Abstract
While during the first trimester of pregnancy natural killer (NK) cells represent the most abundant lymphocyte population in the decidua, their actual function at this site is still debated. In this study we analyzed NK cells isolated from decidual tissue for their surface phenotype and functional capability. We show that decidual NK (dNK) cells express normal surface levels of certain activating receptors, including NKp46, NKG2D, and 2B4, as well as of killer cell immunoglobulin-like receptors (KIRs) and CD94/NKG2A inhibitory receptor. In addition, they are characterized by high levels of cytoplasmic granules despite their CD56bright CD16– surface phenotype. Moreover, we provide evidence that in dNK cells, activating NK receptors display normal triggering capability whereas 2B4 functions as an inhibitory receptor. Thus, cross-linking of 2B4 resulted in inhibition of both cytolytic activity and interferon-γ (IFN-γ) production. Clonal analysis revealed that, in the majority of dNK cell clones, the 2B4 inhibitory function is related to the deficient expression of signaling lymphocyte activation molecule (SLAM)–associated protein (SAP) mRNA. Moreover, biochemical analysis revealed low levels of SAP in the dNK polyclonal population. This might suggest that dNK cells, although potentially capable of killing, are inhibited in their function when interacting with cells expressing CD48.
Introduction
Natural killer (NK) cells represent a lymphocyte population capable of recognizing and lysing not only tumor cells but also virus-infected cells in the absence of previous sensitization.1,2 NK-cell function is regulated by a balance between activating and inhibitory signals.3 Among peripheral-blood NK (pNK) cells, 2 subsets can be identified on the basis of CD56 surface expression: those with low/intermediate levels of CD56 (CD56dim) and those with high levels of CD56 (CD56bright).4 CD56dim NK cells represent the majority (> 90%) of pNK cells, contain abundant cytoplasmic granules, are highly cytotoxic, and mediate antibody-dependent cell cytotoxicity (ADCC) against IgG-coated target cells through the engagement of CD16.5 The phenotypically and functionally distinct CD56bright subset, representing less than 10% of pNK cells, does not express CD16 molecules or expresses low-density CD16 molecules, does not contain cytoplasmic granules, and is poorly cytolytic but has greater cytokine-production capacity.4 In addition, the 2 pNK-cell subsets differ in their expression of killer-cell immunoglobulin-like receptors (KIRs) and CD94/NKG2A receptor. Thus, CD56bright pNK cells have low to absent expression of KIRs, whereas they express high levels of CD94/NKG2A inhibitory receptor. In contrast, most CD56dim pNK cells are KIR positive and express low levels of CD94/NKG2A.4 During the first trimester of pregnancy, NK cells constitute 50% to 90% of the lymphocytes present in the decidua, a tissue in which B and T lymphocytes are infrequent. It is currently believed that in the early stages of gestation, decidual NK (dNK) cells may play an important role in the control of trophoblastic growth, differentiation, and invasion. Decidual NK cells are phenotypically similar to the CD56bright pNK cell subset. However, transcriptional gene-expression profiling has shown that dNK cells express both perforin and granzymes at a similar or even higher level than CD56dim CD16+ pNK cells.6 This observation suggested that dNK cells might have cytotoxic potential. However, conflicting data exist on this functional capability of dNK cells.7-10 Recently, freshly isolated human dNK cells have been shown to display markedly reduced cytotoxicity toward major histocompatibility complex (MHC) class I–negative targets due to their inability to form activating synapses.11
In this study we focused on the contribution of natural cytotoxicity receptors (NCRs) and 2B4 to the cytolytic activity and cytokine production of human dNK cells. NCRs belong to the Ig superfamily. They include NKp46 and NKp30, expressed on both resting and activated pNK cells, and NKp44, expressed only on activated pNK cells.12-15 Thanks to their association with different ITAM-transducing polypeptides, including CD3ζ, FcRγ (NKp30 and NKp46), and KARAP/DAP12 (NKp44), NCRs can deliver intracellular signals that result in induction of cytotoxic activity.14-18 2B4 is characterized by a broad expression on different leukocyte populations (ie, NK cells, γδ T cells, monocytes, basophils, and a subset of memory CD8+ αβ T cells).19 CD48, the natural ligand of 2B4, is widely expressed on human leukocytes.20,21 2B4 is generally considered a coreceptor because it can enhance NK-cell responses delivered by other triggering receptors such as NCRs.22 Upon tyrosine phosphorylation, 2B4 associates with the small cytoplasmic protein termed signaling lymphocyte activation molecule (SLAM)–associated protein (SAP) followed by the recruitment of Fyn tyrosine kinase.23 This molecular interaction is thought to be crucial for preventing 2B4 association with Src homology 2 domain-containing phosphatase 1 (SHP-1).23,24 Notably, depending on the association with SAP or SHP-1, 2B4 mediates opposing functions, activating or inhibitory, respectively. Remarkably, “inhibitory” 2B4 receptor is expressed in vitro by normal NK cells at an early stage of differentiation25 as well as by pNK cells derived from patients with the X-linked lymphoproliferative disease (XLP).26,27
Our present data indicate that NKp46, NKp30, and NKp44 activating NK receptors display normal function in activated dNK cells. However, dNK cell activation, cytolytic activity, and cytokine production were inhibited by the interaction of 2B4 receptor with CD48+ cells. The inhibitory function of 2B4 could be explained by the finding that freshly derived and in vitro–expanded dNK-cell populations, as well as the majority of clones, were characterized by the lack of, or very low level of, SAP.
Patients, materials, and methods
Monoclonal antibodies
The following monoclonal antibodies (mAbs) were used in this study: A13 (IgG1, anti-Vδ1), BB3 (IgG1, anti-Vδ2), BAB281 (IgG1, anti-NKp46), Z231 (IgG1, anti-NKp44), 7A6 and AZ20 (IgG1, anti-NKp30), PP35 and MA344 (IgG1 and IgM, respectively; anti-2B4), GL183 (IgG1, anti-KIR2DL2/L3/S2), 11pB6 (IgG1, anti-KIR2DL1/S1), Z270 (IgG1, anti-NKG2A), BAT 221 (IgG1, anti-NKG2D), UCHT-1 (IgG2a, anti-CD3), and CO202 (IgM, anti-CD48) (produced in our laboratory); D1.12 (IgG2a, anti–HLA-DR) mAb (provided by Dr R. S. Accolla, University of Insubria, Varese, Italy); HP2.6 (IgG2a, anti-CD4) mAb (provided by Dr P. Sanchez-Madrid, Hospital de la Princesa, Madrid, Spain); M-T101 (IgG1, anti-CD1b), TU145 (IgM, anti-CD48), 2H4 (IgM, anti-CCR7), Leu-12 (IgG1, anti-CD19), 19.2 (IgG1, anti-CD206), and DC-SIGN (IgG2b, anti-CD209) mAbs (BD Pharmingen, San Diego, CA); AC144 (IgG1, anti–BDCA-2), AD5-14H12 (IgG1, anti–BDCA-3), and AD5-17F6 (IgG1, anti–BDCA-4) (Miltenyi Biotec, Bergisch Gladbach, Germany); and FITC- and PE-conjugated anti-isotype goat antimouse (GAM) mAbs (Southern Biotechnology, Birmingham, AL). For analysis of surface-marker expression on freshly isolated decidual and peripheral blood mononuclear cells, the following fluorescent-labeled conjugated mAbs were used in different combinations: CD3-PE, CD16-FITC, CD45-FITC, CD80-PE, and CD14-PE (BD Pharmingen); CD1a-PE, CD56-PC5, and CD14-PC5 (Immunotech, Marseille, France); and CD86-FITC (Caltag Laboratories, Burlingame, CA). For intracellular staining, the following mAbs were used: anti–IFNγ-PE (IgG1), anti–granzyme A–PE (IgG1; BD Pharmingen), anti–granzyme B–PE (IgG1; Caltag Laboratories), anti–perforin-PE (IgG2b; Ancell, Bayport, MN), antiactin (rabbit antiserum; Sigma, St Louis, MO), and anti-SAP (FL-128) rabbit antiserum (Santa Cruz Biotechnology, Santa Cruz, CA). FITC-conjugated goat anti–rabbit IgG was purchased from Jackson Immunoresearch (West Grove, PA).
Flow cytometric analysis
The reactivity of mAbs with cell populations was assessed by both indirect and direct immunofluorescence and cytofluorimetric analysis as previously described.28-30 For the intracellular protease staining, freshly isolated decidual mononuclear cells and peripheral-blood mononuclear cells (PBMCs) were analyzed as previously described.30 Cytokine production was assessed using either freshly purified dNK cells or pNK cells. Briefly, purified NK cells were stimulated with recombinant IL-2 (rIL-2) and rIL-12 for 48 hours at 37°C and then incubated with the LCL 221.721 CD48+ B-cell line in the presence of either an anti-CD48 mAb or, as control, an isotype-matched irrelevant mAb for an additional 18 hours at 37°C (NK cells/LCL 721.221 ratio used was 3:1). During the last 4 hours of stimulation, GolgiStop (BD Biosciences, San Jose, CA) was added. Cells were then washed and the intracellular staining using an anti–IFNγ-PE mAb was performed. Cells were analyzed in a FACScan flow cytometer and the analysis was performed using cell CellQuest software (BD Biosciences).
Tissue and cell samples
Samples were obtained at 9 to 12 weeks of gestation from singleton pregnancies of mothers requesting termination of the pregnancy for social reasons or who were undergoing evacuation of retained products of conception following spontaneous pregnancy failure. The study was approved by the relevant institutional review boards and all patients gave their written informed consent according to the Declaration of Helsinki. Decidual tissue was separated from specimens obtained by suction evacuation of the uterus. The total decidual tissue was then minced into fragments of 1 mm3 and digested for 1 hour at room temperature under agitation in PBS with 200 U/mL hyaluronidase (Sigma) and 1 mg/mL collagenase type IV (Seromed, Berlin, Germany). The cell suspension was filtered through sterile stainless-steel 100-μm wire mesh and washed once in PBS. The mononuclear cell population was isolated using Ficoll-Hypaque density gradient (Sigma), washed twice in PBS, and used for cell isolation or fluorescence analysis. Peripheral-blood lymphocytes were isolated from peripheral blood from healthy donors using Ficoll-Hypaque density gradient either directly or after enrichment for NK cells using Rosettesep (StemCell Technologies, Vancouver, BC, Canada).
Isolation, culture, and cloning of dNK and pNK cells
To obtain enriched NK cells, both decidual mononuclear cells and PBMCs were depleted of CD4+, CD14+, CD19+, CD3+,Vδ1+, and Vδ2+ cells by negative selection using appropriate mAbs followed by goat antimouse–coated Dynabeads (Dynal, Oslo, Norway) and immunomagnetic depletion.12,15 CD3– cells were cultured on irradiated feeder cells in the presence of rIL-2 (100 U/mL; Proleukin; Chiron, Emeryville, CA) and 1.5 ng/mL PHA (GIBCO BRL, Carlsbad, CA) to obtain either polyclonal NK-cell populations or, after limiting dilution,12 NK-cell clones.
Cytolytic activity
The following target cells were used in this study: P815 (murine mastocytoma, FcγR+), LCL 721.221 EBV+ B-cell line, Daudi Burkitt lymphoma cell line, and FO1 melanoma cell line. pNK and dNK cell populations and clones were tested for cytolytic activity in a 4-hour 51Cr-release assay, as previously described,12,15 in the absence or presence of various mAbs. The concentrations of the various mAbs were 10 μg/mL for the masking experiments and 0.5 μg/mL for the redirected killing experiments, unless otherwise specified. The effector-target (E/T) ratios used are indicated in the figure legends.
Quantitative analysis of SAP transcript in NK cells
Total RNA was extracted from the polyclonal population and clones using an RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer's instruction, and cDNA synthesis was performed on 500 ng of RNA using hexameric primers. Polymerase chain reaction (PCR) amplifications were performed using TaqMan assay (Applied Biosystem 7700 Sequence Detector; Foster City, CA). A 2-step PCR procedure of 15 seconds at 95°C and 1 minute at 60°C was applied for 40 cycles. The sequences of the primers used for SAP-specific amplification were SAP-Q up (3′AAC AGG TTC TTG GAG TGC TGA GA 5′) and SAP-Q down (3′AGA GGT ATT ACAATG CCT TGA 5′), whereas the SAP-Q probe was 3′AAT AAAAAA TCT CAT TTC AGC ATT TCA GAA GCC AGA 5′-6-FAM. The GAPDH expression was used to normalize the SAP quantity (human GAPDH Endogenous control kit, Applied Biosystem 7700 Sequence Detector). The normalized SAP mRNA transcript of the tested samples was calculated as time-fold of mRNA detected in the NK cell line NK92 (chosen as reference in this study). Each clone was analyzed in 2 independent experiments and each reaction was performed at least in triplicate. For intergroup comparisons, Mann-Whitney test and Kruskal-Wallis test were used as appropriate. All calculations were performed using Prism software package (release 3.00; GraphPad Software, San Diego, CA).
Slot-blot analysis of SAP expression
Postnuclear cell lysates were obtained from polyclonal-activated dNK, pNK, and XLP-NK cells.26 After prewetting a nitrocellulose membrane with TBS for 5 minutes, samples (1 × 106 cells/slot) were loaded under vacuum onto the membrane through the slots using a Minifold-II slot-blot system (Whatman, Schleicher and Schuell, Brentford, United Kingdom). Membrane was soaked in blocking buffer (TBS + 5% BSA) at room temperature for 2 hours and probed with antiactin (Sigma) or anti-SAP (FL-128; Santa Cruz Biotechnology) rabbit antiserum. The HRPO-donkey antirabbit (Amersham, Little Chalfont, United Kingdom) was used as second reagent. The Immun-Star HRP chemiluminescent Kit (Bio-Rad, Hercules, CA) was used for detection. The membrane was then scanned with Chemidoc XRS and bands were quantified by Quantity One software (Bio-Rad).
Results
Expression of informative surface markers and cytotoxic granules in dNK cells
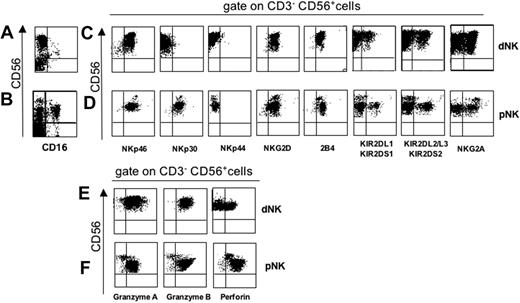
Fresh dNK and pNK cells were analyzed for the surface expression of CD16; CD56; activating receptors including NCRs (NKp46, NKp30, and NKp44), NKG2D, and 2B4; and inhibitory receptors, including KIRs, and CD94/NKG2A. As shown in Figure 1A-B, in contrast to pNK cells, dNK cells did not express CD16. The surface density of NKp46 was comparable in CD56bright CD16– dNK cells (Figure 1C) and in CD56bright pNK cells (Figure 1D), whereas the expression of NKp30 was remarkably lower in CD56bright CD16– dNK cells. Consistent with previous data indicating that NKp44 is virtually absent in freshly isolated pNK cells and is acquired upon NK-cell activation,14 it was absent in CD56bright CD16– dNK cells. NKG2D was expressed at similar levels in both dNK and pNK cells. Similarly, 2B4 was expressed in dNK cells at levels comparable to those of CD56bright pNK cells. Figure 1 also shows that CD56bright CD16– dNK cells (Figure 1C) shared with CD56dim pNK cells (Figure 1D) the expression of KIRs. In addition, similarly to CD56bright pNK cells, CD56bright CD16– dNK cells expressed CD94/NKG2A.
The mechanism by which NK cells kill targets is mostly based on the perforin pathway (ie, it is secondary to the release of cytotoxic perforin and granzymes from intracellular granules upon interaction with target cells). Thus, freshly isolated dNK and pNK cells were assessed for intracellular granzymes and perforin content. As shown in Figure 1, CD56bright CD16– dNK cells exhibited high intracytoplasmic levels of cytolytic granules containing both granzymes A and B, whereas approximately 50% were also positive for perforin (Figure 1E). In contrast, but consistent with previous data,6 CD56bright pNK cells were virtually negative for granzymes and expressed low levels of perforin (Figure 1F). These data suggest that, different from CD56bright pNK cells, dNK cells can mediate cytolytic activity.
Phenotypic analysis of fresh CD56bright CD16– dNK cells. Mononuclear cells were derived from decidua or peripheral blood. (A-B) Cells were analyzed for CD16 and CD56 surface expression. In each sample, 104 cells were acquired for FACS analysis. (C-F) Three-color immunofluorescence analysis with the indicated surface or intracellular markers was performed by gating on CD3– CD56+ cells. Similar results were obtained in 20 different samples analyzed.
Phenotypic analysis of fresh CD56bright CD16– dNK cells. Mononuclear cells were derived from decidua or peripheral blood. (A-B) Cells were analyzed for CD16 and CD56 surface expression. In each sample, 104 cells were acquired for FACS analysis. (C-F) Three-color immunofluorescence analysis with the indicated surface or intracellular markers was performed by gating on CD3– CD56+ cells. Similar results were obtained in 20 different samples analyzed.
Functional analysis of triggering receptors in dNK cells
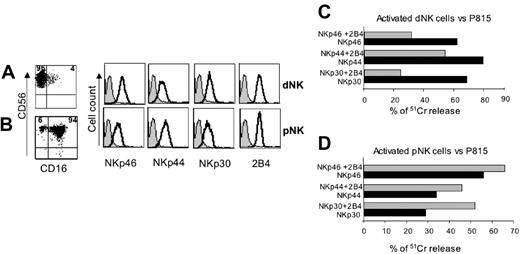
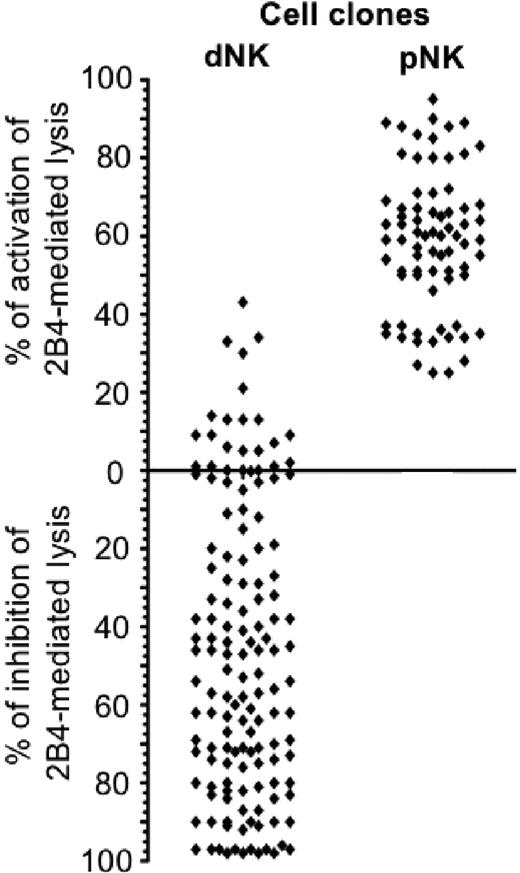
IL-2–activated dNK- and pNK-cell populations were assessed for CD56, CD16, NCRs, and 2B4 surface expression. As shown in Figure 2, activated dNK cells displayed a CD56bright phenotype, whereas CD16 was either absent or expressed at low levels in a small proportion of cells (Figure 2A). On the contrary, the majority of activated pNK cells coexpressed CD56 and CD16 (Figure 2B). NKp46-, NKp30-, and NKp44-triggering receptors, as well as 2B4, were expressed at similar levels on both dNK and pNK cells. Polyclonal dNK-cell populations were then tested for cytolytic activity in a redirected killing assay against the murine FcγR+ P815 target cells. Figure 2C-D shows that dNK cells, upon cross-linking of NCRs, could efficiently kill target cells to an extent similar to pNK cells. However, addition of an anti-2B4 mAb had a dramatically different outcome. Thus, different from pNK cells, the cytolytic activity of dNK cells was sharply inhibited by crosslinking of 2B4. We further analyzed the function of 2B4 coreceptor in a large panel of dNK-cell clones. To this end, dNK clones derived from otherwise healthy women underwent either spontaneous (n = 15) or elective (n = 2) abortion and were tested in a cytotoxicity assay against P815 target cells in the presence or absence of anti-2B4 mAb. More than 140 dNK cell clones were analyzed. As shown in Figure 3, in approximately 50% of dNK cell clones cross-linking of 2B4 resulted in inhibition of the spontaneous lysis of P815 target cells, whereas cytolytic activity was unmodified in 35% and only slightly increased in 15%. Such activating clones were found in the majority of donors analyzed. In contrast, cytolytic activity of pNK cell clones was homogeneously increased upon 2B4 cross-linking.
Engagement of 2B4 inhibits the dNK-mediated cytolytic activity. CD56, CD16, NCRs, and 2B4 surface expression by (A) rIL-2–activated dNK or (B) pNK cells. The percentages of positive cells are indicated in the upper quadrants. Gray profiles represent negative controls. (C) Polyclonally activated dNK or (D) pNK cells were analyzed for cytolytic activity in a redirected killing assay against the FcγR+ P815 target cell line in the presence of mAbs specific for the indicated NCRs either in the absence (▪) or in the presence (▦) of an anti-2B4 mAb. The E/T ratio used was 5:1. All the mAbs used in these experiments were of the IgG1 isotype. Similar results were obtained in 5 independent experiments.
Engagement of 2B4 inhibits the dNK-mediated cytolytic activity. CD56, CD16, NCRs, and 2B4 surface expression by (A) rIL-2–activated dNK or (B) pNK cells. The percentages of positive cells are indicated in the upper quadrants. Gray profiles represent negative controls. (C) Polyclonally activated dNK or (D) pNK cells were analyzed for cytolytic activity in a redirected killing assay against the FcγR+ P815 target cell line in the presence of mAbs specific for the indicated NCRs either in the absence (▪) or in the presence (▦) of an anti-2B4 mAb. The E/T ratio used was 5:1. All the mAbs used in these experiments were of the IgG1 isotype. Similar results were obtained in 5 independent experiments.
Taken together, our results suggest that the majority of dNK cell clones express 2B4 molecules that do not transduce activating signals but rather inhibit natural cytotoxicity.
2B4 inhibits the dNK-mediated killing of CD48+ B-cell lines
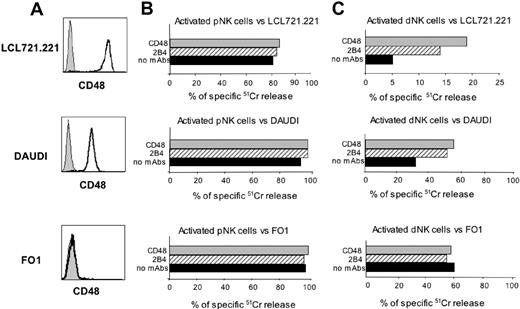
Polyclonal dNK cell populations were further analyzed for their ability to lyse the LCL 721.221 EBV+ B-cell line, the Daudi Burkitt lymphoma cell line, and the FO1 melanoma cell line. All these target cells are HLA class I negative. However, it is of note that while both LCL 721.221 and Daudi cell lines express CD48 (ie, the 2B4 ligand), FO1 is CD48– (Figure 4A). Both LCL 721.221 and Daudi were efficiently lysed by pNK cell populations isolated from healthy donors (Figure 4B) but were resistant to lysis by dNK cell populations (Figure 4C). Since 2B4-mediated recognition of CD48 on target cells could be responsible for inhibition of lysis, we analyzed whether the cytolytic activity of dNK cells could be restored by mAb-mediated masking of CD48 on target cells or of 2B4 on dNK cells. Indeed, mAb-mediated masking of either 2B4 or CD48 (using specific mAbs of IgM isotype) resulted in restoration of lysis of both target cells (Figure 4C). Note that differences in 2B4-mediated inhibition of dNK cell cytotoxicity correlated with different levels of CD48 surface expression in LCL 721.221 versus Daudi cell lines (Figure 4A). In agreement with previous data,2,22 mAb-mediated disruption of the 2B4/CD48 interaction did not modify the pNK cell cytotoxicity against the same CD48+ target cell lines (Figure 4B).
Distribution of inhibitory or activating 2B4 receptors in dNK-cell clones. dNK cell clones (n = 140) and pNK cell clones (n = 65) were assessed for cytolytic activity against the P815 target cells either in the absence or presence of an anti-2B4 mAb. The zero line refers to the spontaneous lysis of P815 by NK-cell clones in the absence of an anti-2B4 mAb. The cytolytic value per each clone represents the percentage of inhibition or activation of the spontaneous lysis obtained in the presence of an anti-2B4 mAb. Each spot represents the mean value of individual clones analyzed in 2 independent tests.
Distribution of inhibitory or activating 2B4 receptors in dNK-cell clones. dNK cell clones (n = 140) and pNK cell clones (n = 65) were assessed for cytolytic activity against the P815 target cells either in the absence or presence of an anti-2B4 mAb. The zero line refers to the spontaneous lysis of P815 by NK-cell clones in the absence of an anti-2B4 mAb. The cytolytic value per each clone represents the percentage of inhibition or activation of the spontaneous lysis obtained in the presence of an anti-2B4 mAb. Each spot represents the mean value of individual clones analyzed in 2 independent tests.
Finally, both pNK and dNK cells were able to kill CD48– FO1 target cells, and, as expected, the presence in the cytolytic assays of mAbs masking either CD48 or 2B4 had no effect on the NK cell–mediated lysis. Taken together, these data indicate that the majority of dNK cells express a 2B4 molecule able to transduce an inhibitory signal, to inhibit NK-cell activation, and to block NK-cell triggering mediated by NCRs and/or NKG2D. In addition, they show that upon disruption of the 2B4/CD48 interaction (by masking either the receptor or the ligand), dNK cells become able to lyse CD48+ target cells.
Analysis of IFN-γ production by CD56bright CD16– dNK cells
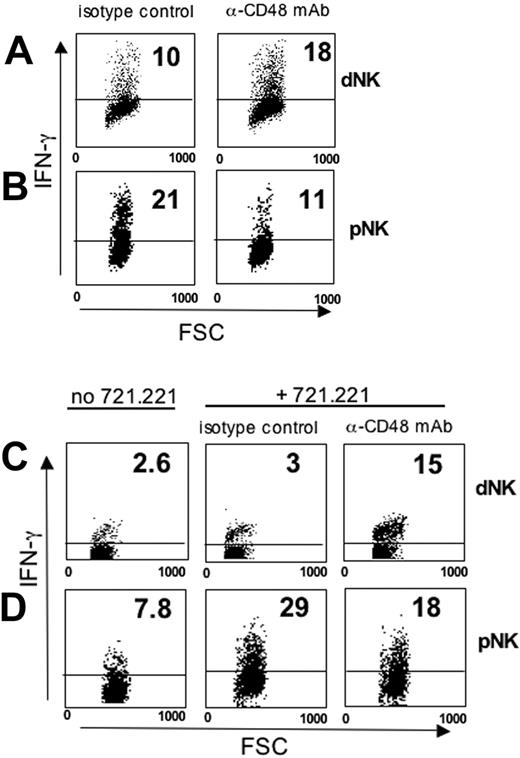
Freshly isolated dNK cells have been shown to produce cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, colony-stimulating factor 1 (CSF1), and tumor-necrosis factor (TNF).31 We analyzed whether crosslinking of 2B4 in dNK cells would result in inhibition of IL-2– or IL-12–induced IFN-γ production. To this end, mononuclear cells (which express CD48, thus providing a suitable source for 2B4 engagement) were isolated either from human decidua or from peripheral blood and cultured for 48 hours in the presence of rIL-2, rIL-12, and a blocking mAb specific for CD48 (or an irrelevant isotype-matched mAb as a control). IFN-γ production was assessed by intracytoplasmic staining of CD3–CD56+ NK cells (Figure 5A-B). Both cytokine-activated dNK (Figure 5A) and pNK cells (Figure 5B) were able to produce IFN-γ. However, mAb-mediated masking of 2B4 expressed by dNK or pNK cells resulted in opposite effects. Thus, in dNK cells it led to enhanced IFN-γ production (Figure 5A), whereas in pNK cells it resulted in decreased IFN-γ production (Figure 5B).
In order to further document the effect of 2B4 on IFN-γ production, we analyzed the effect of culturing freshly purified dNK or pNK cells for 2 days with rIL-2 and rIL-12, either in the presence or absence of the CD48+ LCL 721.221 B-cell line. The experiments containing the LCL 721.221 B-cell line were performed in the presence of either an anti-CD48 blocking mAb or of an irrelevant mAb as control. Also in this case, the disruption of CD48/2B4 interaction in dNK or pNK cells had different outcomes. Thus, in dNK cells, mAb-mediated masking of CD48 on LCL 721.221 cells led to increased IFN-γ production (Figure 5C), whereas in pNK cells it resulted in a partial inhibition of IFN-γ production (Figure 5D). Taken together, these results provide evidence that 2B4 expressed on dNK cells can inhibit not only cytotoxicity but also cytokine production.
2B4 inhibits the dNK-mediated killing of CD48+ B-cell lines. (A) CD48 surface expression by LCL 721.221, Daudi, and FO1 cell lines (mean fluorescence intensities were 710, 75, and 4.5, respectively). Gray profiles represent negative controls. (B) Polyclonally activated pNK or (C) dNK cells were analyzed for their cytolytic activity against LCL 721.221, Daudi, and FO1 target cell lines in either the absence or presence of mAbs specific for 2B4 or CD48. The E/T ratio used was 5:1. Both mAbs used in these experiments were of the IgM isotype. Similar results were obtained in 5 independent experiments.
2B4 inhibits the dNK-mediated killing of CD48+ B-cell lines. (A) CD48 surface expression by LCL 721.221, Daudi, and FO1 cell lines (mean fluorescence intensities were 710, 75, and 4.5, respectively). Gray profiles represent negative controls. (B) Polyclonally activated pNK or (C) dNK cells were analyzed for their cytolytic activity against LCL 721.221, Daudi, and FO1 target cell lines in either the absence or presence of mAbs specific for 2B4 or CD48. The E/T ratio used was 5:1. Both mAbs used in these experiments were of the IgM isotype. Similar results were obtained in 5 independent experiments.
The majority of dNK cells express low levels of SAP
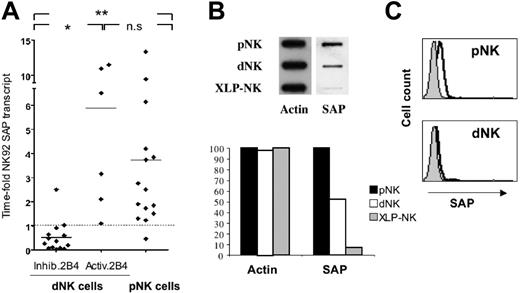
In order to investigate whether the 2B4-mediated inhibitory signal characterizing dNK cells was related to low levels of SAP transcript, a quantitative TaqMan PCR analysis was performed. In this study, the NK92 cell line, characterized by an activating 2B4 receptor,32 was used as reference sample. Results are reported in time-fold the amount of SAP mRNA transcript detected in this NK cell line. First, we analyzed the SAP gene expression in resting dNK cells derived from 2 different donors. In these samples, we detected low levels of SAP transcript (0.1 and 0.4 time-fold NK92 transcript). On the contrary, in resting pNK cells the level of SAP mRNA was higher than that of the reference cell line. We next determined whether the rIL-2–induced activation of dNK cells modified the levels of SAP transcript. To this end, we analyzed 2 short-term rIL-2–activated polyclonal populations of dNK and pNK cells. Results indicated that the presence of rIL-2 did not modify the SAP transcript level in dNK cells, whereas a decrease was detected in pNK cells. Although a previous report demonstrated that treatment with proteases did not modify the expression level of different transcripts,6 we further investigated whether this also occurred under our experimental conditions. Thus, we compared SAP mRNA levels in untreated versus treated pNK cells. No differences could be detected in our experimental setting (untreated pNK cells = 14.13 time-fold NK92 transcript, treated pNK cells = 13.17 time-fold NK92 transcript). Remarkably, protease treatment did not modify the cytolytic activity of pNK cells (data not shown). We further assessed the SAP transcript in 14 dNK-cell clones expressing an inhibitory 2B4 and 6 dNK cell clones expressing an activating 2B4. All these dNK cells were derived from 8 different donors. Fifteen pNK cell clones derived from healthy donors were also included for comparison. As shown in Figure 6A, the inhibitory function of 2B4 correlated with low levels of SAP mRNA expression. Indeed, dNK cell clones characterized by an inhibitory 2B4 receptor transcribed a low level of SAP mRNA, whereas dNK cell clones, expressing an activating 2B4 receptor, displayed a high level of SAP mRNA (P < .01). Notably, the amount of SAP transcript in pNK-cell clones was comparable to that detected in dNK cell clones with an activating 2B4.
Engagement of 2B4 in dNK cells inhibits IFN-γ production. (A) Decidual and (B) peripheral mononuclear cells were cultured in the presence of rIL-2 and rIL-12 for 48 hours with an anti-CD48 blocking mAb (of IgM isotype) or, as control, with an isotype-matched irrelevant mAb. FSC indicates forward scatter. (C) Freshly purified dNK or (D) pNK cells were cultured in the presence of rIL-2 and rIL-12 for 48 hours and then incubated for an additional 18 hours either alone or cocultured with the CD48+ LCL 721.221 cell line. The NK/LCL 721.221 coculture was performed either in the presence of an anti-CD48 blocking mAb (of IgM isotype) or, as control, an isotype-matched irrelevant mAb. Analysis was performed by gating on CD3–CD56+ cells. IFN-γ production by NK cells was evaluated by intracytoplasmic staining. The numbers represent the percentage of IFN-γ–producing NK cells.
Engagement of 2B4 in dNK cells inhibits IFN-γ production. (A) Decidual and (B) peripheral mononuclear cells were cultured in the presence of rIL-2 and rIL-12 for 48 hours with an anti-CD48 blocking mAb (of IgM isotype) or, as control, with an isotype-matched irrelevant mAb. FSC indicates forward scatter. (C) Freshly purified dNK or (D) pNK cells were cultured in the presence of rIL-2 and rIL-12 for 48 hours and then incubated for an additional 18 hours either alone or cocultured with the CD48+ LCL 721.221 cell line. The NK/LCL 721.221 coculture was performed either in the presence of an anti-CD48 blocking mAb (of IgM isotype) or, as control, an isotype-matched irrelevant mAb. Analysis was performed by gating on CD3–CD56+ cells. IFN-γ production by NK cells was evaluated by intracytoplasmic staining. The numbers represent the percentage of IFN-γ–producing NK cells.
We further analyzed the expression of SAP in dNK cells compared with pNK cells. In these experiments cell lysates derived from rIL-2–activated polyclonal dNK or pNK cells were analyzed by slot-blot analysis for their reactivity with an SAP-specific rabbit antiserum. Cells lysates from SAP-deficient polyclonal pNK cells (XLP-NK)26 were used as negative control. According to the data obtained on SAP transcript, the amount of SAP was significantly lower in dNK cells compared with pNK cells (Figure 6B). Finally, rIL-2–activated dNK cells were analyzed by fluorescence-activated cell sorter (FACS) analysis for SAP expression. As shown in Figure 6C, dNK cells were virtually negative, since the intracytoplasmic staining with the SAP-specific antiserum paralleled that detected in SAP-deficient XLP-NK cells.
Analysis of SAP expression in dNK cells. (A) Analysis of SAP mRNA transcript in dNK and pNK cell clones. Total RNA, extracted from dNK and pNK cell clones and converted in cDNA, was subjected to TaqMan-PCR analysis to assay the quantity of SAP mRNA. All values have been normalized to GAPDH transcript and the quantitative PCR (Q-PCR) data are represented as time-fold the normalized SAP transcript detected in the NK92 cell line (used as reference cell line and therefore indicated as 1). Each spot is the mean of 2 independent experiments of Q-PCR analysis performed in triplicates. Statistical analysis of data sets was annotated as follows: *P = .01; **P < .01; and n.s., not significant. (B) Postnuclear cell lysates derived from 1 × 106 dNK, pNK, and XLP-NK cells were spotted on nitrocellulose using a slot-blot system. Membranes were probed with rabbit antisera specific for either actin or SAP. Membranes were then scanned and densitometric analysis of the bands was performed. Densities of actin and SAP in dNK and XLP-NK cells are expressed as percentage of control pNK cells. Data are representative of 3 independent experiments. (C) FACS analysis of SAP expression. Permeabilized rIL-2–cultured XLP-NK, dNK, and pNK cells were stained with the SAP-specific rabbit antiserum. Gray profiles represent negative controls (ie, the antiserum background detected in SAP-deficient XLP-NK cells).
Analysis of SAP expression in dNK cells. (A) Analysis of SAP mRNA transcript in dNK and pNK cell clones. Total RNA, extracted from dNK and pNK cell clones and converted in cDNA, was subjected to TaqMan-PCR analysis to assay the quantity of SAP mRNA. All values have been normalized to GAPDH transcript and the quantitative PCR (Q-PCR) data are represented as time-fold the normalized SAP transcript detected in the NK92 cell line (used as reference cell line and therefore indicated as 1). Each spot is the mean of 2 independent experiments of Q-PCR analysis performed in triplicates. Statistical analysis of data sets was annotated as follows: *P = .01; **P < .01; and n.s., not significant. (B) Postnuclear cell lysates derived from 1 × 106 dNK, pNK, and XLP-NK cells were spotted on nitrocellulose using a slot-blot system. Membranes were probed with rabbit antisera specific for either actin or SAP. Membranes were then scanned and densitometric analysis of the bands was performed. Densities of actin and SAP in dNK and XLP-NK cells are expressed as percentage of control pNK cells. Data are representative of 3 independent experiments. (C) FACS analysis of SAP expression. Permeabilized rIL-2–cultured XLP-NK, dNK, and pNK cells were stained with the SAP-specific rabbit antiserum. Gray profiles represent negative controls (ie, the antiserum background detected in SAP-deficient XLP-NK cells).
Characterization of CD48+ cells present in the decidua
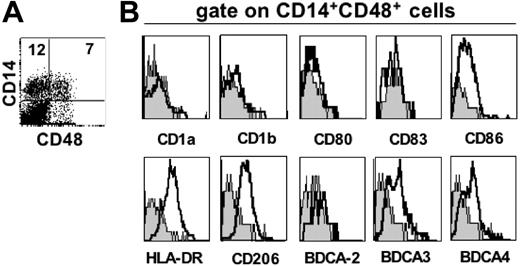
In order to investigate whether particular cell types present in the decidua could potentially interact with the inhibitory 2B4 receptor present on dNK cells, we searched cells that expressed CD48 (ie, the 2B4 ligand). Along this line, we could identify a particularly small cell subset expressing CD14, DC-SIGN (ie, 2 markers of monocytes and iDC), and CD48. In order to gain further information on this cell type, we performed FACS analysis of informative surface markers. Since all cells expressing CD14 were also DC-SIGN positive (data not shown), cells coexpressing CD14 and CD48 were analyzed (Figure 7).33 As shown in Figure 7B, these cells were HLA-DR+, CD206+, BDCA3+, BDCA4+, CD1a–, CD1b–, CD80–, CD83–, BDCA2–, whereas they expressed a low level of CD86. Thus, it is interesting to note that these cells expressed unique phenotypic features being characterized by the expression of markers typical of either macrophages (ie, CD14, DC-SIGN, and CD206),33-35 myeloid DCs (ie, DC-SIGN and BDCA-3),36,37 or plasmacytoid DCs (ie, BDCA-4).37
Discussion
One key question about the fetal-maternal relationship is why the immune system of the mother does not reject fetal tissues. A number of explanations have been provided on different effects and regulatory mechanisms that could explain this phenomenon. Regarding NK cells, a puzzling issue is why NK cells do not kill trophoblastic cells, since the latter cells do not express HLA-A and HLA-B molecules and lose HLA-C after the first trimester of pregnancy; however, it has been shown that they do express the HLA class Ib molecules HLA-E and HLA-G. Since dNK cells express CD94/NKG2A and KIR2DL4 receptors, it has been suggested that the interaction between these inhibitory receptors and HLA class Ib molecules may prevent dNK-cell activation.8,31 Our present study could suggest an additional mechanism involving the 2B4 molecule. Indeed, in this paper we show that although dNK cells are potentially cytolytic and capable of cytokine production, their function can be sharply inhibited by the surface receptor 2B4. We provide evidence that the inhibitory activity of 2B4 observed in the majority of dNK cells correlates with low SAP expression.
In early pregnancy, NK cells characterized by the CD56bright CD16– surface phenotype represent more than 50% of decidual lymphocytes.31 Although their origin is unknown, because of their CD56bright CD16– surface phenotype, they are reminiscent of the minor CD56bright NK-cell population present in the peripheral blood. They also share with these cells the expression of CD94/NKG2A inhibitory receptor. However, dNK cells express higher levels of cytoplasmic cytotoxic granules,38 thus suggesting that they potentially display higher cytolytic activity than CD56bright pNK cells. In this respect, they are similar to CD56dim CD16+ pNK cells. In addition, they can share with CD56dim CD16+ pNK cells the expression of KIRs.39 On the other hand, certain characteristics appear to be unique of CD56bright CD16– dNK cells such as the expression of the activation marker CD6940 and the absence of L-selectin, a molecule involved in leukocyte adhesion and migration.41
Taken together, these observations suggest that CD56bright CD16– dNK cells might derive from CD56bright pNK cells recruited from the blood into the decidua and then induced in situ to acquire their cytolytic machinery by the cytokine- and hormone-rich tissue microenvironment.31 Alternatively, they could originate from CD56dim CD16+ pNK cells that, once activated, acquire a CD56bright phenotype and down-regulate the CD16 surface expression at a tissue level. This point clearly requires further investigation.
Whatever the origin of dNK cells would be, they have the unique property of expressing a functional potential typical of the majority of pNK cells. This is supported not only by the presence of cytolytic granules in their cytoplasm but also by the surface expression of functional activating receptors such as NKp46 and NKG2D. Decidual NK cells also brightly express 2B4, a surface molecule that in “mature” NK cells functions as a coreceptor, allowing an optimal NK-cell activation in cooperation with NCRs and/or NKG2D. In addition, dNK cells had previously been shown to produce abundant cytokines (including GM-CSF, CSF1, IFN-γ, and TNF) when cultured in vitro in the presence of rIL-15.31 Our present data also suggest that dNK cells are capable of producing IFN-γ upon in vitro stimulation with rIL-2 and rIL-12. In contrast with their phenotypic features, suggesting that dNK cells can mediate an efficient cytolytic activity, they were poorly cytolytic against certain target cells. In particular, this occurred with target cells expressing CD48, the molecular ligand of 2B4. On the other hand, they efficiently killed target cells lacking CD48 such as the FO1 melanoma cell line. The impaired cytolytic activity of dNK cells against CD48+ EBV+ B cells was not due to the inability of triggering receptors to deliver activating signals. This was demonstrated by a redirected killing assay (against the murine FcγR+ P815 cell line) in which NCRs were found to display normal activating function.
Phenotypic analysis of freshly isolated CD14+ CD48+ decidual cells. (A) Surface expression of CD48 by CD14+ decidual cells. The percentages of positive cells are indicated in the upper quadrants. (B) Three-color immunofluorescence analysis was performed by staining double-positive cells (ie, coexpressing CD14 and CD48) with mAbs specific for one or another indicated surface markers. Gray profiles represent negative controls.
Phenotypic analysis of freshly isolated CD14+ CD48+ decidual cells. (A) Surface expression of CD48 by CD14+ decidual cells. The percentages of positive cells are indicated in the upper quadrants. (B) Three-color immunofluorescence analysis was performed by staining double-positive cells (ie, coexpressing CD14 and CD48) with mAbs specific for one or another indicated surface markers. Gray profiles represent negative controls.
A conceivable explanation of the inability to kill EBV+ B-cell lines was that in dNK cells, 2B4 molecule delivered inhibitory signals. Indeed, we showed that interruption of the 2B4/CD48 interaction could restore lysis of those target cells expressing CD48. This was also suggested by the finding that addition of an anti-2B4 mAb to the redirected killing assay resulted in inhibition of cytolytic activity.
Although in mature pNK cells 2B4 functions as a triggering coreceptor, it has been shown that in immature NK cells derived in vitro from CD34+ cell precursors, it delivers inhibitory signals.27 This mechanism provides a fail-safe device to prevent killing of bystander cells by immature NK cells that express both activating receptors and cytoplasmic cytotoxic granules in the absence of HLA class I–specific inhibitory receptors.
The inhibitory function of 2B4 was first described in patients affected by XLP.26,27 The molecular defect in these patients is represented by the lack of functional SAP,42 a protein that upon binding to the cytoplasmic portion of 2B4 allows the delivery of activating signals. In the absence of SAP, SHP-1 phosphatase can bind to 2B4 and mediate inhibition of the NK-mediated cytolysis of CD48+ target cells (eg, EBV+ B cells).26 Our data show that 2B4 expressed by dNK cells delivers inhibitory signals and that this function correlates with low or absent SAP expression. Indeed, clonal analysis showed that the majority of, but not all, dNK cell clones were characterized by the lack of or low level of SAP mRNA. Accordingly, such clones did not kill CD48+ target cells, whereas the small percentage of SAP-positive clones could kill. When analyzing polyclonal dNK cell populations, the net result could be inhibition of cytolytic activity, since SAP-deficient dNK cells largely outnumbered the SAP-positive ones. Our present results are in line with data by Koopman et al,6 showing by microarray analysis that in CD56bright CD16– dNK cells, the SAP mRNA was virtually absent.
One may ask what the physiologic role of the inhibitory 2B4 receptor in dNK cells might be. It is of note that the implanted embryo is spared from the attack by the maternal immune system including NK cells. An obvious question is which cell types present in the decidual tissue express the 2B4 ligand (ie, CD48). Trophoblast cell lines do not express CD48.43 If this also holds true for fresh trophoblastic cells, it appears unlikely that 2B4 may serve as a fail-safe mechanism to avoid NK-mediated attack to these cells. On the other hand, we cannot rule out that trophoblast cells may express an additional, still undefined, 2B4 ligand. Remarkably, in this study we described a particular cell type present in the decidua that expresses CD48. These cells are characterized by the DC-SIGN+ HLA-DR+ CD14+ CD1a– CD1b– CD80– CD83– CD86low CD206+ BDCA2– BDCA3+ and BDCA4+ surface phenotype. Thus, it appears that they coexpress markers typical of macrophages (ie, CD14, DC-SIGN, and CD206),33-35 myeloid DCs (ie, DC-SIGN and BDCA-3),36,37 and plasmacytoid DCs (ie, BDCA-4).37 It is conceivable that these cells might correspond to a subset of the previously described cells coexpressing CD14 and DC-SIGN.33 Interestingly, these cells had been reported to outnumber typical DCs in decidual tissue.33 In addition, histochemical analysis revealed that they could be in close association with dNK cells.33 Thus, it is possible that, upon interaction with these particular cells, dNK cells are blocked in their functional capability. One might speculate that the 2B4/CD48 interaction prevents dNK-mediated killing of these cells. As suggested by their surface phenotype, these cells could function as antigen-presenting cells (APCs).33 However, the fact that they weakly express costimulatory molecules (eg, CD86) would imply that they do not efficiently induce T-cell activation but could rather induce T-cell tolerization. Further studies are clearly needed in order to analyze in vitro–purified “decidual APCs.” Aims of future investigations will be to better define (1) the ability of these cells to undergo further maturation in response to cytokines or bacterial products; (2) their actual capability of functioning as APCs; and (3) the net result of their interaction with dNK cells in terms of susceptibility to lysis, cytokine production, and “de novo” expression of informative surface markers.
Authorship
Contribution: P.V. performed most of the experimental work and contributed to paper writing; G.P. designed and coordinated experimental work, analyzed data, and wrote the paper; M.F. performed molecular analysis and contributed to paper writing; E.R. performed molecular analysis; C.B. performed experiments of biochemical analysis and contributed to paper writing; F.B. performed experiments of biochemical analysis; F.P. and P.L.V. recruited study subjects, performed dissections of decidual tissue, and contributed to interpretation of the results; E.F. provided selected samples and contributed to interpretation of the results; M.C. provided selected samples; A.M. contributed to discussion and to paper writing; L.M. contributed to discussion, provided economic support, and wrote the paper; and M.C.M. supervised the project, provided economic support, and contributed to paper writing. P.V. and G.P. equally contributed to the present study.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 24, 2006; DOI 10.1182/blood-2006-04-017343.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants awarded by Associazione Italiana per la Ricerca sul Cancro (AIRC); Istituto Superiore di Sanità (ISS); Ministero della Salute; Ministero dell'Istruzione, dell'Università e della Ricerca (MIUR); Fondazione Compagnia di San Paolo, Turin, Italy; and European Union FP6, LSHB-CT-2004-503319-AlloStem.
The European Commission is not liable for any use that may be made of the information contained.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal