Abstract
We previously compared outcomes after allogeneic peripheral-blood stem cell (PBSC) and bone marrow (BM) transplantation in 706 patients with leukemia. We obtained long-term follow up on 413 of 491 patients who were alive at the time of the initial report: 141 PBSC and 272 BM recipients. Chronic graft-versus-host disease (GVHD) was more frequent after PBSC compared to BM transplantation (RR 1.65, P < .001) yet relapse rates were similar in both groups. Leukemia-free survival rates were higher after PBSC than BM transplantation for patients with advanced chronic myeloid leukemia (33% versus 25%) but lower for those in first chronic phase (41% versus 61%) due to higher rates of late transplant-related mortality. Leukemia-free survival was similar after PBSC and BM transplantation for acute leukemia. These data represent the early experience with PBSC grafts. Long-term outcomes in recipients of more recent transplants are required to better evaluate the role of PBSC grafts relative to BM transplantation.
Introduction
Despite numerous reports comparing transplantation of peripheral-blood stem cells (PBSCs) and bone marrow (BM), long-term results of PBSC transplantation remain largely unknown.1-11 All studies confirm rapid hematologic recovery but data on other end points are less consistent, possibly because of variable periods of follow-up. Most studies have reported a higher risk of chronic graft-versus-host disease (GVHD) with PBSCs, and it is uncertain how this will affect the risk of leukemia relapse or late mortality. Studies indicating a survival advantage with PBSC grafts generally included patients with advanced leukemia; a convincing survival advantage for good-risk patients is not well documented.
In an earlier report, we described higher rates of chronic GVHD after PBSC transplantation but lower transplant-related mortality (TRM) and higher leukemia-free survival (LFS) after PBSC versus BM transplantation for acute leukemia in second complete remission (CR) or chronic myeloid leukemia (CML) in second chronic phase (CP) or accelerated phase (advanced leukemia).1 The median follow-up in that study was 12 months. This report updates that cohort with median follow-up now more than 6 years, allowing better assessment of late outcomes.
Patients, materials, and methods
Patients
Our earlier report described 536 BM and 288 PBSC HLA-matched sibling donor transplantations done in during 1995 and 1996 for patients at least 20 years of age with acute leukemia or CML.1 Outcomes were compared in 462 and 244 of these recipients of BM and PBSC transplants, respectively, who had complete data for prognostic significant variables and posttransplantation outcomes. Follow-up analysis is limited to transplantation teams who provided extended follow-up data on more than 90% of their surviving patients; 100 of 706 (14%) patients in the initial report, transplantations done in 20 centers, did not meet this criterion and were excluded. Consequently, the current report compares outcomes after 398 BM and 208 PBSC transplantations. At the time of our initial report, 318 BM and 173 PBSC recipients were alive. Follow-up information beyond our initial report was available for 272 of 318 (86%) and 141 of 173 (82%) recipients of BM and PBSC transplants, respectively. The median follow-up of the current study population is 84 months (range, 5-120 months) and 76 (range, 3-110 months) months after BM and PBSC transplantation, respectively. There were no differences in overall survival between patients undergoing transplantation in centers with and without extended follow-up.
Statistical methods
The study examined the following outcomes: chronic GVHD, TRM, relapse, treatment failure (death or relapse), and overall mortality. End points and details of statistical methods were described previously.1 Outcomes after BM and PBSC transplantation were compared using Cox proportional hazards regression with the variable for graft type maintained in all steps of model building and the final model.12 Other variables considered in the analysis were age, sex, performance score, disease type and status at transplantation, white cell count at diagnosis, time interval from diagnosis to transplantation, use of total body irradiation (TBI) for conditioning, and type of GVHD prophylaxis. All analyses were performed as previously described using SAS version 9.1 (SAS Institute, Cary, NC).1
Results and discussion
Patient, disease, and transplant characteristics were summarized in Table 1 of our previous report.1 The groups were similar except that PBSC recipients were more likely to receive TBI for conditioning and growth factor to promote hematopoietic recovery and less likely to undergo transplantation for CML in first CP or to receive methotrexate and cyclosporine for GVHD prophylaxis.
Relative risks of TRM, relapse, and treatment failure after PBSC and BM transplantation
. | Previous report . | . | Current report . | . | ||
|---|---|---|---|---|---|---|
| Patient group . | No. BM/no. PBSC . | RR (95% CI); P . | No. BM/no. PBSC* . | RR (95% CI); P . | ||
| TRM† | ||||||
| AL, CR1 | 180/95 | 0.59 (0.33-1.07); .25 | 156/81 | 1.03 (0.62-1.71); .84 | ||
| AL, CR2 | 40/31 | 0.36 (0.13-0.99); .04 | 31/30 | 0.35 (0.13-0.97); .04 | ||
| CML, CP1‡ | 214/91 | 1.33 (0.82-2.15); .27 | 188/76 | 1.61 (1.05-2.46); .03 | ||
| CML, CP2/AP‡ | 28/27 | 0.28 (0.12-0.67); .004 | 23/21 | 0.43 (0.18-1.02); .06 | ||
| Relapse† | ||||||
| AL, CR1 | 180/95 | 0.57 (0.04-7.66); .67 | 156/81 | 0.91 (0.47-1.75); .77 | ||
| AL, CR2 | 40/31 | 0.24 (0.01-4.42); .34 | 31/30 | 1.04 (0.43-2.55); .93 | ||
| CML, CP‡ | 183/95 | 0.48 (0.01-56.97); .76 | 188/76 | 0.49 (0.11-2.21); .36 | ||
| CML, CP2/AP‡ | 28/27 | 1.02 (0.07-14.99); .99 | 23/21 | 0.37 (0.10-1.44); .15 | ||
| TF† | ||||||
| AL, CR1 | 180/95 | 0.76 (0.48-1.21); .25 | 156/81 | 1.01 (0.67-1.51); .97 | ||
| AL, CR2 | 40/31 | 0.40 (0.18-0.92); .03 | 31/30 | 0.60 (0.31-1.16); .13 | ||
| CML, CP‡ | 214/91 | 1.30 (0.82-2.08); .27 | 188/76 | 1.57 (1.05-2.35); .03 | ||
| CML, CP2/AP‡ | 28/27 | 0.29 (0.14-0.59); < .001 | 23/21 | 0.42 (0.20-0.86); .02 | ||
. | Previous report . | . | Current report . | . | ||
|---|---|---|---|---|---|---|
| Patient group . | No. BM/no. PBSC . | RR (95% CI); P . | No. BM/no. PBSC* . | RR (95% CI); P . | ||
| TRM† | ||||||
| AL, CR1 | 180/95 | 0.59 (0.33-1.07); .25 | 156/81 | 1.03 (0.62-1.71); .84 | ||
| AL, CR2 | 40/31 | 0.36 (0.13-0.99); .04 | 31/30 | 0.35 (0.13-0.97); .04 | ||
| CML, CP1‡ | 214/91 | 1.33 (0.82-2.15); .27 | 188/76 | 1.61 (1.05-2.46); .03 | ||
| CML, CP2/AP‡ | 28/27 | 0.28 (0.12-0.67); .004 | 23/21 | 0.43 (0.18-1.02); .06 | ||
| Relapse† | ||||||
| AL, CR1 | 180/95 | 0.57 (0.04-7.66); .67 | 156/81 | 0.91 (0.47-1.75); .77 | ||
| AL, CR2 | 40/31 | 0.24 (0.01-4.42); .34 | 31/30 | 1.04 (0.43-2.55); .93 | ||
| CML, CP‡ | 183/95 | 0.48 (0.01-56.97); .76 | 188/76 | 0.49 (0.11-2.21); .36 | ||
| CML, CP2/AP‡ | 28/27 | 1.02 (0.07-14.99); .99 | 23/21 | 0.37 (0.10-1.44); .15 | ||
| TF† | ||||||
| AL, CR1 | 180/95 | 0.76 (0.48-1.21); .25 | 156/81 | 1.01 (0.67-1.51); .97 | ||
| AL, CR2 | 40/31 | 0.40 (0.18-0.92); .03 | 31/30 | 0.60 (0.31-1.16); .13 | ||
| CML, CP‡ | 214/91 | 1.30 (0.82-2.08); .27 | 188/76 | 1.57 (1.05-2.35); .03 | ||
| CML, CP2/AP‡ | 28/27 | 0.29 (0.14-0.59); < .001 | 23/21 | 0.42 (0.20-0.86); .02 | ||
Relative risk of less than 1.0 indicates an advantage for PBSC transplantation (lower risk of adverse outcome) and a relative risk of greater than 1.0 indicates an advantage for BM transplantation (higher risk of adverse outcome).
TRM indicates transplant-related mortality; TF, treatment failure inverse of LFS; AL, acute leukemia; CR, clinical remission; CML, chronic myeloid leukemia; CP, chronic phase; AP, accelerated phase.
The current report is limited to transplantation centers that provided extended follow-up data on more than 90% of their patients; 100 of 706 patients from 20 centers were excluded. Therefore, the current study includes 606 patients (398 BM and 208 PBSC transplantations).
Model stratified on use of growth factor.
PBSC and BM recipients had similar European Group for Blood and Marrow Transplantation risk score at transplantation.
Chronic GVHD
A total of 401 BM and 208 PBSC transplant recipients were evaluable for chronic GVHD. Chronic GVHD was significantly higher with PBSC recipients (RR = 1.65; 95% CI, 1.28-2.11; P < .001), a bigger difference than observed in our earlier report (RR = 1.30; P = .05). The 6-year probabilities of chronic GVHD were 61% and 45% after PBSC and BM transplantation, respectively. Consistent with other reports,13 among those with chronic GVHD, the severity and pattern of organ involvement were similar in both groups. Chronic GVHD was more frequent in patients 40 years of age or older, regardless of graft type (RR = 1.39, P = .004).
TRM and relapse
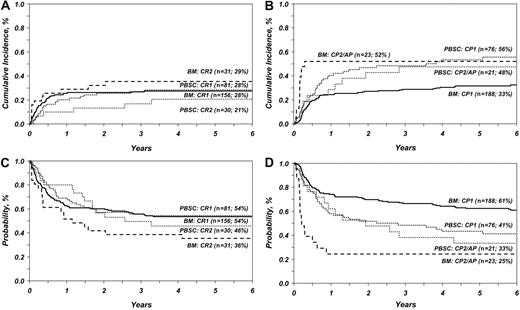
This analysis confirms our earlier observation and those of others that TRM was lower after PBSC transplantation for advanced acute leukemia. However, with longer follow-up, TRM did not differ significantly after PBSC versus BM transplantation for advanced CML (Table 1 and Figure 1). Consistent with our earlier observation, the risk of TRM was similar after PBSC and BM transplantation for early acute leukemia. However, in this study, TRM was higher after PBSC transplantation for early CML. TRM was higher in patients aged 40 years or older regardless of graft type (RR = 1.64, P = .001). Neither this nor our previous analysis showed significant differences in relapse rates after BM and PBSC transplantation. Chronic GVHD was associated with fewer relapses (RR = 0.57, P = .03) in both groups. All published studies2-10 report similar relapse rates after PBSC and BM transplantation except a meta-analysis of 9 randomized trials where relapse rates were lower after PBSC transplantation.11
Overall and leukemia-free survival
The effect of graft type on overall and leukemia-free survival differed by type of leukemia and disease status at transplantation (Table 1 and Figure 1). PBSC transplantation for advanced CML was associated with lower mortality (RR = 0.45, P = .03) and higher LFS than BM transplantation. In contrast, mortality was higher (RR = 1.70, P = .01) and LFS lower after PBSC transplantation for early CML. The 6-year probabilities of overall survival for advanced CML were 25% and 33% after BM and PBSC transplantation, respectively. Corresponding probabilities for early CML were 64% and 45%. Our findings differ from that of the recent meta-analysis,9-11 in which survival rates after PBSC and BM transplantation for early CML were similar.
Consistent with other reports except one meta-analysis,11 overall survival and LFS were similar after PBSC and BM transplantation for early and advanced acute leukemia. The 6-year probabilities of overall survival for early acute leukemia were 57% and 55%, respectively; the corresponding probabilities for advanced acute leukemia were 45% and 49%. The early advantage seen with PBSC transplantation in our earlier study for advanced acute leukemia was not maintained with longer follow-up. Causes of mortality were similar in both groups; recurrent leukemia, GVHD, and infection were the most common causes of death.
Lower early TRM after PBSC transplantation for advanced leukemia may have resulted from rapid hematopoietic recovery. Patients with early leukemia have a lower risk of mortality from transplant-related complications in general and rapid hematopoietic recovery may not produce similar measurable early benefits. In early CML, TRM after BM transplantation is already low, leaving little to gain by faster recovery of hematopoiesis, and higher chronic GVHD with PBSC resulted in higher late mortality. The graft-versus-leukemia (GVL) effect associated with chronic GVHD did not produce measurable survival benefits in either group.
Ours is not a randomized study and subject to bias owing to the complex selection criteria that underlie the choice of graft. Because there are relatively few patients with CML and advanced acute leukemia in both treatment groups, our findings should be interpreted with caution. Further, this report represents the early experience with PBSC transplantation. Nevertheless, this is the first report describing long-term outcomes after PBSC transplantation and indicates that although patients with advanced CML may benefit from using PBSC grafts, the same may not be true of patients with acute leukemia or early CML. These findings should be validated with long-term follow-up of patients enrolled in randomized studies.
TRM and LFS in patients with acute and chronic leukemia after BM and PBSC transplantation. (A) Cumulative incidence of TRM in patients with acute leukemia after BM and PBSC transplantation adjusted for disease status at transplantation (n, number of evaluable patients; %, the 6-year rate of TRM). (B) Cumulative incidence of TRM in patients with chronic leukemia after BM and PBSC transplantation adjusted for disease status at transplantation (n, number of evaluable patients; %, 6-year rate of TRM). (C) Probability of LFS in patients with acute leukemia after BM and PBSC transplantation adjusted for disease status at transplantation (n, number of evaluable patients; %, 6-year rate of LFS). (D) Probability of LFS in patients with chronic leukemia after BM and PBSC transplantation adjusted for disease status at transplantation (n, number of evaluable patients; %, 6-year rate of LFS).
TRM and LFS in patients with acute and chronic leukemia after BM and PBSC transplantation. (A) Cumulative incidence of TRM in patients with acute leukemia after BM and PBSC transplantation adjusted for disease status at transplantation (n, number of evaluable patients; %, the 6-year rate of TRM). (B) Cumulative incidence of TRM in patients with chronic leukemia after BM and PBSC transplantation adjusted for disease status at transplantation (n, number of evaluable patients; %, 6-year rate of TRM). (C) Probability of LFS in patients with acute leukemia after BM and PBSC transplantation adjusted for disease status at transplantation (n, number of evaluable patients; %, 6-year rate of LFS). (D) Probability of LFS in patients with chronic leukemia after BM and PBSC transplantation adjusted for disease status at transplantation (n, number of evaluable patients; %, 6-year rate of LFS).
Authorship
Contribution: all authors contributed equally to the conception, design, and interpretation of data and the final manuscript; M.E., M.-J.Z., and J.P.K. did the statistical analysis; and M.E. and N.S. had primary responsibility for drafting the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 31, 2006; DOI 10.1182/blood-2006-05-024042.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by Public Health Service Grant U24-CA76518-08 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute and K23 CA82350 (J.D.R.) from the National Cancer Institute.
The Human Research Review Committee (HRRC) of the Medical College of Wisconsin, Milwaukee, WI, has approved these data for analysis and publication (HRRC no. 056-87).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal