Abstract
The antiviral thymidine analog azidothymidine (AZT) is used to treat several virus-associated human cancers. However, to date the mechanism of AZT action remains unclear and thus, reasons for treatment failure are unknown. Adult T-cell leukemia/lymphoma (ATL) is an aggressive malignancy of poor prognosis. Here, we report that enduring AZT treatment of T-cell leukemia virus I–infected cells, in vitro and in vivo in ATL patients, results in inhibition of telomerase activity, progressive telomere shortening, and increased p14ARF expression. In turn, this elicits stabilization and reactivation of the tumor suppressor p53-dependent transcription, increased expression of the cyclin-dependent kinase inhibitor p21Waf1, and accumulation of p27kip1, thereby inducing cellular senescence and tumor cell death. While ATL patients carrying a wild-type p53 enter remission following treatment with AZT, those with a mutated p53 did not respond, and patients' disease relapse was associated with the selection of a tumor clone carrying mutated inactive p53.
Introduction
Adult T-cell leukemia (ATL) is etiologically linked to the human T-cell leukemia virus type I (HTLV-I).1,2 HTLV-I–mediated T-cell transformation presumably arises from a multistep oncogenic process in which the virus induces chronic T-cell proliferation, resulting in an accumulation of genetic defects and the deregulated growth of infected cells.3 Since its discovery, many aspects of HTLV-I biology have been uncovered; however, treatment of the disease remains disappointing, with minimal improvement in the overall survival of patients. The poor prognosis of ATL patients is associated with the resistance of neoplastic cells to the conventional combination of high-dose chemotherapy and radiotherapy.4 Recently, a higher response rate following azidothymidine/interferon α (AZT/IFN α) treatment of ATL patients has been reported in several human trials.5-7 However, the mechanism of action of AZT/IFN remains unknown and, therefore, predictive markers for prognostic therapy are not available. Several human cancers have been treated using antiretroviral AZT, including AIDS-related Kaposi sarcoma,8 Kaposi sarcoma–associated primary effusion lymphoma (PEL), Epstein-Barr (EBV)–associated lymphoma,9 and primary central nervous system lymphoma (PCNL).10 In vitro treatment of PEL cell lines with AZT resulted in TNF-related apoptosis-inducing ligand (TRAIL)–dependent apoptosis11,12 along with NF-kB inhibition and apoptosis in EBV-associated Burkitt lymphoma.13 In contrast, remission of ATL patients treated with AZT follows a slow kinetic over several months, suggesting that a nonapoptotic pathway may be involved. These observations prompted us to investigate the long-term potential effects of AZT on telomerase and telomere functions. Since DNA polymerase is unable to replicate the end of linear DNA, every replication cycle leads to a progressive shortening of the telomeric ends and to the limited proliferative capacity of normal cells, termed “replicative senescence.” Activation of human telomerase, an RNA-dependent DNA polymerase that elongates telomeres, has been proposed as a mechanism for avoiding telomere shortening. Consistent with this model, most cancer cells, including HTLV-I–infected cells, have detectable telomerase activity, as opposed to normal somatic cells.14 In some cases, immortalized cells do not have any detectable telomerase activity in spite of long telomere length, suggesting the existence of an alternative mechanism, referred to as ALT and characterized by specific makers.15
Here we report that enduring treatment of HTLV-I–infected cells with AZT result in telomere attrition and reactivation of p53 transcriptional activities leading to senescence of tumor cells. Importantly, in vivo–treated ATL patients responded to therapy only when p53 was wild type in sequence, and inversely disease relapse or absence of response to treatment was associated with mutation and inactive p53. Our results indicate that p53 is a predictive marker and that a response to AZT therapy requires a functional p53 gene.
Patients, materials, and methods
Cell culture
HTLV-I cell lines were maintained in RPMI 1640-10% fetal bovine serum with and without interleukin-2 (IL-2). Cells were treated with 50 μM AZT or 10 μM 2′, 3′-dideoxyguanosine, ddG (CalBiochem, La Jolla, CA). Medium containing AZT or ddG was replaced every 3 days until growth arrest.
Patients
Samples were obtained after informed consent was provided and in agreement with regulations for the protection of human subjects according to National Institutes of Health guidelines. Treatment regimens with AZT and IFN were previously reported.3,4 Patients' clinical status and response to AZT treatment are reported in Table 2. Patients 2 and 7 were initially treated with chemotherapy (cyclophosphamide + vincristine + prednisone), while patients 13 and 14 received yttrium-90 monoclonal antibody therapy. All other patients received AZT therapy combined with IFN-α as a first line of treatment. PR (partial remission) refers to when ATL cells were still detectable by fluorescence-activated cell sorter scanner (FACS) analysis (> 5%), while CR (complete remission) means there were no ATL cells detectable by FACS in the peripheral blood.
Correlation between AZT treatment response and p53 status in ATL patients
. | Diagnosis . | p53 status before initiation of AZT treatment . | Patient response to AZT treatment . | p53 status after AZT treatment . | p53 transcriptional activity . |
|---|---|---|---|---|---|
| ATL1 | Acute | Mut E198G | NR | NA | No |
| ATL2 | Acute | Frameshift, stop aa 148 | NR | NA | No |
| ATL3 | Acute | Mut Q192R | PR | Q192R | Partial |
| ATL4 | Chronic | wt | R | R273H | No |
| ATL5 | Chronic | wt | CR | wt | Yes |
| ATL6 | Smoldering | wt | CR | NA | Yes |
| ATL7 | Lymphoma | Mut P222L; I 232T | NR | NA | No |
| ATL8 | Acute | wt | PR | NA | Yes |
| ATL9 | Acute | wt | PR | NA | Yes |
| ATL10 | Lymphoma | mut | NR | NA | ND |
| ATL11 | Lymphoma | mut | NR | NA | ND |
| ATL12 | Acute | mut | NR | NA | ND |
| ATL13 | Acute | Mut R72P;S166P;R280G | NR | NA | No |
| ATL14 | Chronic | Mut R72P;H178Y;I255V | NR | NA | No |
. | Diagnosis . | p53 status before initiation of AZT treatment . | Patient response to AZT treatment . | p53 status after AZT treatment . | p53 transcriptional activity . |
|---|---|---|---|---|---|
| ATL1 | Acute | Mut E198G | NR | NA | No |
| ATL2 | Acute | Frameshift, stop aa 148 | NR | NA | No |
| ATL3 | Acute | Mut Q192R | PR | Q192R | Partial |
| ATL4 | Chronic | wt | R | R273H | No |
| ATL5 | Chronic | wt | CR | wt | Yes |
| ATL6 | Smoldering | wt | CR | NA | Yes |
| ATL7 | Lymphoma | Mut P222L; I 232T | NR | NA | No |
| ATL8 | Acute | wt | PR | NA | Yes |
| ATL9 | Acute | wt | PR | NA | Yes |
| ATL10 | Lymphoma | mut | NR | NA | ND |
| ATL11 | Lymphoma | mut | NR | NA | ND |
| ATL12 | Acute | mut | NR | NA | ND |
| ATL13 | Acute | Mut R72P;S166P;R280G | NR | NA | No |
| ATL14 | Chronic | Mut R72P;H178Y;I255V | NR | NA | No |
Status of patients before AZT treatment, response to treatment, and status of p53 gene and its transcriptional activity. The criteria for clinical therapeutic response were as follows: complete remission was defined as the disappearance of all measurable and assessable disease, lasting more than 3 months; partial remission was defined as reduction of leukemic cell count, lasting more than 1 month but less than 3 months; and not responding was defined as increase in leukemic cell count. CR indicates complete remission; PR, partial remission; NR, not responding; R, relapse; NA, not available; and ND, not determined.
TRAP assay
Telomerase activity was measured by telomerase repeat amplification protocol (TRAP) assay using Trapeze Telomerase detection kit (Chemicon, Temecula, CA) followed by SYBR-green staining (Molecular Probes, Eugene, OR) and was quantified as previously reported.16 An equal amount of protein lysates in CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid) buffer was used. Results are representative of 3 experiments.
Telomere length analysis
Genomic DNA was isolated, and telomere length was determined by Southern blot analysis using TeloTAGGG telomere length assay kit (Roche, Indianapolis, IN) according to manufacturers' instructions.
Quantitative flow–fluorescence in situ hybridization (Q-FISH)
Peripheral CD25+ T cells were isolated from HTLV-I–infected ATL patients using anti-CD25 magnetic Dynabeads (Dynal Biotech, Lake Success, NY). The telomere length of CD25+ cells from different patients were quantitatively determined by hybridization of Telomere peptide nucleic acid (PNA)/FITC (fluorescein isothiocyanate) probe by flow cytometry (FACS-Calibur, Becton Dickinson, San Jose, CA) using Telomere PNA/FITC kit (DakoCytomation, Carpinteria, CA) as reported17 and according to manufacturer's instructions.
Western blots
Equal amounts of proteins from untreated and long-term AZT-treated MT2 cells were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. All primary and secondary horseradish peroxidase–conjugated antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Bax (N-20), Bcl-xL (H-62), Mcl-1 (S-19), Bcl-2 (N-19), PARP (H-250), caspase-3 (H-277), p14ARF (FL-132), p53 (FL-393), MDM2 (SMP-14), MDMX (D-19), p21WAF (C-19), p27KIP (C-19G), and β-tubulin (D-10) were used as loading control.
Senescence β-gal (SA-β gal) assay
Untreated and long-term AZT-treated MT2 cells were fixed in 2% formaldehyde-0.2% glutaraldehyde for 3 minutes, washed with phosphate-buffered saline, and incubated at 37°C with SA-β-gal staining solution, pH 6.0. Images were captured using a Nikon EFD3 microscope (Boyce Scientific, St Louis, MO) and Nikon camera with an Eplan 100x (160×/0.17) objective. Acquisition software, Image-ProExpress version IV, was from Media Cybernetics (Silver Spring, MD).
PCR and semiquantitative RT-PCR
Reverse transcriptase–polymerase chain reaction (RT-PCR) primers were as follows: p53-forward primer F: (5′-GTCCCCGGACGATATTG-3′), reverse primer R: (5′-CCAGAATGCAAGAAGCCCAG-3′); p21WAF F: (5′-GAAGAAGGGTAGCTGGGGCT-3′), R: (5′-CTCTAAGGTTGGGCAGGGTG-3′); p27KIP F: (5′ TGCCCGAGTTCTACTACAGACC-3′), R: (5′CTTATTCCTGCGCATTGCTCCGC-3′); Bax F: (5′-GGGGACGAACTGGACAGTAA-3′), R: (5′CAGTTGAAGTTGCCGTCAGA-3′); p14ARF exon1 F: (5′-CTGGAGGCGGCGAGAACATGG-3′), R: (5′-GGGCCTTTCCTACCTGGTCTT-3′); p14ARF exon2 F: (5′-GCTCTACACAAGCTTCCTTTCCG-3′), R: (5′-CGGGCTGAACTTTCTGTGCTGG-3′), and GADPH F: (5′-GAAGGTGAAGGTCGGAGTC-3′), R: (5′-GAAGATGGTGATGGGATTTC-3′). Total RNA was isolated by Trizol reagent (Invitrogen, Carlsbad, CA), and RT-PCR was performed using the One-Step RT PCR kit (Qiagen, Valencia, CA).
Inverse LTR-PCR
Proviral integration site was determined by digesting genomic DNA with SmaI, ligation, and inverse PCR with HTLV-I long-terminal-repeat (LTR) primers F: (5′-CCCCAAATATCCCCCGGG-3′) and R: (5′-GGGGCTTATGGTCATTGTC-3′). The amplified products were cloned into pCR2.1 TA vector (Invitrogen) and sequenced.
p53 status and functional assay
Total RNA was isolated from ATL patients, and the p53 hot spot region amplified by RT-PCR using the following primers: F: 5′-CCAGAAAACCTACCAGGGCAG-3′, R:5′-GCTCGCTTAGTGCTCCCTGG-3′, cloned, and sequenced. For p53 full-length RT-PCR and cloning we used F: 5′-CGGAATTCATGGAGGAGCCGCAGTCAGATCC-3′ and R: 5′-CCGCTCGAGTCAGTCTGAGTCAGGCCCTTCTG-3′. For the p53 functional assay, the p53 from MT-2 (wild type) was cloned into pcDNA3.1Zeo vector (Invitrogen), and the hot spot region from pcDNAp53MT2 was substituted by respective ATL p53 hot spot regions using DrdI-BsaI restriction enzymes. Luciferase assays were performed with Luciferase Reporter Assay kit (Promega, Madison, WI) by transfecting Jurkat E-6 cells with p53-responsive pG13Luc and respective ATL pcDNA3.1Zeo-p53.
Results
Inhibition of telomerase induces telomere attrition and cell death in HTLV-I–transformed cell lines
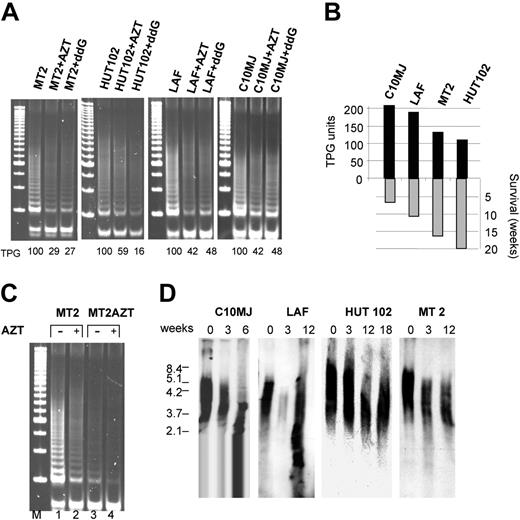
We and others have reported that HTLV-I infection is associated with increased telomerase activity in transformed cells in vitro and in vivo.16,18-20 As expected, treatment of HTLV-I–transformed cell lines with telomerase inhibitors AZT or ddG resulted in a marked reduction of telomerase activity in HTLV-I–infected cells (Figure 1A). As telomerase activity is required for continual proliferation and avoidance of replicative senescence of tumor cells, we evaluated the long-term effects of telomerase inhibition by maintaining HTLV-I cell lines in medium supplemented with AZT or ddG. While short-term treatment had no effect on proliferation or survival (data not shown and Bazarbachi et al21 ), all HTLV-I cell lines stopped growing (Figure 1B), and died after several weeks of culture in the presence of AZT or ddG, as observed by cell counts and trypan blue exclusion (data not shown). Longer survival, as observed in MT-2 and HUT-102, appeared to be associated with a lower telomerase activity compared with C10MJ and LAF (Figure 1B). In contrast, the Jurkat T-cell line, treated under the same conditions, did not enter growth arrest (data not shown), in spite of significant telomere shortening as previously reported.22 HTLV-I–transformed cells do not acquire resistance to AZT-mediated inhibition of telomerase activity, even after a prolonged period of treatment, because residual activity present in long-term–treated cells (Figure 1C, lane 3) can be further inhibited in MT-2 as well as in other HTLV-I cell lines by addition of AZT for 48 hours (Figure 1C, lane 4, and data not shown). Analysis of telomere length by Southern blot revealed a progressive shortening in all HTLV-I cell lines cultured in continuous presence of AZT (Figure 1D).
Telomerase inhibition induces telomere attrition in HTLV-I–infected cells. (A) Telomerase activity (expressed as telomeric product generated, TPG), measured by TRAP assay in HTLV-I cell lines in absence of or presence of telomerase inhibitors AZT (50 μM) and ddG (10 μM) for 72 hours. (B) Correlation between telomerase activity (TPG) and survival of AZT-treated HTLV-I cell lines (in weeks). (C) Inhibition of telomerase activity by AZT in the HTLV-I–transformed MT-2 cell line untreated or treated with AZT for 18 weeks. (D) Southern blot analysis of telomere shortening following AZT treatment.
Telomerase inhibition induces telomere attrition in HTLV-I–infected cells. (A) Telomerase activity (expressed as telomeric product generated, TPG), measured by TRAP assay in HTLV-I cell lines in absence of or presence of telomerase inhibitors AZT (50 μM) and ddG (10 μM) for 72 hours. (B) Correlation between telomerase activity (TPG) and survival of AZT-treated HTLV-I cell lines (in weeks). (C) Inhibition of telomerase activity by AZT in the HTLV-I–transformed MT-2 cell line untreated or treated with AZT for 18 weeks. (D) Southern blot analysis of telomere shortening following AZT treatment.
AZT induces senescence of HTLV-I–infected cells
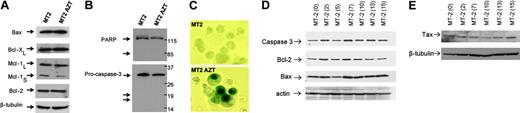
As telomere shortening has been associated with chromosomal instability, causing either apoptosis or senescence,23,24 we investigated the mechanism of tumor cell death in long-term AZT-treated cells. Previous studies reported that EBV-positive Burkitt lymphoma cells from AIDS patients respond in vitro to AZT by undergoing caspase-dependent apoptosis. HTLV-I–infected cells are protected from apoptosis in part through high levels of Bcl-2 and Bcl-xL expression.25 However, the expression of proapoptotic and antiapoptotic proteins remained unchanged in long-term AZT-treated MT-2 cells (Figure 2A).
Absence of procaspase-3 and poly(ADP-ribose) polymerase (PARP) cleavage (Figure 2B), along with negative annexin V staining (data not shown), further confirmed the absence of apoptosis in AZT-treated HTLV-I cells. Instead, we found that HTLV-I cells undergo senescence as shown by the senescence β-galactosidase assay, which was strongly positive in long-term AZT-treated MT-2 cells but not in MT-2 control cells (Figure 2C). This also was confirmed in other treated HTLV-I cell lines (C10MJ, HUT102, and LAF) (data not shown).
Persistent telomerase inhibition is not associated with apoptosis but induces senescence in HTLV-I–infected cells. (A) Western blot analysis for expression of apoptosis regulators in untreated MT-2 or treated with AZT (18 weeks). Equal amounts (50 μg) of each extract were used and confirmed by β-tubulin. (B) Absence of cleavage for procaspase 3 or PARP in AZT-treated MT-2 cells. (C) Senescence β-gal (SA-β gal) assay in MT-2 cells untreated and treated with AZT (18 weeks). A significant amount of senescence was detected only in end of cultures. (D) Western blot analysis of MT-2 cells treated with AZT. Samples were collected at different times after treatment from 0 to 15 weeks. Expression of caspase 3, Bcl-2, and Bax was tested. Actin was used as loading control. (E) Western blot analysis of MT-2 cells treated with AZT. Samples were collected at different times after treatment from 0 to 15 weeks. Expression of Tax was analyzed as described in “Patients, materials, and methods.” Beta-tubulin was used to confirm equal loading.
Persistent telomerase inhibition is not associated with apoptosis but induces senescence in HTLV-I–infected cells. (A) Western blot analysis for expression of apoptosis regulators in untreated MT-2 or treated with AZT (18 weeks). Equal amounts (50 μg) of each extract were used and confirmed by β-tubulin. (B) Absence of cleavage for procaspase 3 or PARP in AZT-treated MT-2 cells. (C) Senescence β-gal (SA-β gal) assay in MT-2 cells untreated and treated with AZT (18 weeks). A significant amount of senescence was detected only in end of cultures. (D) Western blot analysis of MT-2 cells treated with AZT. Samples were collected at different times after treatment from 0 to 15 weeks. Expression of caspase 3, Bcl-2, and Bax was tested. Actin was used as loading control. (E) Western blot analysis of MT-2 cells treated with AZT. Samples were collected at different times after treatment from 0 to 15 weeks. Expression of Tax was analyzed as described in “Patients, materials, and methods.” Beta-tubulin was used to confirm equal loading.
Analysis of samples collected at different intervals in MT-2 cells cultured in the presence of AZT showed no significant differences in the levels of expression of apoptotic markers (Figure 2D). In contrast to previous observations made in HTLV-I–infected cells treated with arsenic trioxide and IFN,26,27 the levels of the viral oncoprotein Tax remain unchanged during AZT treatment (Figure 2E).
Continuing inhibition of telomerase by AZT in HTLV-I–infected cells induces posttranscriptional stabilization of p53
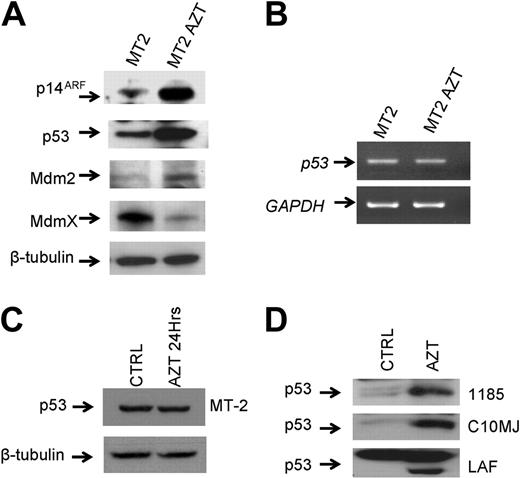
To confirm these findings and to gain further insights into the mechanisms involved, the expression level of p14ARF, a well-known senescence marker,28 was analyzed. We found significant increased expression of p14ARF in long-term AZT-treated MT-2 cells (Figure 3A). Consistent with its ability to prevent Mdm2-mediated degradation of p53,29 levels of p53 expression were considerably increased in AZT-treated cells when compared with control MT-2 cells (Figure 3A). The underlying mechanism of increased p53 expression was posttranscriptional, because the levels of p53 mRNA in MT-2 and AZT-treated MT-2 cells remained similar (Figure 3B). In addition, the effect on p53 protein stabilization was detected only after long-term treatment with AZT, and treatment with AZT for 24 hours did not result in any significant increase in the levels of p53 expression (Figure 3C).
These results exclude a potential direct effect of AZT on p53 and suggest that telomere attrition plays an essential role in increasing the levels of p14ARF and p53 expression. Importantly, long-term effects of AZT on cell cycle arrest and increased expression of p53 were reproduced in 2 independent experiments using MT-2 and C10MJ cell lines and were further confirmed in 3 other HTLV-I cell lines, C10MJ, 1185, and LAF (Figure 3D), suggesting a general mechanism rather than an observation limited to MT-2 cells. Increased expression of p14ARF and p53 following long-term AZT treatment was accompanied by an increase in Mdm2 expression (Figure 3A), and consistent with the previous findings that Mdm2 promotes ubiquitination and proteasome degradation of MdmX,30 our results also revealed a significant decrease in the levels of MdmX expression in long-term AZT-treated MT-2 cells (Figure 3A). Thus, by blocking the ability of Mdm2 to target p53 for degradation, p14ARF causes p53 stabilization and, by stimulating MdmX degradation by Mdm2, p14ARF concurrently eliminates another inhibitor of p53 function.
Continuing inhibition of telomerase by AZT in HTLV-I–infected cells induces posttranscriptional stabilization of p53. (A) Western blot analysis for expression of p53 pathway regulators in untreated MT-2 and AZT-treated (18 weeks) MT-2 cells. Equal amounts (50 μg) of each extract were used and confirmed by β-tubulin. (B) Analysis of p53 mRNA expression by RT-PCR in MT-2 and after culture with AZT for 18 weeks. GAPDH was used as internal control for amplification. (C) Western blot analysis of p53 after 24 hours of AZT treatment. Equal amounts (50 μg) of each extract were used and confirmed by β-tubulin. (D) Increased expression of p53 detected by Western blot in several HTLV-I–infected cell lines (1185, C10MJ, LAF) treated for 4 weeks with AZT.
Continuing inhibition of telomerase by AZT in HTLV-I–infected cells induces posttranscriptional stabilization of p53. (A) Western blot analysis for expression of p53 pathway regulators in untreated MT-2 and AZT-treated (18 weeks) MT-2 cells. Equal amounts (50 μg) of each extract were used and confirmed by β-tubulin. (B) Analysis of p53 mRNA expression by RT-PCR in MT-2 and after culture with AZT for 18 weeks. GAPDH was used as internal control for amplification. (C) Western blot analysis of p53 after 24 hours of AZT treatment. Equal amounts (50 μg) of each extract were used and confirmed by β-tubulin. (D) Increased expression of p53 detected by Western blot in several HTLV-I–infected cell lines (1185, C10MJ, LAF) treated for 4 weeks with AZT.
Analysis of samples collected at different intervals in MT-2 cells cultured in the presence of AZT showed an increase in p53 expression after 7 weeks of treatment and thereafter (data not shown). In contrast, in Jurkat cells treated in the same conditions, p53 levels of expression remained constant (data not shown).
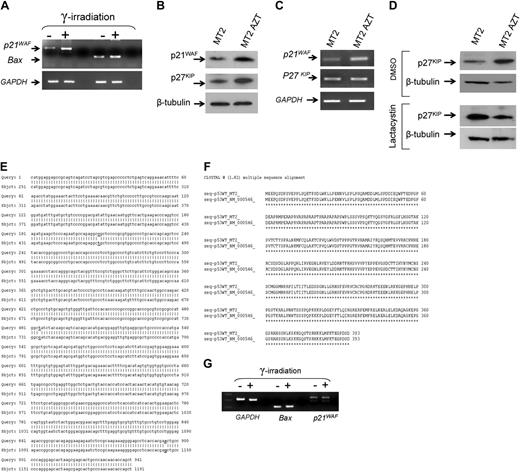
AZT triggers reactivation of p53-dependent transcription and accumulation of cyclin-dependent kinase inhibitors p21waf and p27kip
We hypothesized that the concerted actions of p14ARF, Mdm2, and MdmX may lead to reactivation of p53 transcriptional activities. Previous studies have found that p53 is transcriptionally inactive in HTLV-I–infected cells in vitro and in uncultured ATL cells and that p53-responsive genes are not activated following ionization-radiation in these cells.31,32 To test for reactivation of p53 transcriptional functions, long-term AZT-treated MT-2 cells were subjected to mock or ionization radiation, which produces DNA damage and induces transcription of p53-responsive genes. Our results confirmed the reactivation of p53 transcriptional activities, demonstrated by the induction of p53-responsive genes p21WAF and Bax following ionization radiation (Figure 4A). Spot densitometry indicated a 3.2- and 1.8-fold increase for p21WAF and Bax mRNA expression following irradiation. Proliferation assays, along with propidium iodide staining, revealed that long-term AZT-treated MT-2 cells arrest in all phases of the cell cycle (data not shown), a signature of cyclin-dependent kinase inhibitors (CDKI). Consistently, protein expression levels of both p21WAF and p27KIP were increased significantly following prolonged AZT treatment (Figure 4B).
However, while increased expression of p21WAF occurred at the transcriptional level (Figure 4C), presumably through p53, p27KIP mRNA expression remained comparable in MT-2 control and long-term AZT-treated MT-2 cells, suggesting a posttranscriptional mechanism (Figure 4C). In fact, when MT-2 and AZT-treated MT-2 cells were incubated with lactacystin, a proteasome inhibitor, levels of p27KIP expression significantly increased in MT-2 but not in MT-2 AZT-treated cells (Figure 4D). These findings suggest that the p27KIP degradation pathway is hampered in long-term AZT-treated cells, which allows for its stabilization. Our results are consistent with previous observations that limiting amounts of p27KIP correlate with constitutive activation of the cyclin E-CDK2 complex and increased expression of p27KIP triggers cell cycle arrest.33 We next confirmed that in the original MT-2 cells, p53 was wild type but transcriptionally inactive. To this end, p53 cDNA was amplified, cloned, and sequenced. As shown in Figure 4E, we found 3 nucleotides changed in the p53 sequence from MT-2 cells.
However, these mutations were silent and did not affect the amino acid sequence, which was wild type in MT-2 (Figure 4F). To confirm that p53 transcription was impaired, we gamma-irradiated MT-2 cells and analyzed expression of p53-responsive genes bax and p21waf as reported in Figure 4A. In clear contrast to AZT-treated MT-2 cells, our results demonstrate that p53 is transcriptionally inactivated in untreated MT-2 cells (Figure 4G).
Overall our results suggest that prolonged AZT treatment and inhibition of telomerase activity leads to telomere attrition, restores the functions of p53, and increases expression of senescence markers p14ARF and p21WAF in long-term AZT-treated MT-2 cells.
AZT-based treatment induces telomerase inhibition and telomere shortening in vivo in ATL patients
The effect of AZT in vitro requires several weeks of treatment,34 which parallels the slow kinetics observed in ATL patients treated with AZT.3 Strikingly, the estimated 30% failure rate of AZT treatment in HTLV-I–infected ATL patients35 coincides with the approximate percentage of ATL patients carrying a mutated inactive p53 gene.36 These observations prompted us to investigate whether the effect of AZT in treatment of ATL patients also relied on telomerase inhibition, telomere attrition, and p53 functions.
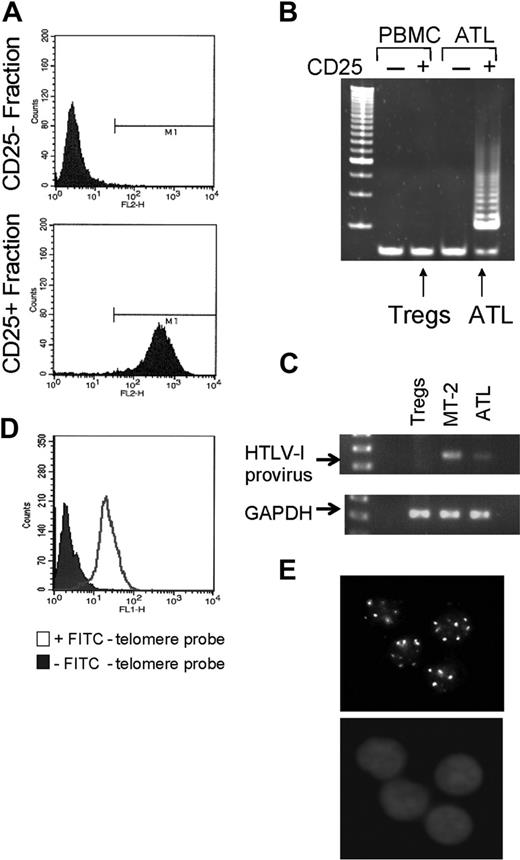
Samples were obtained from 18 ATL patients at different clinical stages, lymphomatous, smoldering, chronic, and acute from 2 different origins (Necker Hospital, Paris, France, and National Institutes of Health, Bethesda, MD). All samples were blind-tested, and in each case the response to therapy was provided only after analysis. In the case of 3 ATL patients (ATL3, ATL4, ATL5) a paired sample, diagnostic and remission/relapse, could be obtained. Due to the intrinsic limitation in the amounts of each of the patient samples available, telomere sizes were measured by Q-FISH37 before and after in vivo AZT treatment. To avoid potential variations between patient's sample origins, we cell-sorted HTLV-I–infected from noninfected cells of 3 uncultured ATL patient samples collected prior to and after several weeks of AZT treatment and compared the relative telomere size in each cellular fraction. As HTLV-I–infected ATL cells overexpress the CD25 activation marker on their surface,38 we used anti-CD25–coupled magnetic beads to sort HTLV-I–infected from their noninfected counterparts. Successful sorting was confirmed by staining each sorted cell population with an anti-CD25 FITC-conjugated antibody, and FACS analysis showed at least 95% purity (Figure 5A). To ensure that CD25+ sorted cells correspond to HTLV-I–infected cells and not uninfected CD25+ regulatory T cells (Tregs), CD25+ and CD25– cells isolated from peripheral blood mononuclear cells (PBMCs) of a healthy individual and those of an ATL patient were tested by TRAP assay. Significant telomerase activity was detected only in the CD25+ population isolated from the ATL patient, and no or very low telomerase activity was detectable in either population isolated from the non–HTLV-I–infected donor (Figure 5B), suggesting that Tregs are mostly telomerase negative and that the CD25+ cell fraction isolated from the ATL patients indeed corresponds to HTLV-I–infected cells.
AZT-mediated reactivation of p53 functions and stabilization of CDKI p21WAF and p27KIP. (A) Expression of p21waf and Bax mRNA before and after ionizing radiation in AZT-treated MT-2 cells (18 weeks). GAPDH was used as internal control for amplification. (B) Western blot analysis for expression of p21WAF and p27KIP in untreated MT-2 or after culture with AZT for 18 weeks. Equal amounts (50 μg) of each extract were used and confirmed by β-tubulin. (C) Analysis of p21WAF and p27KIP mRNA expression by RT-PCR in MT-2 and after culture with AZT for 18 weeks. GAPDH was used as internal control for amplification. (D) Western blot analysis for expression of p27KIP in untreated MT-2 or after culture with AZT for 18 weeks, in absence or presence of proteosome inhibitor lactacystin. Equal amounts of each extract (50 μg) were used and confirmed by β-tubulin. (E) MT-2–derived p53 cDNA nucleotide sequence. (F) p53 amino acid sequence from MT-2 cells compared with wild-type p53. (G) RT-PCR for p53 responsive genes before and after gamma irradiation of MT2 cells.
AZT-mediated reactivation of p53 functions and stabilization of CDKI p21WAF and p27KIP. (A) Expression of p21waf and Bax mRNA before and after ionizing radiation in AZT-treated MT-2 cells (18 weeks). GAPDH was used as internal control for amplification. (B) Western blot analysis for expression of p21WAF and p27KIP in untreated MT-2 or after culture with AZT for 18 weeks. Equal amounts (50 μg) of each extract were used and confirmed by β-tubulin. (C) Analysis of p21WAF and p27KIP mRNA expression by RT-PCR in MT-2 and after culture with AZT for 18 weeks. GAPDH was used as internal control for amplification. (D) Western blot analysis for expression of p27KIP in untreated MT-2 or after culture with AZT for 18 weeks, in absence or presence of proteosome inhibitor lactacystin. Equal amounts of each extract (50 μg) were used and confirmed by β-tubulin. (E) MT-2–derived p53 cDNA nucleotide sequence. (F) p53 amino acid sequence from MT-2 cells compared with wild-type p53. (G) RT-PCR for p53 responsive genes before and after gamma irradiation of MT2 cells.
In fact, integrated HTLV-I provirus was detected by PCR in CD25+ cells isolated from the ATL patient only (Figure 5C). Specific hybridization of the FITC-labeled probe to the telomeric ends was confirmed by microscopic observation of a punctuated pattern consistent with telomere labeling (Figure 5D,E).
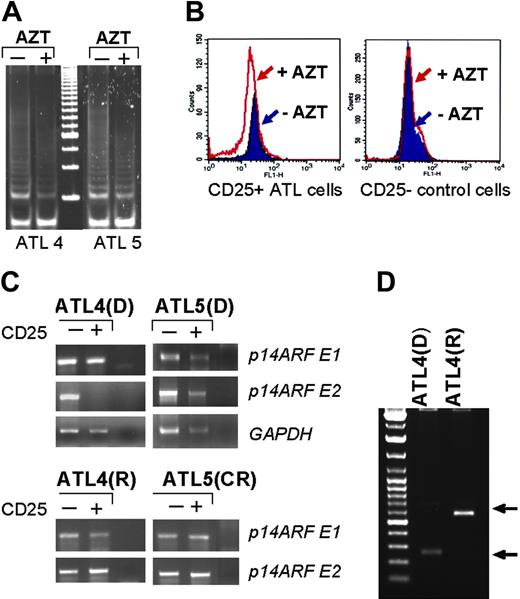
Telomerase activity was readily detected by TRAP assay in all tested ATL patients (data not shown). Treatment with AZT reduced telomerase activity in vivo (Figure 6A). While average telomere size from CD25– cells was not affected by treatment with AZT, a 14% to 30% reduction in telomere length occurred in HTLV-I–infected CD25+ cells after treatment with AZT (Figure 6B and Table 1). Since p14ARF is an important player in p53 functions and overlaps the p16ink locus, a gene frequently methylated, deleted, or mutated in ATL patients,39-42 we analyzed the integrity of the p14ARF gene. At the time of diagnosis p14ARF exons 1 and 2 were present in an ATL5 patient, but p14ARF exon 2 was deleted in HTLV-I–infected cells of an ATL4 patient (Figure 6C). However, a recent study showed that exon 2 is dispensable for p14ARF function and that the amino-terminal 29 residues of p14ARF are sufficient for stabilization of p53.43 Interestingly, analysis of the ATL4 patient's DNA revealed the presence of both p14ARF exons after the course of AZT treatment and patient disease relapse (Figure 6C). These findings suggest that in patient ATL4, treatment with AZT eradicated the initial tumor clone but resulted in the outgrowth of a different tumor clone at relapse. In fact, inverse PCR experiments and sequencing demonstrated a different provirus integration site in ATL4 patient's DNA samples collected at diagnostic and after disease relapse (Figure 6D).
Average telomere size in CD25+ cells before and after AZT treatment, calculated from 2 independent measurements
. | ATL3 . | ATL4 . | ATL5 . |
|---|---|---|---|
| TRF before AZT, kb | 8.7 | 7.6 | 10.2 |
| TRF after AZT, kb | 7.5 | 5.4 | 7.8 |
. | ATL3 . | ATL4 . | ATL5 . |
|---|---|---|---|
| TRF before AZT, kb | 8.7 | 7.6 | 10.2 |
| TRF after AZT, kb | 7.5 | 5.4 | 7.8 |
Analysis of CD25+HTLV-I–infected cells. (A) FACS analysis of the presence of CD25 marker expression from CD25+ and CD25– sorted cells. (B) Telomerase activity detected by TRAP assay in CD25+ and CD25– fractions isolated from an HTLV-I donor and ATL PBMCs. (C) Integrated HTLV-I proviral DNA was detected by PCR using primers in the tax coding region. GAPDH was used as amplification control to ensure proper quality and quantity of extracted DNAs. (D) Staining control of PBMCs in presence or absence of FITC-telomere probe. (E) In situ hybridization of FITC-conjugated telomere probe (top) and DAPI (4′6-diamidino-2-phenylindole 2HCl) (bottom).
Analysis of CD25+HTLV-I–infected cells. (A) FACS analysis of the presence of CD25 marker expression from CD25+ and CD25– sorted cells. (B) Telomerase activity detected by TRAP assay in CD25+ and CD25– fractions isolated from an HTLV-I donor and ATL PBMCs. (C) Integrated HTLV-I proviral DNA was detected by PCR using primers in the tax coding region. GAPDH was used as amplification control to ensure proper quality and quantity of extracted DNAs. (D) Staining control of PBMCs in presence or absence of FITC-telomere probe. (E) In situ hybridization of FITC-conjugated telomere probe (top) and DAPI (4′6-diamidino-2-phenylindole 2HCl) (bottom).
Response of ATL patients to AZT-based therapy correlates with their p53 transcriptional status
As most mutations that inactivate p53-dependent transcription lead to protein stabilization, we investigated p53 stabilization by immunochemistry in 4 ATL patients. Many studies have reported stabilization and increased expression of transcriptionally inactive mutated p53. In 3 patients who did not respond to AZT treatment, p53 expression was readily detectable by immunohistochemistry (Figure 7A). In contrast, in patients responding to AZT, p53 expression was very weak, consistent with a wild-type p53 sequence (Figure 7A). To establish a correlation between the p53 status and the outcome of ATL patients who received AZT therapy, the p53 hot spot region, exons 4 through 9, was cloned by RT-PCR. In each case, we sequenced 2 p53 hot spot clones from 14 ATL patients. For ATL3, 4, and 5, two p53 hot spot clones were sequenced both from before treatment and after remission/disease relapse. Whenever the p53 hot spot region was found to be wild type in sequence, the whole p53 cDNA was then cloned, and 2 additional clones were sequenced to confirm the phenotype.
Our results demonstrate that all ATL patients carrying a wild-type p53 gene responded to AZT treatment and went into partial or complete remission (Table 2). In contrast, all patients carrying a mutated p53 at diagnosis did not respond to AZT treatment and died within a short period of time (Table 2). Thus, a total correlation between p53 and AZT response was found and makes the results highly significant.
AZT-induced telomerase inhibition and telomere shortening in ATL patients. (A) Inhibition of telomerase activity measured by TRAP in vivo in ATL patients before and during treatment with AZT. (B) FACS analysis of telomere size by Flow-FISH in CD25+ and CD25– cells isolated from uncultured ATL patient samples. (C) Detection of exon 1 and 2 of the p14ARF gene by PCR in CD25+ cells from 2 ATL patients before AZT treatment or after AZT treatment; GAPDH amplification was used as control. D indicates diagnosis; R, relapse/death; and CR, complete remission. (D) Detection of provirus integration site by inverse PCR before and after AZT treatment. Arrows indicate different integration sites by inverse PCR.
AZT-induced telomerase inhibition and telomere shortening in ATL patients. (A) Inhibition of telomerase activity measured by TRAP in vivo in ATL patients before and during treatment with AZT. (B) FACS analysis of telomere size by Flow-FISH in CD25+ and CD25– cells isolated from uncultured ATL patient samples. (C) Detection of exon 1 and 2 of the p14ARF gene by PCR in CD25+ cells from 2 ATL patients before AZT treatment or after AZT treatment; GAPDH amplification was used as control. D indicates diagnosis; R, relapse/death; and CR, complete remission. (D) Detection of provirus integration site by inverse PCR before and after AZT treatment. Arrows indicate different integration sites by inverse PCR.
The biological relevance of our finding is further supported in the case of ATL4, in which the initial response to treatment was associated with the presence of a wild-type p53, but as indicated in Table 2, AZT treatment led to the selection of another tumor clone and disease relapse. Strikingly, the clone responsible for the relapse carried a p53 mutated at R273H, a well-characterized mutation that abrogates p53 transcriptional activities. All p53 mutations of unknown function were further tested in a luciferase functional reporter assay after transfection in Jurkat T cells (Figure 7B).
Discussion
Results described here provide a novel link between telomerase targeting, telomere attrition, and reactivation of p53-dependent senescence pathways in HTLV-I–infected cells. While it is established that p53 is inactive in HTLV-I–infected cells, the molecular mechanism of p53 transcriptional inactivation remains a matter of debate.44-50 Our results clearly indicate that the p53 inactivation mechanism is reversible and could therefore be used to develop novel therapeutic interventions. Previous studies have shown that localization and DNA-binding activity of p53 is not altered in HTLV-I cells, and it is more likely that posttranscriptional modification and/or association with specific cellular partners dictates the inhibition. Whether prolonged action of AZT modulates the phosphorylation or the acetylation status of p53 or its interaction with cellular partners warrants further studies.
Response of ATL patients to AZT-based therapy correlates with theirp53transcriptional status. (A) Immunohistochemistry detection of p53 expression in ATL samples collected from AZT responder (top) and nonresponders (bottom) performed as previously described.3 (B) p53 from ATL patients was cloned into pCDNA3.1 and tested in a functional assay. Jurkat T cells were transfected with a p53-responsive vector and p53 expression vectors and luciferase activity detected 24 hours later. Results are representative of 2 independent experiments.
Response of ATL patients to AZT-based therapy correlates with theirp53transcriptional status. (A) Immunohistochemistry detection of p53 expression in ATL samples collected from AZT responder (top) and nonresponders (bottom) performed as previously described.3 (B) p53 from ATL patients was cloned into pCDNA3.1 and tested in a functional assay. Jurkat T cells were transfected with a p53-responsive vector and p53 expression vectors and luciferase activity detected 24 hours later. Results are representative of 2 independent experiments.
Results obtained from patient ATL 4 further underscore the importance of a functional p53 (Figure 6 and Table 2). Initial responses to AZT treatment coincides with the presence of a major tumor clone that is wild-type p53 in sequence. Eradication of that tumor clone, however, led to the emergence of a minor clone carrying a mutated and inactive p53. This selection also coincides with disease relapse and death of the patient. Analyses of the p14ARF locus and the provirus integration sites, at the initiation of treatment and at disease relapse, clearly indicate that the 2 tumor clones present in this patient are distinct.
Several studies have found that transforming growth factor–β (TGF-β) signaling is impaired in HTLV-I–infected cells in vitro and in ex vivo ATL patient samples.51-53 Since prolonged treatment of HTLV-I cells with AZT reduces MdmX protein expression, which has been implicated in inhibition of Smad transactivation and TGF-β signaling,54,55 we tested whether this pathway may be reactivated. However, transfection of MT-2 and AZT-treated MT-2 cells with a TGF-β reporter vector in the absence or presence of exogenous TGF-β did not show any difference in activity (data not shown), suggesting that either MdmX is not involved in TGF-β inhibition or at least that down-regulation of MdmX is not sufficient to restore TGF-β signaling in HTLV-I–transformed cells.
Interestingly, a recent study reported complete response and suppression of HTLV-1 viral load following alemtuzumab therapy in AZT/IFN refractory adult T-cell leukemia.56 Since alemtuzumab is active in a mouse model of ATL57 and in patients with refractory T-cell malignancies or chronic lymphocytic leukemia with mutated or absent p53 gene,58,59 it could be used in combination or in maintenance treatment to prevent emergence of p53-mutated clones in patients responding to AZT therapy. It should be noted that the majority of ATL cases are wild-type p53 and die of their disease despite AZT/IFN or other treatments. In this study, we did not use IFN-α, because AZT has been shown to have some effect on its own in several virus-associated hematologic disorders and to limit the number of variable parameters and facilitate analysis. However, synergies previously observed in vivo between AZT and IFN-α may in part result from the transcriptional inhibition of the hTERT promoter by IFN-α, as significant down-regulation of hTERT mRNA expression and telomerase activity has been reported in leukemic cell lines as well as in primary leukemic cells.36 It is also possible that additional functions of IFN-α may be involved.
Our study identifies p53 status as an essential predictive marker for the response of HTLV-I–infected ATL patients to AZT/IFN treatment and suggests that such therapy may not have any clinical benefits in patients carrying a mutated, inactive p53 gene for which alternative therapies such as allogenic bone marrow transplant, alemtuzumab, or radio-immunotherapy for IL-2Rα60,61 should be considered. Patients treated with chemotherapy or radiotherapy usually select for tumor clones with mutated p53 and become nonresponders to AZT when used in the second line of treatment.35 We propose that for patients carrying wild-type p53, AZT/IFN should be considered as the first line of treatment. Whether the effect of AZT/IFN on AIDS-related Kaposi sarcoma, Kaposi sarcoma–associated primary effusion lymphoma, and AIDS-related primary central nervous system lymphoma in vivo is also p53 dependent is of significant importance and warrants further investigations. In addition, it is possible that other human cancers, such as human papillomavirus (HPV)–associated cervical cancer or breast carcinomas, for which p53 remains wild type, may benefit from AZT/IFN therapy and deserve to be considered.
Prepublished online as Blood First Edition Paper, March 28, 2006; DOI 10.1182/blood-2006-01-0067.
Supported by grant R01CA106258 from the National Cancer Institute (C.N.).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal