The cytokines that function as negative regulators of hematopoiesis are important for at least 3 clinically relevant reasons. First, such pathways can become disrupted in neoplastic transformation. Second, they can also slow engraftment after hematopoietic stem cell transplantation. Third, they can mediate decreases in blood cell production in inflammatory states, such as in anemia of chronic disease. Novel inhibitors of hematopoiesis are therefore of interest because they might be targets for intervention in these disease processes.
The interleukin-17 (IL-17) family of cytokines was recently cloned and characterized by us and others.1-3 Although poorly understood, the IL-17 family has been linked to a number of inflammatory diseases, including rheumatoid arthritis, asthma, allograft rejection, lupus, and antitumor immunity.1-3 It is generally thought that this widely expressed family mediates proinflammatory effects by stimulating the release of multiple other cytokines.1-4 For example, in endothelial cells we have shown that recombinant IL-17D (rIL-17D) protein stimulated production of IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor (GM-CSF),1 and human rIL-17F stimulated production of IL-2, transforming growth factor-β (TGF-β), and monocyte chemoattractant protein-1 (MCP-1).2 In addition, Fossiez et al4 reported that IL-17A induced the expression of G-CSF and GM-CSF by fibroblasts.
We produced purified recombinant protein for 5 of the 6 human IL-17 family members,1 and systematically tested the effects of these on hematopoietic progenitor colony formation under institutional review board (IRB) approval as we described.5 Each of these protein samples was endotoxin free.1 Colony assays were performed in both agar and methylcellulose with the same results using erythropoietin, Steel locus factor (SLF), IL-3, and GM-CSF.5
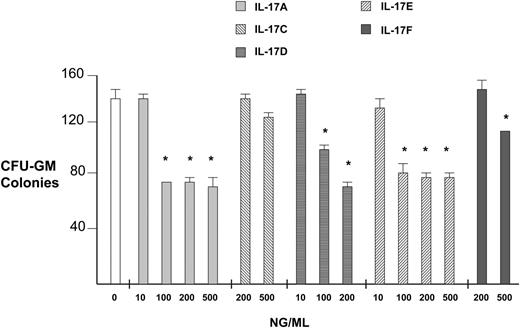
Pure recombinant human IL-17 family members in general had significant inhibitory effects on hematopoietic progenitor colony formation, as shown graphically in Figure 1 for colony-forming unit (CFU)–GM. At a dose of 0.5 μg/mL, IL-17A inhibited CFU-GM, erythroid burst-forming unit (BFU-E), and CFU–granulocyte-erythrocyte-macrophage-megakaryocyte (GEMM) colony formation by an average of 43%, 34%, and 48%, respectively, when compared to isolation buffer–treated cells. At 0.2 μg/mL, IL-17D inhibited CFU-GM, BFU-E, and CFU-GEMM colony formation by 40%, 31%, and 37%, respectively. At 0.5 μg/mL, IL-17E inhibited CFU-GM, BFU-E, and CFU-GEMM colony formation by 42%, 36%, and 42%, respectively. At 0.5 μg/mL, IL-17F inhibited CFU-GM, BFU-E, and CFU-GEMM colony formation by 25%, 10% (not significant), and 27%, respectively. IL-17C did not have a significant effect on progenitor colony formation. We were not able to produce pure recombinant human IL-17B. While IL-17A and E inhibition appear to plateau with higher doses, IL-17D continues to show an increasing inhibition with increasing concentrations.
It is possible that the inhibitory IL-17 family members stimulate production of other inhibitors such as TGF-β, and this mediates their action.1-3 It is also possible that they directly activate nuclear factor-κB (NF-κB) in progenitors,1 which may inhibit proliferation or stimulate apoptosis directly. Since members of this family are highly expressed in chronic autoimmune inflammatory states such as rheumatoid arthritis, asthma, and lupus, it is possible that these cytokines play a role in mediating the myelosuppression seen in those diseases. Perhaps antagonists of these IL-17 family members may reduce the morbidity of these autoimmune diseases, particularly those complicated by myelosuppression.
Inhibition of human myeloid progenitor growth by IL-17 family members. CFU-GM colony formation assays comparing all available IL-17 family proteins at varying concentrations were performed as described.5 IL-17 family cDNAs were isolated by reverse transcriptase–polymerase chain reaction (RT-PCR), subcloned into pCDNA3.1, and mature protein was purified to silver band homogeneity from 293 cell supernatants using nickel affinity columns.1 Control represents treatment with a similar quantity of protein isolation buffer. *P < .005.
Inhibition of human myeloid progenitor growth by IL-17 family members. CFU-GM colony formation assays comparing all available IL-17 family proteins at varying concentrations were performed as described.5 IL-17 family cDNAs were isolated by reverse transcriptase–polymerase chain reaction (RT-PCR), subcloned into pCDNA3.1, and mature protein was purified to silver band homogeneity from 293 cell supernatants using nickel affinity columns.1 Control represents treatment with a similar quantity of protein isolation buffer. *P < .005.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal