Abstract
Lack of allelic exclusion in the T-cell receptor (TCR) α locus gives rise to 2 different TCRs in 10% to 30% of all mature T cells, but the significance of such dual specificity remains controversial. Here we show that human CD4+CD25+ regulatory T (Treg) cells express 2 distinct Vα chains and thus 2 TCRs at least 3 times as often as other T cells. Extrapolating from flow cytometric analysis using Vα2-, Vα12-, and Vα24-specific monoclonal antibodies (mAbs), we estimated that between 50% and 99% of the CD25+ Treg cells were dual specific, as compared with about 20% of their CD25– counterparts. Moreover, both TCRs were equally capable of transmitting signals upon ligation. Cells with 2 TCRs also expressed more FOXP3, the Treg-cell lineage specification factor, than cells with a single TCR. Our findings suggest that expression of 2 TCRs favors differentiation to the Treg-cell lineage in humans and raise the question of the potential functional consequences of dual specificity.
Introduction
Regulatory T (Treg) cells are a specialized population capable of suppressing the function of other T cells and preventing autoimmunity. They form a subset of 5% to 10% of CD4+ T cells and were originally characterized on the basis of CD25 expression.1 More recently, it was shown that expression of the forkhead transcription factor FOXP3 is both required and sufficient for the regulatory phenotype, and it thus appears to be the master regulator of the Treg-cell lineage.2-5 The factors inducing FOXP3 expression or the mechanism by which FOXP3 confers the regulatory phenotype are not yet known. Most FOXP3+ Treg cells develop in the thymus, although they can also be induced peripherally from nonregulatory T cells.1,6 In mice, most of the evidence suggests that Treg-cell development is self-antigen driven and the resultant population autoreactive, the Treg-cell precursors somehow avoiding negative selection in the thymus.7-9 A few recent studies have questioned this interpretation, and it is possible that T-cell receptor (TCR) affinity alone is not sufficient to determine commitment to the Treg-cell lineage.2,10,11 It is assumed that human Treg-cell development and function broadly resembles that of murine Treg cells, but compared with the mouse actual data remain scarce.
While studying the up-regulation of FOXP3 in human thymocytes, we obtained data suggesting that a substantial proportion of thymic CD25+ Treg cells might transcribe both TCR α loci simultaneously. Unlike the TCR β locus, the α locus is not subject to allelic exclusion, and recombination proceeds simultaneously in both alleles until the thymocyte is positively selected.12 It was originally reported that up to 30% of mouse or human peripheral-blood T cells may express 2 α chains,13-15 but several factors are likely to make the actual frequency somewhat lower. Not all secondary α chain rearrangements detectable on the mRNA level result in a functional protein or turn out to have the ability to pair with the β chain expressed by the cell.16-18 Also, several studies have shown that cells positively selected through one αβ TCR can permanently down-regulate the second α chain in an active process termed phenotypic allelic exclusion.19-22 The true frequency of functional dual specificity thus remains an open question exacerbated by the scarcity of TCR variable gene α (Vα)–specific monoclonal antibodies (mAbs) that would allow direct measurements. The significance of dual-specific T cells is also a matter of some controversy, with much of the attention focusing on their potential autoreactivity.23-25 Studies with TCR transgenic mice have suggested that the presence of another TCR competing for intracellular signaling molecules might increase the threshold of negative selection in the thymus, allowing autoreactive T cells to escape deletion.25 Whether this is also true in a normal, polyclonal repertoire is unknown.
Here we show that most human Treg cells express 2 different surface TCRs, suggesting that in the normal thymus dual specificity may in fact be a way to facilitate the survival of autoreactive Treg cells. Our data also provide the first clear example in which the expression of a secondary TCR has an impact on the behavior and fate of the cell within a normal immune system.
Materials and methods
Samples
Thymic tissue was obtained from otherwise healthy children undergoing cardiac surgery (n = 12; age, 7 days to 7 months). Blood was taken from healthy adult volunteers (n = 12; age, 21 to 37 years). All samples were freshly processed. The study was approved by the ethics committee of the Helsinki University Hospital. Informed consent was obtained from the adult volunteers and the parents of the children in accordance with the Declaration of Helsinki.
Cell isolation and flow cytometry
Thymocytes were released by mechanical homogenization. Peripheral-blood mononuclear cells (PBMCs) were collected with Ficoll-Paque (Amersham, Uppsala, Sweden) gradient centrifugation. Selected subsets were isolated using mAbs and magnetic beads (Dynal, Oslo, Norway) according to the manufacturer's instructions. The bound cells were directly lysed or, when necessary, detached by using DETACHaBEADS (Dynal). The purity of the isolated subsets varied depending on the target population. TCR Vα–selected cells were typically 97% to 100% pure; CD25+ or CD25hi cells, about 80%; CD4+CD8+, more than 90%; and CD4+CD8– thymocytes, about 80% (most contaminating cells being CD4–CD8–). In 2 experiments in which cells expressing either 1 or 2 Vα chains were isolated for FOXP3 mRNA analysis, the FACSAria instrument (Becton Dickinson, San Jose, CA) was used for flow cytometric cell sorting.
Conjugated mAbs against human CD3, CD4, CD8, CD25, CD45RO, and αβ TCR were from Becton Dickinson; mAbs against human TCR Vα2 and Vα12 from Pierce (Rockford, IL); and Vα24 from Beckman Coulter (Fullerton, CA). Unlabeled FOXP3 mAb (clone 150D) was a gift from Dr Alison Banham5 (Oxford, United Kingdom), and PE-labeled mAb was purchased from eBioscience (San Diego, CA). Fluorescent second step reagents (Becton Dickinson) were used with the Vα2, Vα24, and FOXP3 mAb. Cell permeabilization for intracellular Vα protein detection was done by using the Fix&Perm kit (Caltag, Burlingame, CA) and for FOXP3 detection by using the FOXP3 permeabilization kit (eBioscience). TCR down-regulation was measured by incubating the cells with Vα-specific mAb for 1 hour at 37°C and analyzing the fluorescence intensity of surface TCR molecules. Flow cytometry was performed with the FACScan instrument (Becton Dickinson).
Because of the extremely low frequency of some of the subsets, we performed several control stainings to exclude the theoretically unlikely possibility that CD4+CD25– and CD4+CD25hi cells would have different levels of nonspecific background staining. The binding of fluorescently labeled nonspecific mouse antibodies and the secondary reagents in the absence of primary mAb was tested. To test the nonspecific binding of the directly conjugated Vα12 mAb, the frequency of Vα12+ CD3– cells was measured. No significant difference between the subsets was observed (Table 1).
Frequency of TCR Vα-chain expression on CD4+CD25+ and CD25- lymphocytes
. | Thymus . | . | . | . | PBMCs . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CD25- . | CD25+ . | P . | CI . | CD25- . | CD25+ . | P . | CI . | ||||||
| Vα2+, % | 1.1 ± 0.7 | 2.8 ± 0.2 | .002 | 1.0-2.3 | 3.1 ± 0.6 | 5.0 ± 0.6 | < .001 | 1.2-2.6 | ||||||
| Vα12+, % | 2.8 ± 0.3 | 3.9 ± 1.0 | .032 | 0.2-2.0 | 2.4 ± 0.6 | 3.1 ± 0.9 | < .001 | 0.4-0.9 | ||||||
| Vα24+, % | ND | ND | ND | ND | 0.3 ± 0.1 | 0.5 ± 0.1 | .002 | 0.1-0.3 | ||||||
| Vα2+12+, % | 0.03 ± 0.02 | 0.09 ± 0.01 | .012 | 0.03-0.09 | 0.02 ± 0.01 | 0.10 ± 0.07 | .011 | 0.02-0.12 | ||||||
| Vα12+ of Vα2+ cells, % | 1.5 ± 0.6 | 4.1 ± 1.2 | .009 | 1.3-4.1 | 0.4 ± 0.2 | 1.5 ± 0.7 | .002 | 0.6-1.6 | ||||||
| Control mAb, single positive, %* | ND | ND | ND | ND | 0.1 ± 0.1 | 0.1 ± 0.2 | NS | 0-0.1 | ||||||
| Control mAb, dual positive, %† | ND | ND | ND | ND | 0.01 ± 0.01 | 0.01 ± 0.01 | NS | -0.01-0.01 | ||||||
| Vα12+CD3-, % | ND | ND | ND | ND | 0.1 ± 0.2 | 0.1 ± 0.1 | NS | -0.1-0 | ||||||
. | Thymus . | . | . | . | PBMCs . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CD25- . | CD25+ . | P . | CI . | CD25- . | CD25+ . | P . | CI . | ||||||
| Vα2+, % | 1.1 ± 0.7 | 2.8 ± 0.2 | .002 | 1.0-2.3 | 3.1 ± 0.6 | 5.0 ± 0.6 | < .001 | 1.2-2.6 | ||||||
| Vα12+, % | 2.8 ± 0.3 | 3.9 ± 1.0 | .032 | 0.2-2.0 | 2.4 ± 0.6 | 3.1 ± 0.9 | < .001 | 0.4-0.9 | ||||||
| Vα24+, % | ND | ND | ND | ND | 0.3 ± 0.1 | 0.5 ± 0.1 | .002 | 0.1-0.3 | ||||||
| Vα2+12+, % | 0.03 ± 0.02 | 0.09 ± 0.01 | .012 | 0.03-0.09 | 0.02 ± 0.01 | 0.10 ± 0.07 | .011 | 0.02-0.12 | ||||||
| Vα12+ of Vα2+ cells, % | 1.5 ± 0.6 | 4.1 ± 1.2 | .009 | 1.3-4.1 | 0.4 ± 0.2 | 1.5 ± 0.7 | .002 | 0.6-1.6 | ||||||
| Control mAb, single positive, %* | ND | ND | ND | ND | 0.1 ± 0.1 | 0.1 ± 0.2 | NS | 0-0.1 | ||||||
| Control mAb, dual positive, %† | ND | ND | ND | ND | 0.01 ± 0.01 | 0.01 ± 0.01 | NS | -0.01-0.01 | ||||||
| Vα12+CD3-, % | ND | ND | ND | ND | 0.1 ± 0.2 | 0.1 ± 0.1 | NS | -0.1-0 | ||||||
Nonspecific background staining was tested by using irrelevant isotype-matched FITC-or PE-labeled mouse IgG. The frequency of cells positive for (*) either FITC or PE or (†) both are shown. The average number of fluorescence-activated cell sorting (FACS) events in the lymphocyte gate was as follows: VαX+ cells, 120 400; Vα2+12+ cells, 698 100; Vα12+ of Vα2+ cells, 149 400.
CI indicates 95% confidence interval for the difference between CD25- and CD25+ cells; ND, not determined; and NS, not significant.
Functional assay
CD25hi cells expressing Vα2, Vα12, or both were isolated from buffy coats (Finnish Red Cross, Helsinki, Finland) by immunomagnetic enrichment followed by cell sorting using FACSAria. They were cocultured with peripheral-blood lymphocytes (PBLs) in RPMI with 10% FCS and supplements using platebound anti-CD3 mAb for stimulation. After 5 days half of the culture supernatant was collected and replaced with RPMI containing 3H-thymidine (1 μCi [37 kBq] per well; Amersham). The cells were harvested 6 hours later with a Skatron harvester (Tranby, Norway) and analyzed with a liquid scintillation counter (Wallac, Turku, Finland). IFN-γ levels were determined using an enzyme-linked immunosorbent assay (ELISA) with anti–human IFN-γ mAb (Endogen, Woburn, MA), as described.26
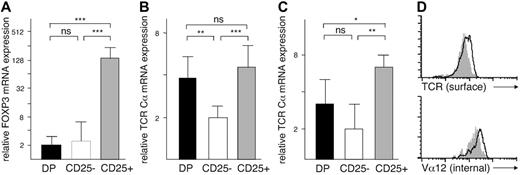
TCR α expression in thymocytes. (A) FOXP3 mRNA levels in CD4+CD8+ (DP: ▪), CD4+CD8–CD25– (CD25–: □), and CD4+CD8–CD25+ (CD25+: ▦) cells. (B) TCR Cα mRNA levels, normalized against β-actin and (C) against GAPDH expression. (D) Surface expression of αβ TCR and intracellular expression of TCR Vα12 chain in CD25– (white profile) and CD25+ (gray profiles) cells. In panels A-C, the mRNA expression levels were determined by quantitative PCR and are shown on a relative scale as mean ± SD. *P < .05, **P < .005, ***P < .001; ns indicates not significant.
TCR α expression in thymocytes. (A) FOXP3 mRNA levels in CD4+CD8+ (DP: ▪), CD4+CD8–CD25– (CD25–: □), and CD4+CD8–CD25+ (CD25+: ▦) cells. (B) TCR Cα mRNA levels, normalized against β-actin and (C) against GAPDH expression. (D) Surface expression of αβ TCR and intracellular expression of TCR Vα12 chain in CD25– (white profile) and CD25+ (gray profiles) cells. In panels A-C, the mRNA expression levels were determined by quantitative PCR and are shown on a relative scale as mean ± SD. *P < .05, **P < .005, ***P < .001; ns indicates not significant.
Quantitative PCR and TCR repertoire analysis
Cells were lysed with Tripure Reagent (Roche, Basel, Switzerland) and RNA collected with RNeasy mini columns (Qiagen, Crawley, United Kingdom). Complementary DNA was synthesized using oligo-dT primers and AMV reverse transcriptase (Finnzymes, Helsinki, Finland). Quantitative polymerase chain reaction (PCR) was done using TaqMan Universal PCR master mix and intron-spanning primer-probe assays (Applied Biosystems, Foster City, CA). The sequences for TCR Cα were GCCTTCAACAACAGCATTATTCCA, CTCGACCAGCTTGACATCACA, and FAM-CAGCCCAGAAAGTTC-5 quencher and, for RAG2, GTGAGCAGCCCCTCTGG, GACTGTTACCATCTGCAGAGACAT, and FAM-CCTTCAGACAAAAATCTAC-5 quencher. The other assays were commercially available from Applied Biosystems (assays-by-demand). The results were normalized against β-actin, or GAPDH when indicated, and quantified by comparing the results with a standard dilution curve.
TCR repertoire analysis has been described.27,28 Briefly, 24 Vβ-specific primers (Sigma, St Louis, MO) were used with a TCR β constant gene (Cβ)–specific primer to amplify the total β chain repertoire, followed by a run-off reaction with an internal FAM-labeled Cβ primer. Similarly, Vα2 or Vα12 primers were used together with a Cα primer and an internal FAM-labeled Cα primer. The fluorescent amplicons were analyzed using an ABI3730 sequencer (Applied Biosystems).
Statistics
Results are shown as mean ± SD. P values were calculated with Student 2-tailed t test, with P values below .05 as the limit for statistical significance. Confidence intervals at the 95% level were calculated for the differences between CD25+ and CD25– cells.
Results
Human CD4+CD8–CD25+ thymocytes express increased levels of TCR Cα mRNA but not TCR proteins
Previous studies have shown that the human CD4+CD8–CD25+ (CD25+) thymocytes express FOXP3 and possess regulatory capabilities.29,30 In quantitative PCR analysis, FOXP3 mRNA levels were 2 orders of magnitude higher in the CD25+ cells than in either CD4+CD8–CD25– (CD25–) or CD4+CD8+ double-positive (DP) cells (Figure 1A). In a further set of experiments we used the TCR Cα gene to normalize the PCR data. We then noted that the CD25+ thymocyte subset consistently expressed more Cα mRNA than the CD25– subset (Figure 1B). The immature DP cells also expressed higher levels of TCR Cα mRNA than the CD25– cells. To make sure that these differences were not caused by variable expression of the β-actin gene used for normalization, we reanalyzed the samples using GAPDH for reference, with similar results (Figure 1C).
The level of TCR expression in the thymus is highly variable and depends on the developmental stage of the cell type.12 To test whether the higher Cα mRNA levels reflected increased expression of TCR proteins, we used flow cytometry to measure the amount of surface αβ TCR in individual cells. In contrast to the quantitative PCR results, the highest intensity of TCR staining was found in the CD25– subset (Figure 1D). The CD25+ cells expressed significantly less TCR protein than the CD25– subset (mean fluorescence intensity, 52 ± 6 and 71 ± 5, respectively; P = .002) but more than the DP cells (24 ± 2). However, the antibody we used only reacts with an intact αβ TCR, so a limiting amount of any component of the TCR/CD3 complex could have restricted the assembly of the TCR. Indeed, it has been reported that human CD25+ thymocytes express less CD3-ϵ protein than the CD25– thymocytes.31 We therefore repeated the analysis using a mAb specific for a single TCR Vα chain, Vα12, and permeabilized the cells to allow the detection of intracellular TCR α protein uncoupled to TCR β/CD3. Both before and after permeabilization, the mean fluorescence intensities of CD25+ and CD25– cells corresponded to the results obtained with the anti-TCR mAb (Figure 1D). Thus, heterogeneity of TCR expression levels did not explain our findings.
There was also no difference in the recombination activity in the α loci between the CD25+ and CD25– thymocytes. As a mark of ongoing TCR gene rearrangement, DP cells expressed high levels of RAG-2 mRNA. In contrast, in both CD25+ and CD25– cells the level of RAG-2 was low, with no difference between them (not shown).
CD25+ thymocytes express 2 productively rearranged TCR Vα genes at an increased frequency
The TCR α locus is not subject to allelic exclusion, and a significant subset of human T cells has been shown to express 2 Vα proteins.14 Several details of the data described under “Human CD4+CD8–CD25+ thymocytes express increased levels of TCR Cα mRNA but not TCR proteins” suggested that the CD25+ thymocytes might transcribe both TCR α loci simultaneously. First, the difference between CD25– and CD25+ cells in Cα mRNA levels was consistently 2-fold. Second, the high Cα mRNA content in the CD25+ population was similar to that of the DP population, which uses both loci while attempting to rearrange a functional α gene and often expresses 2 TCRs.19-21 Third, the frequency of Vα12+ cells was higher in the CD25+ than in the CD25– subset, as would be expected if the former contained more cells expressing 2 TCRs. This was also true for another Vα gene, Vα2 (Table 1). We therefore analyzed the frequency of cells expressing 2 distinct surface TCRs, a task complicated by the small number of Vα gene-specific mAbs available. We used magnetic beads to isolate CD4+CD8– thymocytes and analyzed by flow cytometry the frequency of CD25+ and CD25– cells expressing both Vα2 and Vα12 chains. These cells with 2 TCRs were significantly more common among the CD25+ than the CD25– thymocytes (Table 1; n = 4). We then isolated thymocytes expressing TCR Vα2. Flow cytometric analysis of the Vα2+ cells showed that the frequency of cells also expressing Vα12 was significantly higher in the CD25+ than in the CD25– population.
Peripheral-blood CD4+CD25hi cells express 2 TCRs at a high frequency
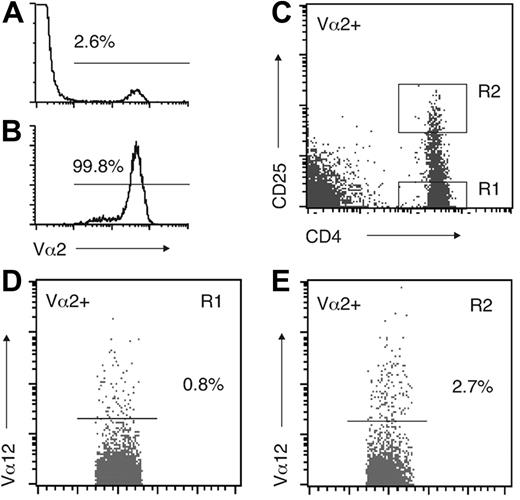
Studies in mice have indicated that surface expression of 2 TCRs is significantly more common in thymocytes than in mature T cells.15,19-21 A crucial question, therefore, was whether the expression of 2 TCRs was retained in mature Treg cells. Quantitative PCR analysis of PBLs showed no significant difference in the TCR Cα mRNA levels between isolated CD4+CD25– and CD4+CD25+ cells (not shown). However, the circulating CD25+ population is heterogeneous, and Treg-cell activity and FOXP3 expression in humans is largely restricted to CD4+ PBLs with a high CD25 expression (hereafter CD25hi cells).5,32 A direct analysis of cells expressing Vα2 and Vα12 simultaneously showed that they were more frequent in the CD25hi subset than in the CD25– subset (Table 1; n = 10). Because the Vα2+Vα12+ population was so small and the analysis easily affected by random variation, we next purified the Vα2+ PBL population. Staining of the isolated cells with the Vα12 mAb revealed a clear difference between CD25hi and CD25– cells (Figure 2). On the average, the frequency of Vα2+ cells also expressing Vα12 was 4 times higher in the CD25hi population than in the CD25– population (Table 1). The third available mAb, specific to Vα24, produced a higher nonspecific background staining than the other 2 mAbs and was therefore not used in these experiments. Also, part of the Vα24+ population belongs to the invariant natural killer T-cell (NKT-cell) subset, although their frequency is usually less than 0.1%.33
The most reliable estimates of the frequency of dual-specific T cells in mice are based on comparing normal mice with mice possessing only one functional TCR α locus.18 The excess Vα expression in the normal animals will then reveal the fraction of cells expressing 2 α chains. Although this approach cannot be applied to dual-specific T cells in humans generally, it can be used to compare 2 different T-cell subsets within the same individual. We therefore measured the frequency of TCR Vα2+,Vα12+, and Vα24+ cells in the CD25hi and CD25– subsets. In each case, the frequency of the Vα+ cells was significantly higher in the CD25hi subset (Table 1). These data indicate that, compared with the CD25– cells, the CD25hi population is enriched in cells expressing 2 α chains.
Expression of TCR Vα12 in isolated Vα2+ PBLs. (A) Vα2 expression in PBMCs. (B) Vα2 expression after immunomagnetic isolation. (C) Gating of CD25– and CD25hi cells within the Vα2+ population. (D) Vα12 expression in Vα2+CD25– and (E) Vα2+CD25hi cells. In panels A, B, D, and E, the cutoff point between positive and negative cells is indicated. A representative example of 7 donors is shown.
Expression of TCR Vα12 in isolated Vα2+ PBLs. (A) Vα2 expression in PBMCs. (B) Vα2 expression after immunomagnetic isolation. (C) Gating of CD25– and CD25hi cells within the Vα2+ population. (D) Vα12 expression in Vα2+CD25– and (E) Vα2+CD25hi cells. In panels A, B, D, and E, the cutoff point between positive and negative cells is indicated. A representative example of 7 donors is shown.
PBLs with 2 TCRs express more FOXP3 than PBLs with one TCR
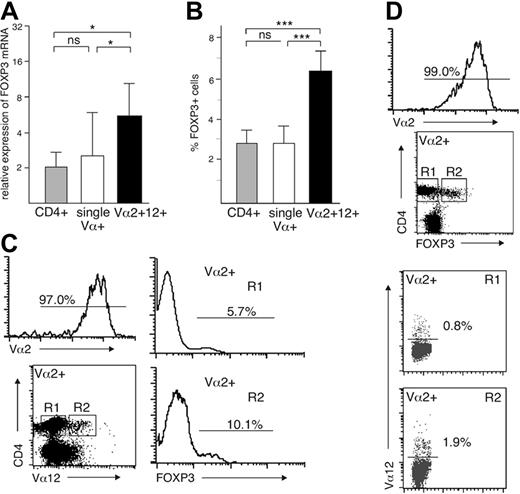
The results described in the preceding sections showed that both in the thymus and peripheral blood the CD4+CD25+ population consists of cells expressing 2 TCRs at a much higher frequency than the CD4+CD25– cells. However, although the CD25hi PBL subset is highly enriched in Treg cells, with 95.7% of them expressing FOXP3,5 we wanted to exclude the possibility that some of the dual-specific CD25hi cells might be activated T cells and not Treg cells. We therefore analyzed the expression of FOXP3, the Treg lineage–determining gene, in cells with 1 or 2 TCRs. We isolated cells expressing both Vα2 and Vα12, regardless of any other cell-surface marker, and compared them with CD4+ cells or cells expressing either Vα2 or Vα12 alone. The cells with 2 surface TCRs expressed significantly more FOXP3 mRNA than the 2 other populations tested (Figure 3A). There was no significant difference between CD4+ cells and cells with a single TCR. These results were confirmed by intracellular staining with anti-FOXP3 mAb, which showed that FOXP3+ cells were significantly more common among cells with 2 TCRs than among cells with a single TCR or the whole CD4+ population (Figure 3B-C). Similarly, among isolated Vα2+ cells, cells also expressing Vα12 were significantly more frequent in the CD4+FOXP3+ subset than in the CD4+FOXP3– subset (1.5 ± 0.5 versus 0.7 ± 0.4, P = .015; Figure 3D). Taken together, our data provide strong evidence that the human CD25hi FOXP3+ Treg-cell population is highly enriched in cells expressing 2 surface TCRs.
Vα2 and Vα12 are representative of the whole repertoire
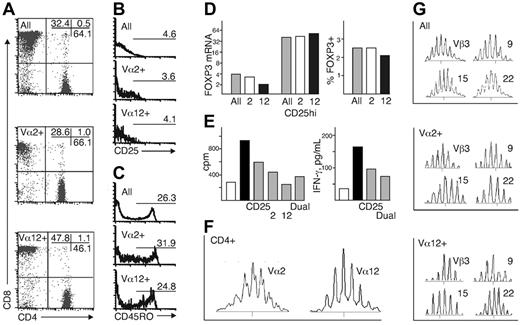
Because our analysis of the dual-specific Treg cells relied mostly on extrapolation from populations expressing Vα2 or Vα12, representing in our donors 5% to 7% of the whole repertoire, we performed a careful analysis of this subset to show that these Vα chains are typical and representative of the TCR α repertoire. Flow cytometric analysis of PBLs from 6 donors showed that Vα2+ cells did not have a preference for recognition of either major histocompatibility complex (MHC) class I or class II, with a CD4/CD8 ratio of 1.6, compared with 1.3 in all T cells, while Vα12+ cells favored recognition of MHC I (CD4/CD8 ratio, 0.6; Figure 4A). Within the CD4+ population the frequency and intensity of CD25 expression was similar in Vα12+ and Vα2+ cells to that of cells expressing other Vα genes (Figure 4B). CD45RO, a marker reported to differentiate between regulatory and nonregulatory CD25+ T cells in humans,4 was also expressed at roughly the same frequency by Vα2+,Vα12+, and other CD4+ T cells (Figure 4C). Analysis of FOXP3 mRNA and protein showed comparable levels in all T cells and Vα2+ cells, while Vα12+ cells had a slightly lower level of expression, most likely explainable by the overrepresentation of CD8+ cells among Vα2+ cells (Figure 3D). In isolated CD25hi cells the expression of FOXP3 was identical in Vα2+,Vα12+, and other T cells. Furthermore, when cultured together with other T cells, isolated CD25hi cells expressing Vα2, Vα12, or both suppressed anti-CD3–triggered responses as efficiently as CD25hi cells in general (Figure 4E).
We then analyzed the TCR repertoire of Vα2+ and Vα12+ cells. The TCR α chain repertoire showed a polyclonal, Gaussian distribution of CDR3 lengths in both Vα2 and Vα12 genes (Figure 4F), similar to that of other Vα chains. We next isolated cells expressing Vα2 or Vα12 and used a panel of 24 Vβ gene-specific primers to analyze the total TCR β repertoire used by these subsets. In both populations, the β chain usage was similar to that of the T-cell population in general, with a mostly polyclonal pattern of CDR3 lengths with occasional clonal expansions and underrepresented rearrangements (Figure 4G). These data show that with the exception of a biased MHC recognition by the Vα12+ subset, the cells expressing Vα2 or Vα12 are indistinguishable from the T-cell population and repertoire in general, and both Vα chains pair freely with β chains.
FOXP3 expression in single- and dual-specific peripheral-blood T cells. (A) FOXP3 mRNA and (B) protein levels in CD4+, single Vα+, and Vα2+Vα12+ PBLs (n = 8; mean ± SD). (C) A representative example of intracellular FOXP3 staining in isolated Vα2+ cells also expressing Vα12 (n = 8) and (D) of the frequency of dual-specific Vα12+ cells within the CD4+FOXP3– and CD4+FOXP3+ subsets of isolated Va2+ cells (n = 4). *P < .05, ***P < .001; ns indicates not significant.
FOXP3 expression in single- and dual-specific peripheral-blood T cells. (A) FOXP3 mRNA and (B) protein levels in CD4+, single Vα+, and Vα2+Vα12+ PBLs (n = 8; mean ± SD). (C) A representative example of intracellular FOXP3 staining in isolated Vα2+ cells also expressing Vα12 (n = 8) and (D) of the frequency of dual-specific Vα12+ cells within the CD4+FOXP3– and CD4+FOXP3+ subsets of isolated Va2+ cells (n = 4). *P < .05, ***P < .001; ns indicates not significant.
Phenotype, regulatory function, and TCR repertoire of Vα2+ and Vα12+ PBMCs. (A) CD4/CD8 ratio and expression of (B) CD25 and (C) CD45RO within the CD4+ subset. (D) Expression of FOXP3 mRNA and protein (TCR αβ+: ▦;Vα2+: □;Vα12+: ▪). (E) Suppression of T-cell proliferation and IFN-γ production by CD25hi cells expressing Vα2, Vα12, or both, with total CD25hi cells as a control (nonstimulated PBMCs: □; anti-CD3–stimulated PBMCs: ▪; anti-CD3–stimulated PBMCs cocultured at a ratio of 1:1 with the indicated CD25hi subsets: ▦). The cell numbers in the cultures were adjusted to the number of CD25hi Vα2+Vα12+ cells obtained (3000 to 4000). (F) TCR Vα2 and Vα12 repertoire in CD4+ cells. (G) Part of the TCR β repertoire in isolated Vα2+ and Vα12+ cells. The results in panels D and E are the mean and, in other panels, a representative example of 2 to 4 independent experiments.
Phenotype, regulatory function, and TCR repertoire of Vα2+ and Vα12+ PBMCs. (A) CD4/CD8 ratio and expression of (B) CD25 and (C) CD45RO within the CD4+ subset. (D) Expression of FOXP3 mRNA and protein (TCR αβ+: ▦;Vα2+: □;Vα12+: ▪). (E) Suppression of T-cell proliferation and IFN-γ production by CD25hi cells expressing Vα2, Vα12, or both, with total CD25hi cells as a control (nonstimulated PBMCs: □; anti-CD3–stimulated PBMCs: ▪; anti-CD3–stimulated PBMCs cocultured at a ratio of 1:1 with the indicated CD25hi subsets: ▦). The cell numbers in the cultures were adjusted to the number of CD25hi Vα2+Vα12+ cells obtained (3000 to 4000). (F) TCR Vα2 and Vα12 repertoire in CD4+ cells. (G) Part of the TCR β repertoire in isolated Vα2+ and Vα12+ cells. The results in panels D and E are the mean and, in other panels, a representative example of 2 to 4 independent experiments.
Most human Treg cells express 2 TCRs
Estimates of the fraction of T cells expressing 2 TCRs are complicated by the limited choice of reagents and the low frequency of the populations actually measured. Because of this we used several approaches to calculate the frequency of CD25hi cells with 2 TCRs, each based on a different set of experimental data. A previously used method18 extrapolates from staining with 2 Vα-specific mAbs: frequency = (A × 100 × 100)/(B × C), where A, B, and C are the frequency of Vα2+Vα12+ cells, Vα2+ cells, and Vα12+ cells, respectively. In our donors, this equation gave 15% as the overall frequency of T cells with 2 TCRs.
As the second approach we compared the frequency of single-specific Vα12+ T cells in the total T-cell population with the frequency of dual-specific Vα12+ T cells within the Vα2+ population: frequency = (A × 100)/(B–B × 0.15), where A is the frequency of Vα12+ cells in the Vα2+ population, B the frequency of Vα12+ cells in the total population, and 15% the frequency of dual-specific cells in the total population.
The measurements based on the excess staining of the CD25hi cells with Vα-specific mAb can also be used to estimate the frequency of CD25hi cells with 2 TCRs: frequency = (A–[B–B × 0.15]) × 100/(B–B × 0.15), where A and B are the frequency of the VαX gene in the CD25hi and CD25– subsets, respectively, and 15% again the overall frequency of dual-specific cells.
These 3 complementary methods produced reasonably consistent estimates (Table 2). Within the CD25– population, the frequency of cells with 2 TCRs was roughly 20% and thus comparable to T cells in general. A significantly higher fraction of the CD25hi cells was dual specific, the 3 equations producing estimates ranging from 50% to 99%. Although extrapolations and therefore tentative, these calculations strongly suggest that most human CD25hi Treg cells express 2 TCRs.
Estimated frequency of CD25hi and CD25- PBMC expressing 2 TCRs
. | Dual specific . | . | . | |
|---|---|---|---|---|
| Basis of calculation . | CD25hi, % . | CD25-, % . | P . | |
| Staining with Vα2 and Vα12 mAb | 93.9 ± 47.2 | 21.5 ± 18.0 | .008 | |
| Vα12 expression in Vα2+ population | 90.0 ± 36.3 | 25.5 ± 15.6 | .004 | |
| Excess Vα2 expression in CD25hi cells | 99.3 ± 50.8 | NA | NA | |
| Excess Vα12 expression in CD25hi cells | 50.3 ± 20.5 | NA | NA | |
| Excess Vα24 expression in CD25hi cells | 82.3 ± 48.6 | NA | NA | |
. | Dual specific . | . | . | |
|---|---|---|---|---|
| Basis of calculation . | CD25hi, % . | CD25-, % . | P . | |
| Staining with Vα2 and Vα12 mAb | 93.9 ± 47.2 | 21.5 ± 18.0 | .008 | |
| Vα12 expression in Vα2+ population | 90.0 ± 36.3 | 25.5 ± 15.6 | .004 | |
| Excess Vα2 expression in CD25hi cells | 99.3 ± 50.8 | NA | NA | |
| Excess Vα12 expression in CD25hi cells | 50.3 ± 20.5 | NA | NA | |
| Excess Vα24 expression in CD25hi cells | 82.3 ± 48.6 | NA | NA | |
NA indicates not applicable.
Both TCR α chains on Treg cells are functional
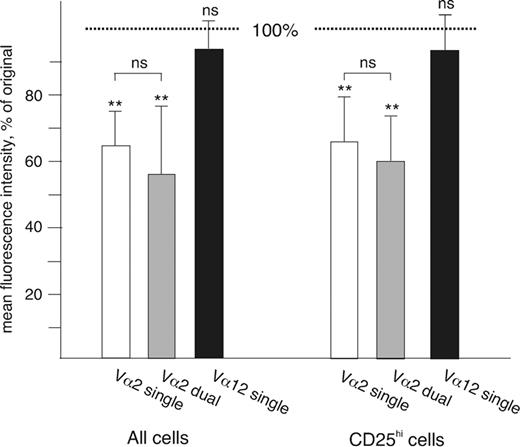
TCRs transmitting signals are rapidly internalized following engagement by a ligand.14,34 To test whether both TCRs on the surface of the dual-specific Treg cells were capable of signal transduction, we used mAb against Vα2 to stimulate the cells for 1 hour at 37°C and then compared the down-regulation of the engaged TCR in Vα2+ single- and Vα2+Vα12+ dual-specific cells. The nonengaged Vα12 was used as a negative control. In cells expressing only Vα2, the mean fluorescence intensity of surface Vα2 decreased to 64% of the original in CD4+ cells and to 65% in the CD4+CD25hi cells, while the intensity of Vα12 remained stable (94% in both cases; Figure 5). The 1-hour incubation also had no measurable effect on the overall level of CD3 expression either in CD4+ cells or in CD25hi cells (not shown). We then measured the down-regulation of Vα2 in cells also expressing Vα12. In dual-specific CD4+ cells, Vα2 intensity decreased to 57% and in CD25hi Treg cells to 60%, indicating that there was no significant difference in TCR down-regulation between single- and dual-specific T cells either in the CD25hi population or in the CD4+ cells in general. Similarly, engagement of Vα12 on CD25hi cells led to its down-regulation in both single-specific and Vα2+Vα12+ cells (to 69% and 60%, respectively), while Vα2 intensity was unaffected (not shown). These results suggest that both TCRs expressed by the dual-specific CD25hi Treg cells are as efficient as TCRs expressed by single-specific T cells in transmitting signals when bound by a ligand.
TCR down-regulation in cells expressing 1 or 2 Vα chains. The mean fluorescence intensity of TCR Vα expression was measured before and after incubation for 1 hour with an anti-Vα2 mAb. The results are shown as the relative decrease in fluorescence as compared with the original intensity (indicated by the dotted line; Vα2 on Vα2+Vα12– cells: □;Vα2 on Vα2+Vα12+ cells: ▦;Vα12 on Vα2– Vα12+ cells: ▪). The asterisks above the error bars indicate the statistical significance of the change in fluorescence (ns indicates not significant, **P < .005); the comparison between single- and dual-specific Vα2+ cells is also shown. There was no statistically significant difference between CD25hi and other CD4+ cells (n = 6).
TCR down-regulation in cells expressing 1 or 2 Vα chains. The mean fluorescence intensity of TCR Vα expression was measured before and after incubation for 1 hour with an anti-Vα2 mAb. The results are shown as the relative decrease in fluorescence as compared with the original intensity (indicated by the dotted line; Vα2 on Vα2+Vα12– cells: □;Vα2 on Vα2+Vα12+ cells: ▦;Vα12 on Vα2– Vα12+ cells: ▪). The asterisks above the error bars indicate the statistical significance of the change in fluorescence (ns indicates not significant, **P < .005); the comparison between single- and dual-specific Vα2+ cells is also shown. There was no statistically significant difference between CD25hi and other CD4+ cells (n = 6).
Discussion
Despite the well-established fact that many T cells express 2 TCRs, their development and function is in practice viewed in terms of monospecificity, and there have been no reports of dual specificity having an impact on the normal immune system. On theoretical grounds, it has been argued that the second α chain is often nonfunctional, either because it cannot pair with the β chain or because the second TCR has not been positively selected and does not recognize self-MHC.15-22 Our data on human Treg cells thus provide the first clear instance in which the presence of a second TCR influences the fate of the dual-specific cell in a nontransgenic setting. The data are based on the surface expression of 2 TCRs and verified by flow cytometry, so the pairing of both α chains to the β chain has demonstrably taken place. Upon engagement by mAb, the TCR complexes on dual-specific Treg cells were also internalized quite as efficiently as TCRs on single-specific T cells, suggesting that both receptors were capable of transmitting signals. The antigens recognized by human Treg cells are not yet known, so it cannot be shown directly that both receptors participate in Treg-cell selection or responses. However, the greater part of TCR repertoire is biased for MHC recognition even before selection,35 so it is likely that most of the secondary TCRs have at least the potential to respond to MHC-bound antigens. Furthermore, the fact itself that Treg cells are so greatly enriched in cells expressing 2 TCRs suggests that dual specificity does have an active role in directing cells to the regulatory lineage.
Previous studies on dual-specific T cells, and especially attempts to measure their frequency, have often been criticized for their reliance on the small number of Vα-specific mAbs available. Also, the fact that in many cells the presence of secondary Vα mRNA does not result in surface protein expression15-22 means that mRNA-based approaches to study a wider range of Vα genes are not reliable. Our data, too, address directly only part of the repertoire, ranging from 5% to 7% in different donors, although the measurement of excess Vα expression does provide a more comprehensive view of the secondary α chains within the CD25hi subset.18 Therefore, the actual frequency of dual-specific cells may be somewhat different from our estimates, but the relative difference between Treg cells and other T cells should not be affected. Furthermore, our detailed analysis of the cells expressing Vα2 or Vα12 showed that they are representative of the TCR α repertoire in general. Both populations had a normal surface phenotype, including the expression of CD25 and CD45RO, expressed FOXP3 at roughly the same level, contained CD25hi cells with a regulatory function, and possessed a fully polyclonal repertoire of both α and β chains. It is therefore highly unlikely that our observations would apply only to the 2 Vα genes studied. Our analysis of the expression of Vα24 also supported our conclusions, the reservations concerning this α chain notwithstanding. It therefore seems that the Treg-cell population in humans is indeed highly enriched in cells expressing 2 TCRs.
Because random recombination of the second TCR α locus will produce a functional chain in not more than one third of the cells, the high frequency of 2 productive α genes in CD25+ Treg cells is likely to be the result of intrathymic selection. A substantial body of evidence indicates that Treg cells are selected by self-antigens and recognize them with a high affinity.1,7-9 It is not known how thymocytes expressing such autoreactive TCRs avoid negative selection, especially because the up-regulation of FoxP3 in itself does not appear to provide protection from deletion.9 It has been postulated that the affinity of Treg-cell TCRs to self-antigens falls just below the threshold of negative selection1 but, opposing this view, it was recently demonstrated that these TCRs are capable of triggering tissue damage if expressed by FoxP3– cells.9 Furthermore, a substantial fraction of the autoreactive effector cells in FoxP3– scurfy mice seems to use TCRs, which in FoxP3+ mice are largely restricted to Treg cells. These results suggest that there is little difference in the TCR affinity between autoreactive FoxP3+ thymocytes that survive and FoxP3– thymocytes that are mostly deleted, again raising the question as to how the autoreactive Treg cells avoid negative selection. The finding that most human Treg cells express 2 α chains may provide at least part of the solution. Studies in transgenic mice have shown that the intracellular competition of 2 α chains for pairing with a single β chain allows the cell to down-modulate the autoreactive TCR, thereby avoiding negative selection, and still to be positively selected through the other TCR.25 Our data suggest a scenario in which a developing thymocyte with an autoreactive TCR is deleted unless saved by the presence of a second α chain, in which case it is recruited to the Treg-cell lineage and upregulates FOXP3. The real physiologic significance of the lack of allelic exclusion in the α locus may therefore be to create a selection window for autoreactive Treg cells.
Findings suggesting a role for dual specificity in the development of Treg cells have also been reported from studies on mouse strains transgenic for an autoreactive TCR. In several settings the autoreactive TCR induces more efficient Treg-cell development when placed in a recombination-competent background that allows endogenous TCR rearrangements.36-40 For example, in mice expressing a transgenic TCR specific for myelin basic protein, neither the transgenic TCR+ cells in Rag-deficient mice nor the endogenous TCR+ cells in Rag-sufficient mice were effective Treg cells.40 Only those cells that expressed both an endogenous and the transgenic autoreactive TCR were capable of suppressing the autoimmune encephalitis. In these models, too, the presence of a secondary TCR seems to provide the opportunity for the transgenic TCR to escape deletion and direct the developing thymocyte into the Treg-cell lineage. Nevertheless, in other settings it has been possible to generate Treg cells in TCR transgenic mice in the Rag-deficient background,41-43 and analysis of mice unable to express 2 different Vα chains showed that they have normal Treg cells.44 It is therefore clear that murine Treg cells can develop from monospecific precursors, but in the normal human immune system dual specificity appears to strongly favor differentiation into the Treg-cell lineage.
Does the potential dual specificity influence Treg-cell function? Previous studies on the functional significance of dual-specific T cells have often reached conflicting conclusions. In some settings activation of dual-specific T cells has indeed been associated with autoimmunity,23-25 while in others dual-specific T cells appear to be neutral or even protective.18,45,46 These contradictions may be in large part due to the transgenic models used, which, although convenient, may reveal more of the particular TCRs than of dual-specific T cells in general. In a normal immune system the effect of having 2 TCRs on T-cell function is unknown, and without data on the antigens recognized by human Treg cells, the effect of dual specificity on Treg-cell function must remain speculative. However, one paradox of Treg-cell biology is that their development is dependent on high-affinity interactions with self-antigens in the thymus, but at least some of them can subsequently respond to exogenous antigens in the periphery.47 For example, in mice infected by leishmania, most thymus-derived Treg cells in the lymph nodes draining the infection site are specific to the parasite.48 This apparent conflict between thymic selection and peripheral activation has been explained by crossreactivity, antigen-independent bystander suppression, or peripheral induction of adaptive subsets of Treg cells, but our data suggest a further possibility. Because most Treg cells express 2 TCRs, the secondary repertoire is likely to be as diverse as the positively selected self-reactive repertoire,31 with many potentially useful specificities. Some of the Treg cells regulating responses to exogenous antigens may have been selected through the autoreactive TCR but function in the periphery through the other. In other situations the same cells might be activated through the autoreactive TCR to prevent autoimmunity. In this way dual specificity may increase the range and flexibility of Treg-cell responses.
Despite intensive study, many aspects of Treg-cell commitment and function remain unclear. The dual specificity of this population provides putative answers to some questions but also raises new ones, which will require more information on the antigens recognized by human Treg cells. In particular, such data will help in resolving how signals from the 2 TCRs are integrated during Treg-cell selection and activation. Our findings open new avenues of investigation into the development and function of human Treg cells and also provide the first demonstration of dual specificity having an impact on the normal immune system.
Authorship
Contribution: H.T. and T.P.A. designed and performed the research and wrote the paper, and J.S. contributed clinical samples.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, 8/22/2006; DOI 10.1182/blood-2006-04-016105.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from the Academy of Finland, Finnish Cultural Foundation, and Helsinki University Science Foundation and research funds of the Helsinki University Hospital.
We thank A. Banham for providing the anti-FOXP3 mAb, A. Miettinen for reviewing the manuscript, S. Sarna for advice on statistics, and O. Vaarala for performing the IFN-γ measurements.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal