Abstract
Heteroclitic peptide modifications increase immunogenicity, allowing generation of cytotoxic T lymphocytes (CTLs) against weakly immunogenic tumor-associated antigens (TAAs). A critical issue is whether T cells generated against heteroclitic peptides retain the ability to recognize and kill tumor cells expressing the original weak TAAs, and whether there is a lower threshold of binding affinity of the native peptides, below which such CTLs can still kill primary tumor cells. To examine this we used a model examining the ability of native and heteroclitic immunoglobulin (Ig)–derived peptides to generate CTLs that can kill chronic lymphocytic leukemia (CLL) cells. We demonstrate that CTLs generated against heteroclitic peptides have enhanced killing of CD40-activated B cells pulsed with either heteroclitic (P < .001) or native peptide (P = .04) and primary CLL cells (P = .01). The novel finding reported here is that the rate-limiting factor appears to be the ability to generate CTLs and that once generated, CTL lysis of primary tumor cells is independent of the binding affinity of the native peptide. These findings have implications for vaccination strategies in malignancies and are currently being further examined in vivo in murine models.
Introduction
A primary requirement for successful tumor vaccine development is the identification of tumor-associated antigens (TAAs). The first TAA identified was the idiotype (Id) of the immunoglobulin (Ig) expressed in malignant B cells,1-3 and Id vaccination strategies induce specific humoral and cellular immune responses that can lead to tumor regression or rejection.4-10 A bioinformatics approach has been applied to the identification of T-cell epitopes from a variety of candidate TAAs, including proteinase 3,11 MUC-1,12 melanoma antigen 3,13 and telomerase,14 as well as the Id of malignant B cells.15,16 A conceptual drawback to targeting Id has been the need to create an individualized reagent for each patient. In chronic lymphocytic leukemia (CLL), subsets of patients have been identified with remarkably similar antigen receptors,17-22 and shared Ig framework region (FR)–derived peptides represent important targets for cross-reactive Id therapy, offering the advantage of a less patient-specific immunotherapeutic strategy in B-cell malignancies.16 A major limitation of this method is the generally low immunogenicity and low binding affinity of these peptides to major histocompatibility complex (MHC) class I and class II molecules. Heteroclitic peptide modifications can increase immunogenicity of low-binding peptides while leaving T-cell recognition residues intact,23 and these “heteroclitic peptides” lead to improved ability to generate cytotoxic T lymphocyte (CTL) responses against primary tumors.15,24,25 Heteroclitic modifications as a strategy to enhance immune responses have been tested in several tumor antigens,26,27 and agonist analogs of subdominant epitopes, once optimized for binding class I molecules, can be used effectively to recruit a nontolerized CTL repertoire.28 For this reason, many epitope-based strategies for cancer aim to use optimized analogs of low- or poor-affinity epitopes.24 Efficient antitumor immunity has been induced in patients with cancer using these peptide variants in conjunction with interleukin-2 (IL-2),25 demonstrating the value of such peptides as immunotherapeutics. A potential limitation of heteroclitic peptides is that resulting CTLs must still be capable of killing the tumor cells that express the lower-binding native affinity peptides.
The aim of the present study was to determine whether there is a lower threshold of binding affinity of the TAA peptide that allows exploitation of the heteroclitic technology. We modeled this in studies examining the ability of native and heteroclitic Ig FR peptides to generate CTLs that can kill CLL cells that express the native peptide. We demonstrate that whereas increased binding affinity of the heteroclitic peptides correlates with the ability to generate CTLs, once generated, these CTLs can kill targets expressing even weakly immunogenic peptides. These findings suggest that the rate-limiting factor is the ability to generate CTLs, and that once this has been overcome by the use of heteroclitic peptides these CTLs retain their capacity to kill the tumor cells expressing even very weakly binding native peptides.
Patients, materials, and methods
Patients and healthy donors
Peripheral blood was collected from 25 healthy donors and 8 patients with previously untreated CLL at Dana-Farber Cancer Institute and cryopreserved. In 4 patients the variable region of the immunoglobulin heavy-chain genes (IgVH) was mutated, and in 4 patients IgVH was unmutated. All participants provided signed informed consent, and the study was approved by the Institutional Review Board at Dana-Farber Cancer Institute.
Epitope prediction analysis and bioinformatics
RNA was isolated from 5 × 106 cells from patients with CLL. The Ig heavy chains were polymerase chain reaction (PCR) amplified, and both strands were sequenced as previously described.29 The derived IgVH-deduced protein sequences were reviewed for nonameric and decameric peptides that could potentially bind to MHC class I molecules as previously described.15,16,18 Two independent computer prediction analysis tools were used to examine the binding of peptides to HLA-A*0201 molecules: the BIMAS algorithm (http://thr.cit.nih.gov/molbio/hla_bind) and the SYFPEITHI database.30 The present analysis was confined to human leukocyte antigen (HLA)–A*0201 (HLA-A2), since HLA-A2 is the most common HLA allele and expressed in approximately 50% of our patients. Ongoing studies are under way analyzing HLA-A3–, HLA-A11–, HLA-A24–, and HLA-B7–binding peptides. This set of HLA types covers more than 75% of our tumor patients. When examining allogeneic T-cell responses against Id-derived peptides, the potential role of minor histocompatibility antigens expressed by the lymphoma cells also needs to be determined. All peptides were selected from unmutated Ig-shared FR peptides.16,18 Heteroclitic peptides were designed by conservative amino acid substitutions of MHC-binding residues expected to enhance the affinity toward the MHC class I allele, as predicted by using the computer algorithms. Alterations were made to amino acids at positions 1 or 2 (or both) and 9 or 10 by substitution with lysine at position 1, leucine at position 2, and valine at position 9 or 10.
Peptide synthesis
Peptides were synthesized at New England Peptide (Gardner, MA) and purified to more than 75% by reverse-phase high-performance liquid chromatography (HPLC), as confirmed by mass spectrometry. A high-binding peptide derived from melanoma antigen 3 (FLWGPRALV) was used as a positive control for HLA-A*0201–binding ability. Lyophilized peptides were dissolved in phosphate-buffered saline (PBS) at a concentration of 1 mg/mL and were stored in aliquots at –80°C until use.
Peptide-binding assay
A cellular peptide-binding assay using the transporter associated with the antigen-processing–deficient cell line T2 was used to determine binding of peptides to HLA-A2 as described previously.14 Briefly, T2 cells (1 × 106/mL in serum-free medium) were pulsed with 50 μg/mL peptide and 5 μg/mL β-2 microglobulin (Sigma, St Louis, MO) for 15 to 18 hours at 37°C. HLA-A2 expression of T2 cells was then measured by using monoclonal antibody (mAb) BB7.2 (American Type Culture Collection [ATCC], Manassas, VA). Fluorescence index (FI) was calculated as the mean fluorescence intensity (MFI) of HLA-A*0201 on T2 cells as determined by fluorescence-activated cell-sorting analysis, using the formula FI = (MFI [T2 cells with peptide]/MFI [T2 cells without peptide])–1.
Screening for peptide-specific HLA-restricted CTLs
We used an established T-cell expansion system to screen peptide-specific autologous CTL responses.16 Purified HLA-A2+ CD8+ T cells were primarily stimulated with irradiated monocyte-derived, peptide-pulsed dendritic cells that were pulsed prior to stimulation with native or heteroclitic peptide for 2 hours in the presence of β2-microglobulin. T cells were then restimulated weekly using irradiated autologous CD40-activated B cells pulsed with the corresponding native or heteroclitic peptide for 2 hours in the presence of β2-microglobulin.31 After 3 to 5 stimulations, the cytotoxicity of the CTL line was assessed in standard chromium (51Cr) release assays, where effector cells are incubated with 51Cr-labeled target cells. The release of 51Cr in the culture supernatant is related to target cell death. CD40-activated B cells were incubated with Mage-3 (positive control), the specific native and heteroclitic peptide, an irrelevant peptide (negative control), or nonpulsed CD40 B cells; nonstimulated primary CLL cells were used as target cells. CTL activity was titrated at 3 different effector-target ratios (30:1, 10:1, and 3:1), with 5000 K562 cells used as a control for natural killer cell activity. In all experiments, results represent killing of the peptide-pulsed CD40-activated B cells subtracted from that of the unpulsed CD40-activated B cells. Specific lysis was calculated as the percentage of specific 51Cr release by using the following formula: (E–S/T–S) × 100, where E is experimental 51Cr release, S is the spontaneous 51Cr release, and T is the total 51Cr release by 2% Triton X-100. The purity of cell populations was determined by dual-color fluorescence-activated cell-sorting analysis using directly conjugated mAbs against CD3, CD4, CD8, CD19, CD20, CD56, and CD14 as well as respective isotope-matched control IgG.
Statistical analysis
The Wilcoxon signed-rank test32 was used to test for increased tumor lysis and binding affinity. We report the Hodges-Lehmann estimate of the median33 percentage increase in tumor lysis and binding affinity along with the respective 95% confidence intervals (CIs). In addition, we report the Hodges-Lehmann estimate of the median ratio of tumor lysis with its associated 95% CI. Spearman rank correlation was used to assess the correlation among the binding prediction methods and between binding affinity and tumor lysis. P values are 2-sided. No adjustment was made for multiple testing.
Results
We have previously demonstrated that CTLs can be generated against FR-derived peptides more readily using heteroclitic peptides rather than native peptides.15,16 Here we examined the separate issue of whether there was a lower threshold of binding of the native peptide that could be killed by CTLs that are generated against heteroclitic peptides. We selected 10 native peptides with very low binding affinity (FIs lower than 0.5) and 7 native peptides with low to intermediate binding affinity (FIs between 0.6 and 1.3), and synthesized these 17 native nonameric or decameric peptides (Parker score: range, 0.001-181 minutes, median, 23 minutes; Rammensee score: range, 10-26, median 19) as well as their corresponding heteroclitic counterparts (Parker score: range, 49-4047 minutes, median, 403 minutes; Rammensee score: range, 15-29, median, 19). There was a correlation between predicted bindings assessed by the Parker score and the Rammensee score (Figure 1A; Spearman rho = 0.62; P < .001) and between measured and predicted binding affinities as assessed by both computer prediction tools, the Parker score (Figure 1B; Spearman rho = 0.66; P < .001) and the Rammensee score (Figure 1C; Spearman rho = 0.58; P < .001). The results of the measured binding are representative of at least 3 independent experiments performed on a total of 34 peptides.
Heteroclitic modifications significantly increased the predicted and measured binding affinity compared with the native peptide as assessed by the Parker score (median change of predicted half-time of dissociation to HLA class I molecules, 366 minutes; 95% CI, 236-666 minutes; P < .001; median increase of 37-fold), the Rammensee score (median change of the score, 4.5; 95% CI, 1.0-7.5; P = .004; median increase of 1.3-fold), and measured binding affinity (median, 3.3-fold increase; median change of the FI, 1.2; 95% CI, 0.75-1.7; P < .001). Representative results showing the FI of 2 native peptides and their heteroclitic counterparts are shown in Figure 2.
Peptides require a threshold of binding for successful generation of CTLs
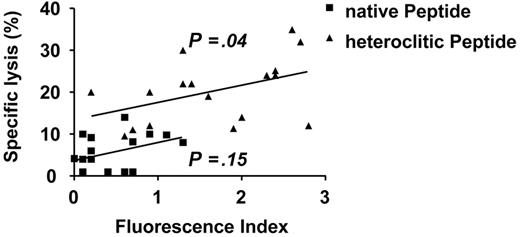
The native peptides selected for study generated CTLs that were ineffective in killing native peptide-pulsed CD40-activated B cells (median specific lysis, 4%; range, 1%-14%; Figure 3). The binding affinity of these weakly immunogenic native peptides did not correlate with the specific lysis achieved by CTLs generated against them as predicted by the Parker score (Spearman rho = 0.16; P = .54) and the Rammensee score (Spearman rho = 0.36; P = .15), and as measured by the T2 binding assay (Spearman rho = 0.37; P = .15; Figure 4).
Predicted and measured binding affinities. Correlation between predicted half-life of binding of 17 Ig-derived native (▪) and 17 heteroclitic (▴) peptides to HLA-A*0201 of (A) predicted binding as assessed by the Parker score and the Rammensee score, (B) predicted binding according to the Parker Score and measured binding as calculated by the FI, and (C) predicted binding according to the Rammensee score and measured binding as calculated by the FI.
Predicted and measured binding affinities. Correlation between predicted half-life of binding of 17 Ig-derived native (▪) and 17 heteroclitic (▴) peptides to HLA-A*0201 of (A) predicted binding as assessed by the Parker score and the Rammensee score, (B) predicted binding according to the Parker Score and measured binding as calculated by the FI, and (C) predicted binding according to the Rammensee score and measured binding as calculated by the FI.
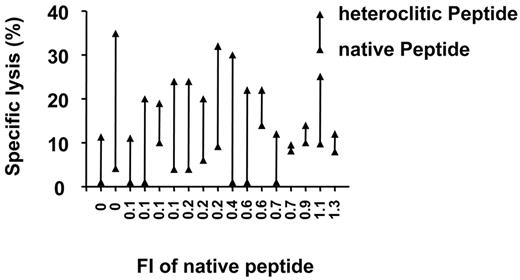
In an attempt to overcome this limitation and increase the pool of immunogenic Ig-derived peptides we generated peptides with alterations at the HLA-A*0201 binding motif or anchor residues. T-cell lines generated against the heteroclitic peptides demonstrated significantly enhanced killing of peptide-pulsed antigen-presenting cells (APCs; median specific lysis, 20%; range, 10%-35%) compared with that induced by the native peptides (median percentage change in specific lysis, 15%; 95% CI, 10%-20%; P < .001; median increase of 5-fold) as shown in Figures 3 and 5.
There was a correlation for the heteroclitic peptides between FI and the ability to induce CTL responses (Figure 3; Spearman rho = 0.50; P = .004). CTLs generated against the heteroclitic peptides demonstrated enhanced cytotoxicity against APCs pulsed not only with the heteroclitic peptide against which they were generated, but also against APCs pulsed with the native peptide from which they were generated (Figure 3; median specific lysis, 17%; range, 10%-20%; median percentage change in specific lysis, 7%; 95% CI, 4%-13%; P = .04; median increase of 2-fold).
Specific lysis of primary tumor cells by successfully generated CTLs is independent of the original binding affinity of the native peptides
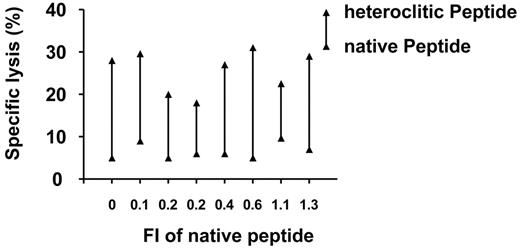
The most important question we wished to address was whether the CTLs generated against the heteroclitic peptides could kill the CLL cells, which express only the native peptide. As shown in Figure 6, T-cell lines generated against the heteroclitic peptide were significantly more effective at killing CLL cells (median specific lysis, 28%; range, 18%-31%) in 8 patients in which killing of the CTLs generated against the native peptide was poor (median specific lysis, 6%; range, 5%-10%; median percentage change in specific lysis, 19%; 95% CI, 14%-24%; P = .01; median increase of 4-fold).
FACS analysis of T2 binding assay. (A) HLA-A2 surface expression on 1 × 106 T2 cells without peptide (negative) and up-regulation of HLA-A2 expression after preincubation with the native peptide (FI = 1.1) and the heteroclitic peptide (FI = 2.4). (B) HLA-A2 surface expression on 1 × 106 T2 cells without peptide (negative) and up-regulation of HLA-A2 expression after preincubation with the native peptide (FI = 0.2) and the heteroclitic peptide (FI = 2.7). The results are representative of at least 3 independent experiments performed on a total of 34 peptides.
FACS analysis of T2 binding assay. (A) HLA-A2 surface expression on 1 × 106 T2 cells without peptide (negative) and up-regulation of HLA-A2 expression after preincubation with the native peptide (FI = 1.1) and the heteroclitic peptide (FI = 2.4). (B) HLA-A2 surface expression on 1 × 106 T2 cells without peptide (negative) and up-regulation of HLA-A2 expression after preincubation with the native peptide (FI = 0.2) and the heteroclitic peptide (FI = 2.7). The results are representative of at least 3 independent experiments performed on a total of 34 peptides.
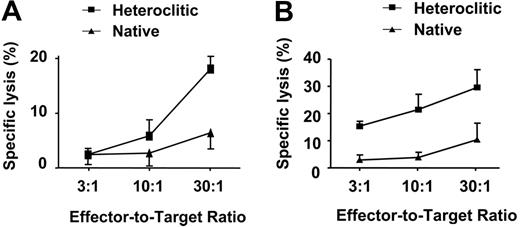
Representative results for 3 different effector-target ratios are shown in Figure 7A-B, demonstrating that CTLs generated against the heteroclitic peptide were able to kill CLL cells in cases where CTLs generated against the native peptide did not. The specificity of killing of these CTLs was demonstrated by the finding that these CTLs killed only APCs pulsed with the appropriate native or heteroclitic peptide or CLL cells that express this peptide, and not control cells that did not express either peptide. There was no significant correlation between the specific lysis of CLL cells and the original binding affinity of the native peptide, both as measured by T2 binding assay (Spearman rho = 0.38; P = .36) and as predicted by the Parker score (Spearman rho = 0.22; P = .60) and the Rammensee score (Spearman rho = 0.02; P = .95). T-cell lines generated against the heteroclitic peptide were more effective at killing CLL cells, even when the native peptides had low binding affinity (Figure 6). The surprising finding is that therefore, once CTLs are generated, the rate of cytotoxicity appears independent of the binding affinity of the original peptide from the TAA.
Killing of CD40-activated B cells. Killing of CD40-activated B cells pulsed with native peptides by T cells generated against the native peptide (Native-Native), killing of CD40-activated B cells pulsed with heteroclitic peptides by T cells generated against the heteroclitic peptide (Heteroclitic-Heteroclitic), and killing of CD40-activated B cells pulsed with native peptides by T cells generated against the heteroclitic peptide (Heteroclitic-Native). The top lines in the boxplot represent the 75% quartile, and the bottom line represents the 25% quartile, with the middle line showing the median. The results of the specific lysis are at an effector-target ratio of 30:1 and are mean values from experiments done in triplicate with samples from 3 different donors.
Killing of CD40-activated B cells. Killing of CD40-activated B cells pulsed with native peptides by T cells generated against the native peptide (Native-Native), killing of CD40-activated B cells pulsed with heteroclitic peptides by T cells generated against the heteroclitic peptide (Heteroclitic-Heteroclitic), and killing of CD40-activated B cells pulsed with native peptides by T cells generated against the heteroclitic peptide (Heteroclitic-Native). The top lines in the boxplot represent the 75% quartile, and the bottom line represents the 25% quartile, with the middle line showing the median. The results of the specific lysis are at an effector-target ratio of 30:1 and are mean values from experiments done in triplicate with samples from 3 different donors.
Killing of APCs pulsed with native or heteroclitic peptides. Comparison between specific lysis of APCs pulsed with native (▪) and heteroclitic (▴) peptides by CTLs generated against the corresponding peptide and their calculated binding (FI). The results of the specific lysis are at an effector-target ratio of 30:1 and are mean values from experiments done in triplicate with samples from up to 3 different donors; the results of the measured binding are representative of at least 3 independent experiments performed on a total of 34 peptides. Diagonal lines represent best-fit regression analysis, with B value given.
Killing of APCs pulsed with native or heteroclitic peptides. Comparison between specific lysis of APCs pulsed with native (▪) and heteroclitic (▴) peptides by CTLs generated against the corresponding peptide and their calculated binding (FI). The results of the specific lysis are at an effector-target ratio of 30:1 and are mean values from experiments done in triplicate with samples from up to 3 different donors; the results of the measured binding are representative of at least 3 independent experiments performed on a total of 34 peptides. Diagonal lines represent best-fit regression analysis, with B value given.
Discussion
Targeting of TAA peptides is hampered as an immunotherapeutic strategy by the generally low immunogenicity of such peptides, and heteroclitic protein modifications represent a promising strategy to enhance immunogenicity and subsequent generation of successful CTLs. The present study, modeled using peptides derived from the Ig FR in CLL, demonstrates recognition of naturally processed tumor peptides, even with extremely low binding to MHC class I, when higher-affinity analogs are used as “in vitro immunogens.” The results suggest that the important threshold to overcome is the ability to generate specific T cells and that once such cells are generated, the binding affinity of the native peptide to MHC class I is not rate limiting in the capacity of these cells to kill primary tumor cells.
The immunogenicity of a peptide is related to its binding affinity for class I molecules.34-39 Human CTL epitopes encoded by human papillomavirus (HPV), such as E6 and E7, with a high binding affinity to HLA-A*0201 (inhibitory concentration [IC50] < 50 nM) were immunogenic both in vivo and in vitro, whereas HPV16-derived peptides with low binding affinities (IC50 > 500 nM) were not capable of inducing CTL responses, indicating that the MHC binding affinity of a peptide has a major impact on its immunogenicity.40,41 The rate of dissociation of MHC/peptide complexes appears to be the most important binding parameter, with highly immunogenic peptides displaying slower off-rates.42,43 Consequently, heteroclitic peptides with higher binding affinities than their native counterparts showed significant enhanced killing of target cells, when pulsed with the corresponding heteroclitic peptide or the native peptide. More important, CTLs generated against such heteroclitic peptides also resulted in increased specific lysis of the corresponding primary tumor cells, in keeping with our preliminary data.15 These data suggest an important role of HLA-binding affinity in determining both immunogenicity and recognition of naturally processed antigen production with respect to significant enhanced killing by CTLs. Our data are in keeping with recent findings correlating the stability of the peptide–MHC–T-cell receptor complex with the killing of target cells.44 A potential mechanism underlying this phenomenon is the ability of analog peptides to stimulate a faster polarization of lytic granules to the immunologic synapse, to reduce dependence on CD8 binding, and to induce greater numbers of cross-reactive CTLs to the native peptide.44
Increased killing of CD40-activated B cells pulsed with the heteroclitic peptide by CTLs generated against the heteroclitic peptide compared with CD40-activated B cells pulsed with the native peptide by CTLs generated against the native peptide. Results shown are at an effector-target ratio of 30:1 and are mean values from experiments done in triplicate with samples from 3 different donors.
Increased killing of CD40-activated B cells pulsed with the heteroclitic peptide by CTLs generated against the heteroclitic peptide compared with CD40-activated B cells pulsed with the native peptide by CTLs generated against the native peptide. Results shown are at an effector-target ratio of 30:1 and are mean values from experiments done in triplicate with samples from 3 different donors.
Increased killing of nonstimulated CLL cells by CTLs generated against heteroclitic peptides compared with native peptides. Results shown are at an effector-target ratio of 30:1 and are representative of at least 3 experiments performed against CLL cells from a total of 8 patients.
Increased killing of nonstimulated CLL cells by CTLs generated against heteroclitic peptides compared with native peptides. Results shown are at an effector-target ratio of 30:1 and are representative of at least 3 experiments performed against CLL cells from a total of 8 patients.
The design of heteroclitic peptides remains dependent upon prediction models of binding. To date it remains controversial as to how reliable the predicted binding corresponds to experimental binding and to what extent the binding affinity translates into immunogenicity and subsequent generation of effective CTLs. Several reports analyzing non-Ig–derived peptides have demonstrated poor correlation between predicted and actual binding as determined by peptide-binding assay or by complex stabilization.45-50 Our own results point to the increasing efficiency of matrix-driven epitope deduction analyzing Ig-derived peptides because Rammensee scores, Parker scores, and binding in the T2 binding assay correlated highly. However, the peptide-binding assay still remains an obligatory step in T-cell epitope identification.15 In addition, once CTLs were generated using heteroclitic modifications, the specific lysis of primary CLL cells was independent of the original binding affinity of the native peptides, suggesting that regardless of the strategy, the important step is to generate CTLs, and that once these cells have been generated, they are capable of killing primary tumor cells. It is also well recognized that other factors likely contribute to immunogenicity and the strength of T-cell responses, including T-cell avidity, T-cell tolerance, and frequency, proteosomal cleavage, and modulation by various lymphokines.51-55
Killing of nonstimulated CLL cells by CTLs generated against native and heteroclitic peptides at 3 different effector-target ratios. The 2 results shown in panels A and B are mean ± SD and are representative of at least 3 experiments performed against CLL cells from a total of 8 patients.
Killing of nonstimulated CLL cells by CTLs generated against native and heteroclitic peptides at 3 different effector-target ratios. The 2 results shown in panels A and B are mean ± SD and are representative of at least 3 experiments performed against CLL cells from a total of 8 patients.
To date it is not known whether somatically mutated or somatically unmutated regions of the IgVH or complementarity-determining region (CDR)– and/or FR-derived peptides are more important for targeting. Similar numbers and predicted binding affinities of HLA-binding motifs were found in both mutated and germline IgVH.18,56 Id peptides covering somatically mutated peptides are tumor specific and have the potential to be immunogenic, since there is no need to break tolerance.56 Studies have demonstrated that CDR3 may be an important target for lytic T cells.57,58 All of the peptides we examined were derived from the FR region and were not mutated, since this approach allows us to examine killing of multiple patient samples; we have shown that up to 30% of FR peptides were shared among patients with B-CLL.18 These shared, commonly expressed Ig molecules may be important targets for cross-reactive idiotypic therapy and could potentially form the basis of widely applicable Ig-directed immunotherapy in B-cell malignancies. Thus emphasis could be shifted from a patient-specific immunotherapeutic approach to the targeting of Ig as a disease of tissue-specific self-antigen. A major concern is that some normal B cells would also express targeted FR-derived peptides. Clinical trials with the anti-CD20 antibody rituximab in which virtually all B cells are eliminated have not revealed severe side effects caused by B-cell deficiency. However, one has to clearly differentiate between a vaccination approach with the induction of an active immune response and loss of B cells after passive administration of an antibody. Although the Ig peptides were shared, previously we have not been able to demonstrate killing of normal B cells using this approach.59 This could be due either to an altered processing of the Ig-derived peptides in normal B cells, or it might reflect the fact that only a small proportion of normal B cells will express a particular shared FR region sequence. CTLs generated against the epithelial cell adhesion molecule (Ep-CAM) killed tumor cells that express Ep-CAM, yet they did not kill normal epithelial tissue that also expressed Ep-CAM,60 suggesting that the normal cells may not process and present such peptides efficiently. Ongoing in vivo murine experiments are addressing the question of whether immune responses against the FR will result in a depletion of the normal B-cell repertoire. Data suggesting that this may not occur have come from studies using a vaccine targeting VH peptides expressed by the dominant dextran-specific B-cell clonotype, which demonstrated that this had no effect upon the magnitude of the normal B-cell response to dextran.61 Therefore, knowledge of T-cell epitopes and their immunodominant features can be useful for the generation of antigen-specific T cells for adoptive immunotherapy. Regardless of the potential use of FR-derived peptides in vaccine development targeting Id's, this has been a useful model to examine the question being addressed in the present study.
In conclusion, our findings confirm an increasing rate of immune responses with increasing binding affinity. However, once an immune response is initiated, the rate of cytotoxicity appears to be independent from the binding affinity of the native peptide. Thus, the important threshold to overcome is the ability to generate specific T cells; once such cells are generated, the binding affinity of the peptide to MHC I is not rate limiting in the capacity of these cells to kill primary tumor cells. These findings may have important implications for vaccination strategies in B-cell malignancies and warrant in vivo evaluation of this model in CLL. Although these studies were modeled using Id-derived peptides and by the killing of CLL cells, the confirmation that heteroclitic modifications represent a promising strategy to render low-binding peptides into productive antigens that can serve as a target for a T-cell–mediated response has potential implications for the design of all peptide antigens.
Authorship
The experiments were designed and performed by K.M.Z. and J.G.G., with statistical plan and analysis by D.Z. and D.N. All authors contributed to the writing of the manuscript.
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 10, 2006; DOI 10.1182/blood-2006-04-014415.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from the National Institutes of Health (CA81534; J.G.G.) and from the Deutsche Krebshilfe and Dr Mildred Scheel Stiftung (K.M.Z.).




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal