Abstract
Adult T-cell leukemia (ATL) was first described in 1977. A link between ATL and human T-cell leukemia virus type 1 (HTLV-1) was clearly established in the early 1980s. Over the years, many aspects of HTLV-1–induced cellular dysfunctions have been clarified. However, the detailed mechanism behind ATL occurrence remains unsolved. Presently, we are still unable to explain the absence of viral Tax protein (thought to play a central role in T-cell transformation) in more than 50% of ATL cells. A novel HTLV-1 HBZ protein, encoded on the negative strand, was characterized by our group and is currently the subject of intensive research efforts to determine its function in viral replication and/or pathophysiology. Recently, 4 studies reported on the existence of different HBZ isoforms and have investigated on their function in both ATL cells or animal models. One report suggests that the HBZ gene might have a bimodal function (at the mRNA and protein levels), which could represent an uncharacterized strategy to regulate viral replication and proliferation of infected T cells.
The clinical entity termed adult T-cell leukemia (ATL) was established nearly 30 years ago.1 The etiologic agent of this fatal neoplastic disease of T lymphocytes was identified in the early 1980s as being human T-cell leukemia virus type 1 (HTLV-1)2,3 and was the first retrovirus linked to a form of cancer in humans. Availability of the full sequence indicated that its genome was quite stable,4 but further argued that its accessory genes played an intricate role in controlling its own replication. The viral Tax protein, encoded by one of these accessory genes, can support the preferential growth of infected cells by transactivating HTLV-1 mRNA expression and transcription of many cellular genes, including IL2, IL2R, and the oncogene c-Fos. Direct evidence was obtained indicating that Tax harbors oncogenic properties.5,6 Over the years, we and others succumbed to the excitement of identifying new Tax partners and demonstrated that Tax represses the lck gene through an E-Box in the lck gene promoter, and induced transcriptional activation of VCAM1 through an NF-κB binding site located in the VCAM1 promoter and E2F-1 through an activating transcription factor (ATF) site positioned in the E2F-1 promoter.7-10 In recent years, the increasing complexity of the molecular crosstalk between Tax and cellular proteins that regulate cell-signaling cascades and transcription was a precious source of information that led to several models of cellular dysfunction associated to Tax expression and aberrant regulation of cyclin, kinases, tumor suppressors, and transcription factors.11-15 An increasing amount of intriguing reports focused on the ability of Tax to force T-cell progression from G1 to S phase of the cell cycle (reviewed in Mesnard and Devaux16 and Basbous et al17 ). Tax was also shown to deregulate the G2-to-M transition by interacting with the mitotic checkpoint protein Hs-MAD1,18 which explained multinucleated giant cells and chromosomal abnormalities frequently present in ATL cells. At first glance, the complex pattern of Tax interaction with several cellular partners19 and Tax pleiotropic actions on cyclic AMP response element binding protein (CREB)/ATF–, serum response factor (SRF)–, and nuclear factor κB (NF-κB)–associated pathways seemed to support the conventional theoretical model, whereby Tax plays a central role in ATL induction.
However, Tax transgenic mice develop a wide range of tumors, such as neurofibrosarcomas, mesenchymal tumors, and mammary adenomas, or even skeletal bone abnormalities including osteolytic bone metastases. Surprisingly, no leukemias or lymphomas were identified in these mouse models.20-24 Only one report has indicated that mice expressing Tax through the granzyme B promoter developed large granular lymphocytic leukemia.25 It is, however, known that Tax is likely a central actor in the induction of HTLV-1–associated myelopathy (or tropical spastic paraparesis, HAM/TSP). In this neurodegenerative disease, most patients carry nonmalignant T cells randomly infected with HTLV-1. Yet, efforts made to understand the striking observation that T-cell transformation and clonal proliferation of ATL cells is usually associated with a Tax-low or Tax-negative phenotype26 have not yet answered these questions satisfactorily. This lack of Tax expression can be the result of genetic or epigenetic phenomena such as deletion of the 5′ long terminal repeat (LTR)27 or methylation of this region.28 For almost a decade, the HTLV-1 research community faced a difficult challenge in determining which of the numerous identified candidate molecules could turn on the leukemogenic process driven by the viral Tax protein. However, despite all the hypotheses that were put forward, there are still no real answers to: Why is HTLV-1 infection lifelong, and why does it induce a lethal disease in very few (0.2%-5%) patients? Why does leukemia occur 10 to 50 years after infection? And, why should Tax expression be down-regulated during the late stages of this disease? The apparent paradox between the oncogenic properties of Tax and its low expression in ATL remains to be explained.
A few years ago, an mRNA encoding for a fascinating novel open reading frame (ORF) in the minus strand of HTLV-1 was suggested to exist,29 and was later characterized by our group in HTLV-1–positive T-cell lines.30 Indeed, we demonstrated that this minus-strand RNA is transcribed by a functional promoter present within the antisense strand of the HTLV-1 3′ end and that this unconventional mRNA encodes for HBZ (HTLV-1 bZIP factor), a protein that contains an N-terminal transcriptional activation domain and a leucine zipper motif in its C-terminus.30,31 Furthermore, our results led us to conclude that HBZ interacts with CREB-2, resulting in inhibition of Tax-dependent viral transcription.30 HBZ protein/CREB-2 interaction is thus an efficient way to down-regulate viral transcription while HBZ mRNA levels are likely 20- to 50-fold lower than Tax mRNA levels.
Beside the peculiar encoding capacity revealed by these studies indicating that this retrovirus capitalized on its ability to encode a protein through the complementary strand of the genomic RNA, thereby resulting in a global increase in the ORF number accommodated within a limited proviral genome size (almost 8.5 kb), it was commendable to readdress an important question: why would such a small genome, which should focus its coding capacity on proteins essential for its replication, use a fancy genetic strategy that precisely inhibits Tax transactivation, for encoding the HBZ protein? Functions inherent to HBZ thus seemed contradictory with the hypothesis that Tax expression is required for preferential growth of infected cells and T-cell malignancy.
A simple explanation could be that, although Tax is necessary to promote proliferation of HTLV-1–infected cells and to inhibit apoptosis, Tax might be detrimental to viral replication. Indeed, Tax expression induces a strong anti-Tax cytotoxic T-lymphocyte response in infected patients, and down-regulation of Tax expression may be advantageous to evade the immune surveillance. HTLV-1 carriers with a high proviral load (correlated with high anti–HTLV-1 antibody titer) are more prone to develop ATL when the anti-Tax immune response is weak.32 Although this hypothesis meets the criteria to justify the presence of the HBZ-ORF in the HTLV-1 genome, is it still arguable that HBZ would have been selected at such a high genetic cost, only to turn off the expression of another accessory viral protein?
Both the HBZ and Tax genes are found in the genome of the simian T-cell leukemia virus type 1 (STLV-1), which shares a common ancester with HTLV-1, indicating that HBZ has not been recently acquired; that is, once the virus adapted to humans (STLV-1 and HTLV-1 are considered to have diverged around 50 000 years ago33 ) it did not tolerate genetic drift, resulting in its silencing. In the HTLV-2 genome, a human retrovirus related to HTLV-1, Tax also exists but, surprisingly, HTLV-2 lacks the HBZ-ORF. Moreover, in contrast to HTLV-1 and STLV-1, which both cause lymphoid malignancy in the host, no association between HTLV-2 infection and cancer has been yet evidenced. There has been only one reported case of a patient carrying HTLV-2 who developed a variant of hairy cell leukemia. Does this evidence indicate that the HBZ protein and/or HBZ mRNAare critical for ATL induction?
To answer this question, searching for HBZ function and HBZ-cellular partners might be a possible avenue. Yet, the fact that the HBZ-binding region in CREB-2 was a bZIP domain suggests that other proteins with bZIP domains could possibly interact with HBZ. Evidence promptly emerged indicating that HBZ can form complexes with the bZIP transcription factors JunB, c-Jun,34 and JunD,35 which led to speculation that HBZ could trigger aberrant expression of cellular genes through these new complexes. The Jun family members are known to regulate gene expression by interacting with the AP-1 site present in the promoter region of several cellular genes, including IL-2. Therefore, it was not surprising that HBZ suppressed both transactivation by c-Jun and HTLV-1 transcription,30 a result confirmed by different research groups.36,37 Morever, HBZ promotes c-Jun degradation through a proteasome-dependent pathway.36 In conclusion, not only can HBZ control Tax production at the transcriptional level, but it can also modulate Tax activity in infected cells by attenuating AP-1 signal transduction. Yet, are these observations sufficient to claim that the discovery of HBZ improves our understanding of ATL induction?
Previous studies showed that T-cell lines transformed by HTLV-1 display high AP-1 activity with increased levels of mRNAs coding for c-Jun and c-Fos.38 This result may be linked to our observation that HBZ can sometimes be poorly expressed in certain HTLV-1 leukemic T-cell lines, an observation recently confirmed by Satou et al.37 A likely explanation is that HBZ plays another role besides simply inhibiting Tax. Satou et al have presented additional experiments in favor of this hypothesis. Indeed, suppression of HBZ gene transcription by short interfering RNA (siRNA) significantly decreased proliferation of ATL cells. Although it cannot be totally excluded that these siRNAs might have also modulated the expression of cellular mRNA coding for bZIP proteins or hybridized with viral sense mRNA coding for auxillary proteins other than HBZ, these results further suggest that HBZ could be critical for the development of ATL cells. The ghost of a smile occurs along with the demonstration that, in contrast to Tax mRNA, HBZ mRNA is expressed in all fresh ATL cells tested from a large panel of cell clones.37 Is this sufficient evidence for considering that HBZ offers the answer that retrovirologists have been looking for for years to explain ATL induction, or does it only move the question a step further?
Satou et al37 conducted additional experiments using an impressive series of mutants designed to shut down HBZ protein expression while maintaining HBZ mRNA expression, or aimed at altering the secondary structure of the RNA without, theoretically, affecting protein synthesis. First, they replaced the ATG of HBZ by a TTG, which is expected to block HBZ protein synthesis, and surprisingly, observed that TTG HBZ could still promote proliferation. They concluded that the proliferative function was linked to the mRNAand not to the protein. However, a few controls would have been interesting to include in these experiments before considering that a novel concept is definitively established; it is worth noting that, in Table 2 of Satou et al,37 TTG HBZ did not seem to be functionally inactive, and both wild-type HBZ and TTG HBZ modulated cellular gene expression. Second, the HBZ coding segment was modified by generating silent mutations (SM HBZ) (altering the RNA secondary structure with no amino acid change). In contrast to wild-type HBZ, this mutant did not support cell proliferation. It was then concluded that HBZ mRNA directly promoted T-cell proliferation, whereas the HBZ protein inhibited Tax-mediated transactivation through the 5′ LTR. However, confirmation of the conclusions drawn from this study will need to include experiments on the detection of the HBZ protein in wild-type or mutated HBZ-expressing cells. In the upcoming years, it will be of the highest importance to identify whether different functions should be ascribed to HBZ protein and HBZ mRNA, which is a promising challenge. Pertaining to this point, experiments by Arnold et al39 clearly indicated that HBZ inhibition of Tax-mediated trancription did not result from an RNA interference effect on Tax/Rex or Gag/Pol.
Assuming that either HBZ protein or HBZ mRNA is responsible for ATL induction, is there any in vivo evidence supporting this hypothesis? Unfortunately, the answer is not so clear. Recently, both HBZ transgenic mice and HTLV-1ΔHBZ–infected rabbits have been studied. The HBZ transgene apparently promotes a CD4+ T-lymphocyte proliferation in transgenic mice, albeit at a modest level.37 On the other hand, in cell culture, HBZ-deficient HTLV-1 viruses were indistinguishable from wild-type virus, indicating that HBZ is dispensable for infectivity and cellular immortalization of primary T lymphocytes. However, infection of rabbits with HBZ-deficient HTLV-1 viruses revealed that in vivo HBZ is important and functions to enhance infectivity, replication, and persistence.39
Although Tax transactivation is suppressed by HBZ and Tax is expected to play a central role in E2F1 gene expression, Satou et al37 reported that, through reverse transcriptase–polymerase chain reaction (RT-PCR) analysis, E2F1 was overexpressed in ATL cells that lack Tax. They suggested that the HBZ protein suppresses Tax transactivation of E2F1, whereas the HBZ mRNA increased E2F1 gene transcription and, subsequently, T-cell proliferation. If this bimodal function of HBZ turns out to be correct, it would represent a major breakthrough in this long eventful story. If not, a period of intense brainstorming and of experimentally productive approaches will be required to reassess the function of HBZ in ATL and to determine which viral compound or compounds might support ATL induction. In addition, the HBZ story recently reached a higher level of complexity when 4 research groups simultaneously reported31,37,39,40 that different HBZ isoforms might be expressed. Immunochemistry experiments performed in primary ATL cell lines demonstrated that HBZ has an exclusive nuclear distribution.40 However, despite high sequence similarity, each HBZ isoform is targeted to distinguishable subnuclear structures.40,41 This particular subnuclear localization could explain why antibodies to HBZ have not been detected in the sera of HTLV-1 carriers.
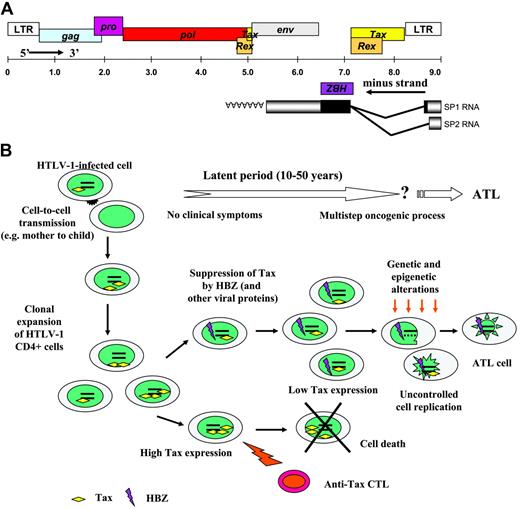
Simplified schematic model showing events from infection with HTLV1 to onset of ATL. Panel A illustrates the HTLV-1 genome organization, including the HBZ open reading frame. Panel B is a schematic presentation of the hypothetical flow of events occurring between the initial infection with HTLV-1 and the onset of ATL. Although the occurrence of ATL is limited in patients infected with HTLV-1, ATL remains an aggressive form of adult leukemia with no actual effective treatment. As the presence of the HBZ protein has been demonstrated in several clones derived from infected patients and because its function could be associated with HTLV-1–related pathogenesis, this viral protein thus becomes an interesting new target for ATL treatment.
Simplified schematic model showing events from infection with HTLV1 to onset of ATL. Panel A illustrates the HTLV-1 genome organization, including the HBZ open reading frame. Panel B is a schematic presentation of the hypothetical flow of events occurring between the initial infection with HTLV-1 and the onset of ATL. Although the occurrence of ATL is limited in patients infected with HTLV-1, ATL remains an aggressive form of adult leukemia with no actual effective treatment. As the presence of the HBZ protein has been demonstrated in several clones derived from infected patients and because its function could be associated with HTLV-1–related pathogenesis, this viral protein thus becomes an interesting new target for ATL treatment.
Several viral accessory proteins (p12I, p27I, p13II, p30II) are encoded in the pX region between the env gene and the 3′ LTR of HTLV-1,1,42 many of which modulate Tax expression. It is likely that some of these proteins work synergistically with HBZ to modulate viral and cellular gene expression and ultimately promote ATL cell proliferation. Yet, is HBZ-mediated suppression of Tax simply required for ATL cells to evade the anti-Tax CTL response? Are the different isoforms of HBZ playing equivalent functions toward ATL progression? Does HBZ permit infected cells to survive long enough to go through genetic alterations and epigenetic changes, transforming infected T cells into ATL? How important is HBZ for T cells to progress into ATL? Further investigations are required to answer these questions. Searching for somatic alterations43-46 or epigenetic variations47 in genes such as p53, p16INK4, and p27KIP1 in ATL cells and studying their transcriptional deregulation48,49 permitted the identification of many putative genes implicated in disease progression. Consequently, it remains almost impossible to characterize the cascade of events that leads to ATL. An appealing and so far poorly explored target is JunD, which can form complexes with HBZ.35 Leukemic cells from patients with ATL appear to induce AP-1 activity through a Tax-independent mechanism, and supershift experiments have established that AP-1 complexes in fresh leukemic cells contained JunD but not c-Fos, FosB, Fra-1, Fra-2, c-Jun, and JunB.38,50 JunD is considered to be an inhibitor of cellular proliferation/transformation but it would be of high interest to determine whether HBZ might alter JunD function into a positive regulator of ATL cell proliferation.
Is it possible to lay the foundation for a model explaining ATL induction? In the first stage of the infection, Tax is overexpressed, but most high Tax–expressing clones are killed by CTL activity (Figure 1). Moderate/low Tax expression may then be a prerequisite to evade immune surveillance and IL-2–dependent proliferation. At this step, HBZ might contribute to T-cell transformation. Clones, which become Tax-independent, will likely be driven toward the avenue that drives to leukemia. Not surprisingly, many more questions arise from each advance and several parts of the puzzle are still lacking to determine how the progression to ATL can be turned on.
Authorship
Contribution: C.D. designed the format of the article and wrote the draft; J.M.M. and B.B. critically reviewed the text and the figure. All authors contributed to writing the manuscript.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 17, 2006; DOI 10.1182/blood-2006-03-007732.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal