
We first analyzed whether freshly isolated peripheral-blood NK cells could regulate the IFN-α production by pDCs. In these experiments pDCs were cocultured with autologous NK cells in the presence or in the absence of CpG-A (TLR9 ligand). After 24 hours of culture, supernatants were evaluated by ELISA for IFN-α content. As shown in Figure 1A, although pDCs alone produced IFN-α in response to CpG-A, the amount was significantly up-regulated when pDCs were cultured in the presence of NK cells. The magnitude of this effect was dependent, in different donors, on the NK/pDC ratio as well as on the concentration of CpG-A. For example, the representative donor shown in Figure 1A displayed a higher NK-dependent IFN-α release at a 10:1 NK/pDC ratio when high doses of CpG-A were used. At lower doses, the optimal effect was observed at a 5:1 ratio. Although not shown, no IFN-α release could be detected in the absence of CpG-A. These data are in agreement with those reported by Gerosa et al.14
Freshly isolated NK cells promote IFN-α and IL-6 release by pDCs. (A) Peripheral-blood–derived pDCs were cultured in the presence or in the absence of resting autologous NK cells at the NK/pDC ratios of 10:1 or 5:1, stimulated by CpG-A that was added at the concentrations of 5 μg/mL or 0.5 μg/mL. After 24 hours of culture, supernatants were harvested and analyzed for IFN-α content by specific ELISA. ▪, IFN-α concentrations in supernatants collected from cell cultures stimulated by CpG-A at the higher dose of 5 μg/mL; □, supernatants collected from cell cultures stimulated with 0.5 μg/mL; ▦, supernatants collected from unstimulated cell cultures (ie, in the absence of CpG-A). Histograms on the right show IFN-α release at the NK/pDC ratio of 5:1, and histograms on the left indicate IFN-α release at the NK/pDC ratio of 10:1. NK cells cultured alone did not produce IFN-α either in the presence or in the absence of CpG-A. A representative experiment of 10 performed is shown. (B) pDCs were precultured in the presence (right panels) or in the absence (left panels) of autologous resting NK cells for 18 hours and then cultured for 6 hours in the presence of brefeldin A with the indicated stimuli (medium, IL-3 + CpG-A, IL-3 + CpG-B). Then, TNF and IL-6 production by pDCs was evaluated by intracellular double immunofluorescence and cytofluorimetric analysis. The percentage of cytokine-producing pDCs is indicated in the corresponding square. (C) Supernatants collected from parallel NK/pDC cocultures run for 72 hours of culture (without the addition of brefeldin A) were analyzed for IL-6 content with Bioplex. As control, supernatants from cultures containing pDCs alone or NK cells plus IL-3 and CpG-A have also been analyzed. (D) The purity of pDCs after FACS is shown as a double fluorescence staining where pDCs are labeled with anti–BDCA4-PE and anti–CD19-FITC mAbs. (E) The purity of NK cells after FACS is shown as a double fluorescence staining where NK cells are labeled with anti–CD56-PC5 and anti–CD3-FITC. Data are representative of 6 independent experiments that gave comparable results.
Freshly isolated NK cells promote IFN-α and IL-6 release by pDCs. (A) Peripheral-blood–derived pDCs were cultured in the presence or in the absence of resting autologous NK cells at the NK/pDC ratios of 10:1 or 5:1, stimulated by CpG-A that was added at the concentrations of 5 μg/mL or 0.5 μg/mL. After 24 hours of culture, supernatants were harvested and analyzed for IFN-α content by specific ELISA. ▪, IFN-α concentrations in supernatants collected from cell cultures stimulated by CpG-A at the higher dose of 5 μg/mL; □, supernatants collected from cell cultures stimulated with 0.5 μg/mL; ▦, supernatants collected from unstimulated cell cultures (ie, in the absence of CpG-A). Histograms on the right show IFN-α release at the NK/pDC ratio of 5:1, and histograms on the left indicate IFN-α release at the NK/pDC ratio of 10:1. NK cells cultured alone did not produce IFN-α either in the presence or in the absence of CpG-A. A representative experiment of 10 performed is shown. (B) pDCs were precultured in the presence (right panels) or in the absence (left panels) of autologous resting NK cells for 18 hours and then cultured for 6 hours in the presence of brefeldin A with the indicated stimuli (medium, IL-3 + CpG-A, IL-3 + CpG-B). Then, TNF and IL-6 production by pDCs was evaluated by intracellular double immunofluorescence and cytofluorimetric analysis. The percentage of cytokine-producing pDCs is indicated in the corresponding square. (C) Supernatants collected from parallel NK/pDC cocultures run for 72 hours of culture (without the addition of brefeldin A) were analyzed for IL-6 content with Bioplex. As control, supernatants from cultures containing pDCs alone or NK cells plus IL-3 and CpG-A have also been analyzed. (D) The purity of pDCs after FACS is shown as a double fluorescence staining where pDCs are labeled with anti–BDCA4-PE and anti–CD19-FITC mAbs. (E) The purity of NK cells after FACS is shown as a double fluorescence staining where NK cells are labeled with anti–CD56-PC5 and anti–CD3-FITC. Data are representative of 6 independent experiments that gave comparable results.
Following coculture with freshly isolated peripheral-blood NK cells, pDCs displayed slight increments of surface molecules expressed during maturation such as CD83. Expression of CD86 remained substantially unchanged (not shown). These data are in agreement with a previous report on pDC maturation induced by IL-2–activated NK cells.14
We also analyzed whether following coculture with fresh NK cells, pDCs could enhance their production of IL-6 and TNF-α. In these experiments, pDCs were preincubated for 18 hours with or without NK cells and then triggered with different stimuli for 6 hours in the presence of brefeldin A. Cells were assessed for cytokine production by intracellular staining and cytofluorimetric analysis. As shown in Figure 1B, NK cells were capable of enhancing the IL-6 production induced by different stimuli (such as IL-3 + CpG-A and IL-3 + CpG-B). In addition, they could also directly promote IL-6 production in unstimulated pDCs. This effect was dependent on NK cells because pDCs did not produce IL-6 when cultured alone. Consistent with these data, NK cells induced increments of IL-6 production by pDCs also when determined by Bioplex analysis (Figure 1C). On the contrary, NK cells did not promote any significant TNF production by pDCs (Figure 1B). Moreover, cytofluorimetric analysis showed that IL-6–producing pDCs were distinct from those producing TNF. The purity of the cell populations analyzed in these experiments rules out the possibility that contaminating cell populations might be responsible for the observed cytokine production (Figure 1D-E).
Altogether our data support the notion that NK cells can exert a regulatory control on the IFN-α production by pDCs in response to microbial stimuli as well as on the release of proinflammatory cytokines such as IL-6. Notably, IL-6 production can be promoted even in the absence of TLR-dependent activation.
The NK-dependent IFN-α release by pDCs is up-regulated by IL-12
Previous studies suggested that IL-2 allows NK cells to exert a regulatory effect on pDC functions.14 This consists of the induction of cell maturation and in increases of the IFN-α production. Although we have provided evidence that even resting NK cells can enhance pDC effector functions, it is possible that this effect may be further modulated at the site of inflammation by locally produced cytokines. Thus, it is conceivable that, in the course of immune responses, pDCs interact with cytokine-exposed NK cells. This can occur at different sites including peripheral tissues and lymph nodes.15,17-20 Notably, whereas in lymph nodes the effect of NK cells on pDC function may be modulated, at least in part, by IL-2 released by primed T cells, the IL-2 itself may not have any significant effect on NK-cell function during innate inflammatory responses occurring in peripheral tissues. In this context intracytoplasmic IL-2 was demonstrated at early stages of DC responses to pathogen-derived products only when DCs were generated in the presence of IL-15 but not in the presence of IL-4.21 Thus, in inflamed tissues, IL-12, but not IL-2, is likely to represent one of the key cytokines released by antigen-presenting cells (APCs). Along this line, when NK cells were exposed to IL-12 for a short time (compatible with the time window of an early innate response) this resulted in abundant IFN-γ release in response to activating stimuli acting on natural cytotoxicity receptors (NCRs).22 Thus, we further analyzed whether NK cells that had been exposed to exogenous IL-12 could modulate pDC function. In these experiments, NK cells incubated overnight with IL-12 were extensively washed prior to coculture with autologous pDCs in the presence of different doses of CpG-A. These NK cells could promote IFN-α release by pDCs (Figure 2A) to a much greater extent than resting NK cells or IL-4–exposed NK cells.
To analyze whether the increased IFN-α release was mediated by soluble factors or by cell-to-cell contacts, the experiments were performed also under conditions in which resting or short-term IL-12–activated NK cells were cultured separated from pDCs by a semipermeable membrane.
As shown in Figure 2B, under these conditions, the effect of NK cells on pDCs was sharply reduced, particularly in the case of IL-12–exposed NK cells. However, it is also evident that even when NK cells and pDCs were separated by a semipermeable membrane, pDCs remained capable of releasing almost 10-fold more IFN-α compared with controls cultured in the absence of NK cells. Altogether these data indicate that cellular contact, or at least cell proximity, although not strictly required, can promote optimal induction of pDC activation and IFN-α release by NK cells. These results are in line with previous data on IL-2–activated NK cells cocultured with pDCs.14
These results prompted us to analyze whether any known receptor-ligand interaction was involved in the NK/pDC cross talk resulting in IFN-α release. Thus, blocking mAbs directed against the major activating NK-cell receptors (NKp46, NKp30, 2B4, NKG2D) or major adhesion molecules (including LFA-1 and DNAM-1) were used to assess whether they could inhibit the NK-dependent IFN-α release by pDCs. Although not shown, none of these antibodies had an inhibitory effect.
IL-12–pulsed NK cells enhance the IFN-α release by pDCs, in the presence of CpG-A, in a contact-dependent manner. (A) Purified resting NK cells were cultured overnight in the presence or in the absence of rhIL-12 or, as control, with rhIL-2. Cells were then washed and cocultured with autologous pDCs in the presence of CpG-A at 2 different concentrations (5 μg/mL or 0.5 μg/mL). After 24 hours of culture, supernatants from pDCs alone and from NK/pDC cocultures were collected and analyzed for IFN-α content by specific ELISA. ▪, IFN-α content in supernatants collected from cell cultures stimulated by CpG-A at the final concentration of 5 μg/mL; □, IFN-α content in supernatants collected from cell cultures stimulated by CpG-A at the final concentration of 0.5 μg/mL. The NK/pDC ratio was 10:1. A representative experiment of 10 performed is shown. (B) pDCs were cultured, in the presence of CpG-A (5 μg/mL), with either resting or short-term IL-12–activated NK cells together or separated by a semipermeable membrane. ▪, supernatants collected from NK cells and pDCs cultured together in a 24-well plate; ▦, supernatants harvested from transwell. NK/pDCs ratio was 10:1. A representative experiment of 4 performed is shown.
IL-12–pulsed NK cells enhance the IFN-α release by pDCs, in the presence of CpG-A, in a contact-dependent manner. (A) Purified resting NK cells were cultured overnight in the presence or in the absence of rhIL-12 or, as control, with rhIL-2. Cells were then washed and cocultured with autologous pDCs in the presence of CpG-A at 2 different concentrations (5 μg/mL or 0.5 μg/mL). After 24 hours of culture, supernatants from pDCs alone and from NK/pDC cocultures were collected and analyzed for IFN-α content by specific ELISA. ▪, IFN-α content in supernatants collected from cell cultures stimulated by CpG-A at the final concentration of 5 μg/mL; □, IFN-α content in supernatants collected from cell cultures stimulated by CpG-A at the final concentration of 0.5 μg/mL. The NK/pDC ratio was 10:1. A representative experiment of 10 performed is shown. (B) pDCs were cultured, in the presence of CpG-A (5 μg/mL), with either resting or short-term IL-12–activated NK cells together or separated by a semipermeable membrane. ▪, supernatants collected from NK cells and pDCs cultured together in a 24-well plate; ▦, supernatants harvested from transwell. NK/pDCs ratio was 10:1. A representative experiment of 4 performed is shown.
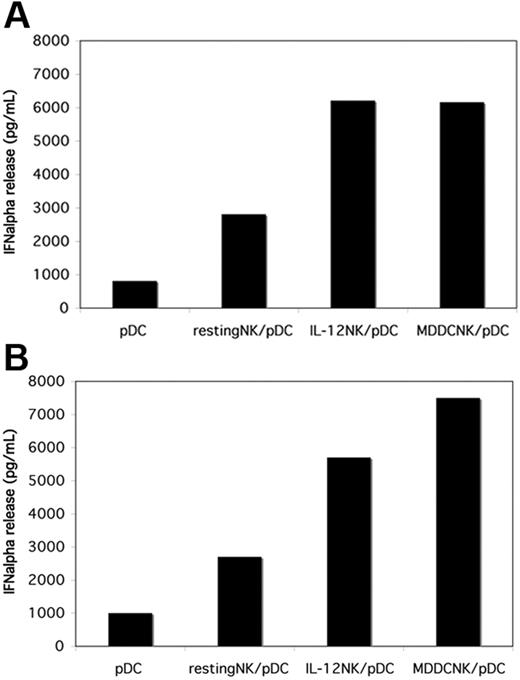
NK cells cocultured with LPS-stimulated MDDCs are more efficient in promoting IFN-α release by pDCs. Freshly isolated NK cells from 2 different donors (A-B) were cultured with allogeneic iMDDCs in the presence of LPS for 18 hours. Then NK cells were negatively separated from MDDCs by immunomagnetic depletion and cocultured with autologous pDCs in the presence of CpG-A (5 μg/mL). Either resting NK cells or short-term IL-12–activated NK cells were comparatively cocultured with pDCs. After 24 hours of culture, supernatants were collected and analyzed for IFN-α concentrations by specific ELISA. The NK/pDC ratio was 10:1.
NK cells cocultured with LPS-stimulated MDDCs are more efficient in promoting IFN-α release by pDCs. Freshly isolated NK cells from 2 different donors (A-B) were cultured with allogeneic iMDDCs in the presence of LPS for 18 hours. Then NK cells were negatively separated from MDDCs by immunomagnetic depletion and cocultured with autologous pDCs in the presence of CpG-A (5 μg/mL). Either resting NK cells or short-term IL-12–activated NK cells were comparatively cocultured with pDCs. After 24 hours of culture, supernatants were collected and analyzed for IFN-α concentrations by specific ELISA. The NK/pDC ratio was 10:1.
NK cells cocultured with LPS-stimulated MDDCs acquire the ability to promote IFN-α release by pDCs
In response to microbial stimuli MDDCs release large amounts of IL-12. Thus, we analyzed whether on direct interaction with LPS-stimulated MDDCs NK cells would acquire the ability to promote IFN-α production by pDCs. To this end, resting NK cells were first cocultured for 18 hours with immature MDDCs in the presence of LPS. Subsequently NK cells, separated from MDDCs, were cocultured with autologous pDCs in the presence of CpG-A. The IFN-α released in culture supernatants was evaluated by ELISA. As shown in Figure 3, MDDC-NK cells induced substantial increments of IFN-α release by TLR9-engaged pDCs. The amounts of IFN-α production paralleled or even exceeded that induced by NK cells exposed to rhIL-12 (Figure 3A-B) or to rhIL-2 (not shown). These results prompted us to investigate whether other soluble factors in addition to IL-12 might be responsible for the acquisition by NK cells of their ability to promote IFN-α production. Although not shown, the use of anti–IL-12 antibodies reduced only, in part, this ability, thus suggesting that indeed other still undefined factors might be involved in this process.
pDC-activated NK cells acquire the ability to kill iMDDCs but not pDCs
IL-2–activated NK cells are capable of killing autologous iMDDCs.4,5,23 This functional capability was also acquired by NK cells that had been cocultured with pathogen-pulsed MDDCs24 as well as by NK cells shortly exposed to IL-12 (but not to IL-4).22 These data suggested that the NK-mediated killing of iMDDCs may play a role in the editing process occurring during DC maturation. Thus, NK cells would select DCs capable of optimal T-cell priming by eliminating those that failed to undergo maturation during immune responses.8,10
It has previously been shown that NK cells cocultured with pDCs in the presence of pathogens become activated and acquire the ability to kill tumor cells.14,15 We then asked whether, under the same culture conditions, NK cells would also become capable of killing pDCs or MDDCs or both. In these experiments, NK cells, cultured for 24 hours together with autologous pDCs and CpG-A, were evaluated for their cytolytic activity against allogeneic peripheral-blood–derived pDCs or iMDDCs. As shown in Figure 4A, these NK cells did not kill freshly isolated peripheral-blood pDCs. This finding is in line with the high survival of pDCs cocultured with NK cells that has been reported by others.14 On the contrary, NK cells pre-exposed to pDCs displayed a strong cytotoxic activity against iMDDCs (Figure 4B), whereas exposure of NK cells to CpG-A alone had no effect (not shown). The IFN-α released by pDCs during the NK/pDC coculture could be responsible for this effect. To evaluate this possibility, we analyzed whether the addition of neutralizing mAbs specific for IFN-α and for the IFN receptor had any effect. As shown in Figure 4, a significant inhibition of NK-mediated cytotoxicity could be detected, although in no instance could complete inhibition be achieved. The cytolytic activity of pDC-activated NK cells was compared to that mediated by polyclonal NK cells that had been exposed for 24 hours to exogenous IL-2. As expected, these cells efficiently killed iMDDCs. However, similar to pDC-activated NK cells, they did not lyse pDCs.
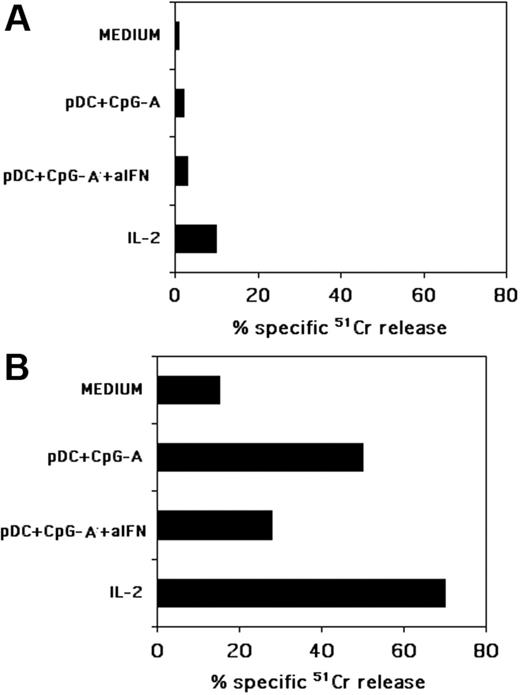
NK cells cultured with pDCs, in the presence of CpG-A, kill iMDDCs but not pDCs. Freshly isolated NK cells were cocultured with autologous pDCs and CpG-A in the presence or in the absence of a mixture of neutralizing antibodies specific for IFN-α and for its receptor (indicated as aIFN). After 24 hours of coculture NK cells were assessed for their cytotoxic activity against allogeneic, freshly blood-derived pDCs (A) or against allogeneic iMDDCs (B) in a 4-hour 51Cr-release assay. As a control, the same NK cells, activated or not with rhIL-2 for 24 hours, were tested for cytotoxicity against either pDCs or iMDDCs. The effector-to-target (E/T) ratio was 20:1. Each value represents the mean of triplicate experiments. The SD did not exceed 5%. Data are representative of at least 6 different experiments.
NK cells cultured with pDCs, in the presence of CpG-A, kill iMDDCs but not pDCs. Freshly isolated NK cells were cocultured with autologous pDCs and CpG-A in the presence or in the absence of a mixture of neutralizing antibodies specific for IFN-α and for its receptor (indicated as aIFN). After 24 hours of coculture NK cells were assessed for their cytotoxic activity against allogeneic, freshly blood-derived pDCs (A) or against allogeneic iMDDCs (B) in a 4-hour 51Cr-release assay. As a control, the same NK cells, activated or not with rhIL-2 for 24 hours, were tested for cytotoxicity against either pDCs or iMDDCs. The effector-to-target (E/T) ratio was 20:1. Each value represents the mean of triplicate experiments. The SD did not exceed 5%. Data are representative of at least 6 different experiments.
Although not shown, short-term exposure of NK cells to IL-12 resulted in a cytolytic pattern similar to that of pDC-activated or IL-2-activated NK cells.
NK-mediated killing of IL-3–activated pDCs
Peripheral-blood pDCs could be resistant to NK-cell–mediated lysis because, unlike iMDDCs,8 they express high levels of surface HLA class I molecules25,26 (Figure 5A). In addition, they could lack ligands recognized by NK-cell triggering receptors or by adhesion molecules. For example, pDCs have been shown to lack MICA and ULPB1-4 (not shown), that is, the specific ligands for the activating receptor NKG2D.27 They also lack both the poliovirus receptor PVRα (CD155; not shown) and nectin-2 (CD112; Figure 5A), that is, the ligands of the DNAM-1 receptor.28 On the other hand, blood pDCs express ICAM-1,25 which plays a crucial role in cell-to-cell adhesion on interaction with LFA-1. In addition they express CD48 (Figure 5A), a ligand recognized by the 2B4 receptor.29-31
Remarkably, blood pDCs express high levels of the IL-3 receptor α chain (CD123) and can be activated by rhIL-3, a cytokine that represents a survival and maturation factor for these cells.16 It has been shown that IL-3–activated pDCs display an increased surface expression of both HLA II and costimulatory molecules and acquire APC morphology and function.32,33 As shown in Figure 5B, pDCs treated with IL-3 for 48 hours were more susceptible to NK-mediated lysis than freshly isolated blood pDCs. This difference, however, could be appreciated only in the presence of anti–HLA class I mAbs to remove inhibition mediated by the HLA class I–specific inhibitory NK receptors. Nevertheless, as shown in Figure 5 the NK-mediated lysis of IL-3–treated pDCs (Figure 5B) was significantly lower than lysis of immature or mature allogeneic MDDCs (Figure 5C). Smaller increments of susceptibility to NK-mediated cytotoxicity were observed also after treatment of pDCs with other stimuli, including CpG or CD40L (not shown).
A possible explanation of the finding that IL-3–activated pDCs become more susceptible to NK-mediated lysis would be that IL-3 could induce the de novo expression of ligands for activating NK receptors on their surface. Indeed, pDCs, after exposure to IL-3, expressed nectin-2 (Figure 5A), a DNAM-1 ligand. DNAM-1, together with NKp30, are involved in the NK-cell–mediated lysis of MDDCs.34 Therefore, we analyzed whether DNAM-1, NKp30, and, in general, NCRs had a role also in the NK-mediated killing of IL-3–activated pDCs. As mentioned, IL-2–activated (allogeneic) NK-cell populations could lyse IL-3–activated pDCs only in the presence of anti–HLA class I mAbs (Figure 5B). Accordingly, aimed at evaluating the involvement of activating NK receptors, experiments were performed in the presence of anti–HLA class I mAb. As shown in Figure 6, masking of either DNAM-1 or NKp30 could partially inhibit pDC killing, whereas masking of NKp46 or NKp44 did not affect NK-mediated pDC lysis (data not shown). A role for DNAM-1 in pDC killing was further substantiated by the partial inhibition observed in the presence of anti–nectin-2 mAb. The simultaneous masking of both NKp30 and DNAM-1 molecules virtually abolished pDC lysis, thus indicating a prominent role for these receptors in NK/pDC cytotoxic interactions. Although not shown, masking of 2B4 or CD48 with specific mAbs did not inhibit the NK-mediated killing of pDCs.
Discussion
Our present study provides evidence that resting peripheral-blood NK cells, on interaction with pDCs, become capable of promoting the release of IFN-α and IL-6 by pDCs. This effect could be further increased by different cytokines including IL-12, released by MDDCs during the early phases of innate immune responses. Indeed, a remarkable enhancement of the ability to promote IFN-α production by pDCs was detected in NK cells that had been cocultured with LPS-stimulated MDDCs. In this case, however, additional soluble factors released by MDDCs are likely to complement the activity of IL-12. In turn, NK cells cocultured with pDCs stimulated via TLR9 (with CpG-A) acquired cytolytic activity against immature MDDCs. This effect, which is largely IFN-α dependent, suggests the existence of an indirect interaction between the 2 different DC types that exploit NK cells as a bridging element to mutually regulate their function in response to pathogen invasion. Moreover, the finding that NK cells could promote the release of IFN-α and IL-6 from pDCs suggests that the NK/pDCs cross talk could also play a role in the control of B-cell responses. Indeed, pDC-derived cytokines (ie, IL-6 and IFN-α) were shown to induce human B-cell differentiation into plasma cells and to produce immunoglobulins.35 Notably, to release amounts of IL-6 sufficient to induce B-cell responses, pDCs needed to be signaled via CD40.35 Our present data suggest that NK cells can provide an alternative, CD40-independent, mode of pDC activation resulting in relevant levels of IL-6 release.
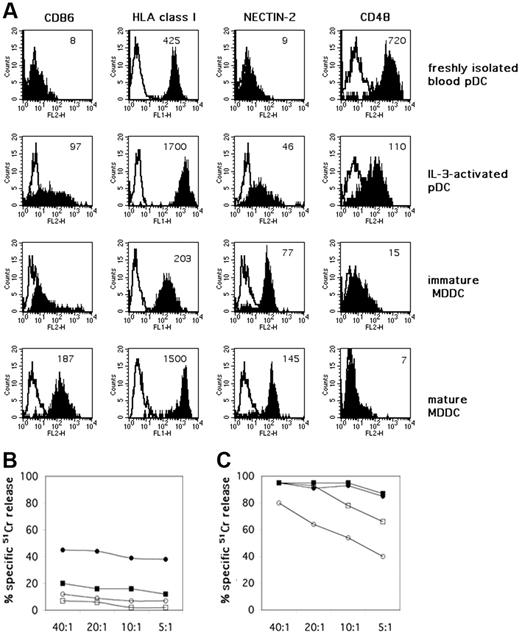
Exposure to IL-3 up-regulates functionally relevant surface molecules on pDCs and modulates their resistance to NK-mediated killing. (A) Peripheral-blood–derived pDCs were evaluated by immunofluorescence and cytofluorimetric analysis for the surface expression of CD86, HLA class I molecules, nectin-2, and CD48 both immediately after isolation from PBMCs and after 48 hours of culture in the presence of rhIL-3. The same surface markers were comparatively analyzed on iMDDCs and on matured MDDCs, induced by LPS. White profiles indicate isotypic negative control. Mean fluorescence intensity (MFI) is indicated in each histogram. One representative experiment is shown. (B) The same peripheral-blood–isolated pDCs were analyzed for their susceptibility to lysis by allogeneic polyclonal NK-cell lines at different E/T ratios. Specific 51Cr-release by pDCs was assessed both immediately after their isolation (□) and after 48 hours of culture in the presence of rhIL-3 (○). The cytolytic activity of polyclonally activated NK cells against pDCs was also evaluated in the presence of anti–HLA class I mAbs (▪ for freshly isolated pDCs, • for IL-3–activated pDCs, respectively). Each value represents the mean of triplicate experiments. The SD did not exceed 5%. Results are representative of at least 6 different experiments. (C) MDDCs were analyzed for their susceptibility to the same allogeneic polyclonally NK-cell lines that were used as effector cells against pDCs, at different E/T ratios. Specific 51Cr release was assessed against both iMDDCs (□) and LPS-matured MDDC (○). The cytolytic activity of polyclonally activated NK cells was also evaluated in the presence of anti–HLA class I mAbs (▪ iMDDCs, • mature MDDC, respectively). Each value represents the mean of triplicate experiments. The SD did not exceed 5%. Results are representative of at least 6 different experiments.
Exposure to IL-3 up-regulates functionally relevant surface molecules on pDCs and modulates their resistance to NK-mediated killing. (A) Peripheral-blood–derived pDCs were evaluated by immunofluorescence and cytofluorimetric analysis for the surface expression of CD86, HLA class I molecules, nectin-2, and CD48 both immediately after isolation from PBMCs and after 48 hours of culture in the presence of rhIL-3. The same surface markers were comparatively analyzed on iMDDCs and on matured MDDCs, induced by LPS. White profiles indicate isotypic negative control. Mean fluorescence intensity (MFI) is indicated in each histogram. One representative experiment is shown. (B) The same peripheral-blood–isolated pDCs were analyzed for their susceptibility to lysis by allogeneic polyclonal NK-cell lines at different E/T ratios. Specific 51Cr-release by pDCs was assessed both immediately after their isolation (□) and after 48 hours of culture in the presence of rhIL-3 (○). The cytolytic activity of polyclonally activated NK cells against pDCs was also evaluated in the presence of anti–HLA class I mAbs (▪ for freshly isolated pDCs, • for IL-3–activated pDCs, respectively). Each value represents the mean of triplicate experiments. The SD did not exceed 5%. Results are representative of at least 6 different experiments. (C) MDDCs were analyzed for their susceptibility to the same allogeneic polyclonally NK-cell lines that were used as effector cells against pDCs, at different E/T ratios. Specific 51Cr release was assessed against both iMDDCs (□) and LPS-matured MDDC (○). The cytolytic activity of polyclonally activated NK cells was also evaluated in the presence of anti–HLA class I mAbs (▪ iMDDCs, • mature MDDC, respectively). Each value represents the mean of triplicate experiments. The SD did not exceed 5%. Results are representative of at least 6 different experiments.
Previous data indicated that NK cells express functional TLR3 and TLR9.36 However, poly I:C or CpG resulted in up-regulation of several functional activities only when exposed to proinflammatory cytokines. For example, NK-cell activation in response to poly I:C required the simultaneous presence of IL-12, derived from MDDCs.36 Similarly, in the present study, we show that CpG stimulation is not sufficient to activate NK cells in the absence of IFN-α–producing pDCs. Indeed, NK cells could acquire cytolytic activity against immature MDDCs only when exposed to CpG-stimulated pDCs.
Remarkably, although capable of up-regulating the cytolytic function of NK cells against tumors and iMDDCs, pDCs remained substantially resistant to NK-cell–mediated lysis. Resistance of pDCs to lysis is only, in part, the result of the high level of expression of HLA class I molecules because they were resistant to lysis also in the presence of anti–HLA class I mAbs. A more likely explanation could be that pDCs lack ligands for major activating receptors expressed by NK cells. Along this line, pDCs cultured in the presence of exogenous IL-3 become more susceptible to NK-mediated lysis and up-regulate some ligands recognized by activating NK receptors. Indeed, blocking of both NKp30 and DNAM-1 receptors could abolish the NK-mediated lysis of IL-3–induced pDCs. Although the ligand for NKp30 expressed by DCs is still undefined, nectin-2, a ligand for DNAM-1, was up-regulated in pDCs after exposure to IL-3. Thus, the NK-mediated killing of pDCs, similar to that of MDDCs, may be regulated by the same pairs of receptor/ligand interactions. However, even after exposure to IL-3, lysis of pDCs was much lower than that of MDDCs. Moreover, lysis of IL-3–cultured pDCs required the disruption of HLA class I/inhibitory receptor interaction by appropriate mAbs. Notably, as shown in Figure 5B-C, even in the presence of anti–HLA class I mAbs, killing of IL-3–cultured pDCs by allogeneic NK-cell populations remained much lower compared with the killing of immature or mature MDDCs derived from the same donor. This could suggest that, unlike MDDCs, which require NK cells to mediate an efficient editing process, pDCs appear to require NK cells only for enhancing their production of IFN-α and IL-6. Recent studies have also shown that, unlike in vitro–generated MDDCs, peripheral-blood myeloid DCs are essentially resistant to NK-mediated lysis. The reason for this difference is not known but may depend on the different state of activation of these 2 cell types. Indeed, exposure of myeloid DCs to inflammatory cytokines within inflamed tissues may not only negatively modulate the expression of HLA class I molecules, but also up-regulate the expression of NKp30 (or DNAM-1)–specific ligands.
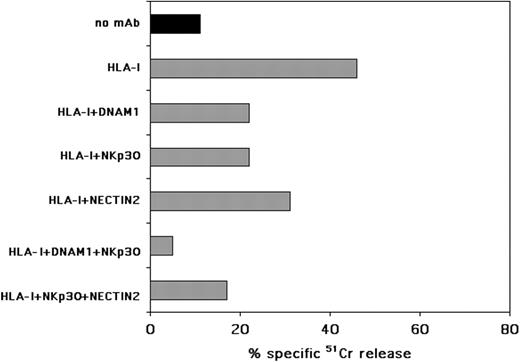
IL-3–activated pDCs are recognized and lysed by NK cells through the NKp30 and DNAM-1 triggering receptors. pDCs were cultured for 48 hours in the presence of rhIL-3 and then assessed for their susceptibility to lysis by allogeneic activated polyclonally NK cells in a 51Cr-release assay, either in the absence or in the presence of the mAbs to the indicated molecules. The E/T ratio was 20:1. Each value represents the mean of triplicate experiments. The SD did not exceed 5%. One representative experiment of 10 performed is shown.
IL-3–activated pDCs are recognized and lysed by NK cells through the NKp30 and DNAM-1 triggering receptors. pDCs were cultured for 48 hours in the presence of rhIL-3 and then assessed for their susceptibility to lysis by allogeneic activated polyclonally NK cells in a 51Cr-release assay, either in the absence or in the presence of the mAbs to the indicated molecules. The E/T ratio was 20:1. Each value represents the mean of triplicate experiments. The SD did not exceed 5%. One representative experiment of 10 performed is shown.
Because IL-3 regulates nectin-2 expression on pDCs, one could ask which cell types would provide this cytokine to pDCs in vivo. Although it has been reported that IL-3 is released by a CD8+ T-cell subset expressing a memory phenotype,37 these cells cannot play any substantial role at the initiation of innate immune responses when most of the NK/pDC interactions are actually occurring. At these early stages, other cells would release IL-3 and favor pDC phenotypic progression. These cells include mastocytes and eosinophils, present in peripheral tissues where they can rapidly respond to pathogenic threats and interact via cell-to-cell contact or cytokine release with other cells of the innate immunity, including NK cells and DCs.12 On the other hand, the actual physiologic significance of the cytolytic interaction between NK cells and pDCs remains unknown. In particular, because the NK-mediated killing of pDCs is blocked by inhibitory receptor/HLA class I interactions, the only possible situation in which killing would occur is represented by the occurrence of a KIR mismatch as in the case of a haplotype mismatched bone marrow transplantation.38
During the initial steps of an innate immune response, the production of IFN-α by pDCs is strongly up-regulated when NK cells and pDCs are stimulated via the same TLRs. As discussed, under these conditions NK cells acquire the ability to select MDDCs undergoing maturation. MDDC maturation is initiated by different stimuli, including the uptake and engulfment of particles of virus-infected cells resulting from NK-mediated killing. Also double-stranded RNA, a viral product released on killing of virus-infected cells, can simultaneously activate MDDCs and NK cells via TLR3. The consequent rapid release of IL-12 from MDDCs is crucial to promote IFN-γ production by TLR-stimulated NK cells. In turn, NK cells will induce further IFN-α production by pDCs. These events together with the NK-mediated editing program of MDDCs would eventually result in the selection of mature MDDCs that, after migration to lymph nodes, will mediate antigen cross-presentation to virus-specific CD8+ cytotoxic T lymphocytes (CTLs). Thus, NK cells would be essential for bridging pDC- and MDDC-dependent responses during early innate immunity to viruses. It should be considered that several in vivo models have indicated that DCs and NK cells are crucial in the early phases of CMV infection.39-43 Moreover, a crucial role for NK cells in dictating a Th1 type of immune response against various pathogens has been well documented. Thus, in mice NK cells are required not only for innate resistance but also for generation of protective Th1 immunity against listeriosis, chlamydial infection, and leishmaniasis.44-47 Moreover the Th1-dependent production of IgG2a in response to keyhole limpet hemocyanin was impaired in NK-deficient animals.48 It is possible that NK cells, thanks to their editing program of MDDCs undergoing maturation, prevent the survival of faulty MDDCs that have not appropriately up-regulated MHC class I and II molecules and costimulatory molecules.10 Lack of NK cells would interfere with Th1 responses because of an impaired elimination of faulty MDDCs that, after expression of CCR7, may reach lymph nodes and prime inappropriate, low-affinity T cells. Indeed, the in vivo default mechanism of CD4+ T cells in the absence of NK cells appears to be strongly biased toward the acquisition of a Th2 phenotype.49,50 In humans, direct evidence for the close contact between NK cells and MDDCs as well as for the ability of NK cells to kill iMDDCs after migration to peripheral tissues has been provided in atopic skin lesions induced by the Malassezia yeast.51 Finally, it is of note that the encounter between NK cells and different DC subtypes is not limited only to pathogen-invaded peripheral tissues but occurs also in secondary lymphoid compartments. Indeed, it has been demonstrated that a particular type of NK cell is localized in parafollicular T-cell areas of lymph nodes52,53 and that NK cells and pDCs colocalize in T-cell areas of inflamed tonsils.54 These NK cells, characterized by the CD56bright, CD16–, NKG2A+ phenotype, are poorly cytotoxic but are capable of releasing high levels of IFN-γ. Thus, it is conceivable that these NK cells are directly involved in determining the Th1 functional phenotype during the early phases of T-cell priming as strongly suggested by recent studies.55
Authorship
M.D.C. and C.R. participated in designing and performing the research; A.T. provided analytical tools and analyzed data; L.M. analyzed data and participated in writing the article; A.M. participated in designing the research and wrote the article; all authors checked the final version of the manuscript.
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, July 27, 2006; DOI 10.1182/blood-2006-02-004028.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro (AIRC), Istituto Superiore di Sanità (ISS), Ministero della Salute–RF 2002/149, Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST), Ministero dell'Istruzione dell'Università e della Ricerca (MIUR), Fondo per gli Investimenti per la Ricerca di Base (FIRB–MIUR) progetto-codRBNE017B4, European Union FP6, LSHB-CT-2004-503319-Allostem (the European Commission is not liable for any use that may be made of the information contained) and Fondazione Compagnia di San Paolo. M.D.C. is a recipient of a fellowship awarded by Federazione Italiana Ricerca sul Cancro (FIRC). C.R. is funded by the Department of Experimental Medicine (DIMES), University of Genoa, and by European Molecular Biology Organization (EMBO) long-term fellowship.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal