Abstract
The Stroke Prevention Trial in Sickle Cell Anemia (STOP) was a randomized trial to evaluate whether chronic transfusion could prevent initial stroke in children with sickle-cell anemia at high risk as determined by transcranial Doppler (TCD). The trial demonstrated a large benefit of transfusion and was halted early. After termination of the trial, patients participated in a posttrial follow-up study. More patients in the transfusion group (70%) elected transfusion for primary stroke prevention compared with those on standard care (45%). Six patients with persistently abnormal TCD results developed stroke. A minority with initially abnormal TCD results remained stroke-free without transfusion. Except for lower baseline and follow-up TCD velocities compared with those with stroke, no predictive features of this apparent lower-risk subgroup could be determined. TCD results at last testing in 108 patients that did not have stroke were: normal (44.4%), conditional (26.9%), abnormal (22.2%), and inadequate (6.5%). Patients on transfusion were more likely to have normal TCD results. Transfusion resulted in iron overload and alloimmunization, but no infection. The study provides new information on acceptance rates and long-term effects of transfusion. Persistent TCD elevation signals ongoing stroke risk. Reduction in TCD results over time without transfusion is observed in some patients and requires further study.
Introduction
Stroke is a devastating complication of sickle-cell disease (SCD), particularly during childhood, with an estimated 11% of patients with homozygous SCD having a stroke by the age of 20 years.1 The use of transcranial Doppler (TCD) ultrasonography identifies children with SCD who are at high risk for stroke.2,3 The stratification of stroke risk by TCD served as the basis for the development and testing of a therapeutic strategy using blood transfusion for the primary prevention of stroke in a randomized multicenter trial initiated in 1995 called the Stroke Prevention Trial in Sickle Cell Anemia (STOP).4 The clinical trial demonstrated such a significant benefit of chronic red-cell transfusion in reducing the risk of a first stroke by 90%, that it was halted before its scheduled closure on the advice of the study's data and safety monitoring board. Based on these findings, the National Heart, Lung, and Blood Institute issued a Clinical Alert recommending TCD screening of children with SCD and consideration of chronic transfusion to prevent stroke for those who are identified to be at high risk for stroke.5
Despite its proven efficacy for primary prevention of stroke in SCD, chronic transfusion is not without associated costs, risks, and complications, including iron overload, alloimmunization, and transmission of bloodborne infectious pathogens. Consequently, the STOP investigators embarked on a second randomized clinical trial, STOP II (Optimizing Stroke Prevention in Sickle Cell Anemia), in 2000 to determine whether transfusion therapy could be safely withdrawn after at least 30 months of transfusions in patients who have converted to normal TCD velocities.6
In the interval between the early termination of the STOP trial in September 1997 and the initiation of the STOP II trial in June 2000, the STOP cohort was asked to participate in a posttrial follow-up study to allow further clinical observation of the patients and acquire extended TCD, magnetic resonance imaging (MRI), and magnetic resonance angiography (MRA) data. In this report, we describe the treatment choices of the STOP cohort after the clinical trial, provide extended analysis of the stroke rate, demonstrate the longitudinal changes in TCD velocities, and compare the transfusion experience during and after the trial. The MRI and MRA data were previously reported,7,8 and are excluded here.
Patients, materials, and methods
STOP trial
The design of the randomized trial is described in detail elsewhere.4 Briefly, 1934 children ages 2 to 16 years with sickle-cell anemia (Hb SS) or sickle β° thalassemia without a history of clinical stroke were screened by TCD at 14 STOP trial centers to determine the risk of stroke. Risk stratification of stroke by TCD was based on the time-averaged mean of the maximum velocity (TAMMvel) in the distal internal carotid artery or proximal middle cerebral artery, and interpreted as follows: “normal,” all mean velocities less than 170 cm/sec; “conditional,” at least 1 mean velocity of 170 to 199 cm/sec with none greater than or equal to 200 cm/sec; “abnormal,” at least 1 mean velocity of 200 cm/sec or higher; or “inadequate,” when no readings from the right- or left-middle cerebral artery and internal carotid artery could be obtained, unless 1 side is abnormal. To enter the study, each patient had to have abnormal results on 2 Doppler studies, not necessarily obtained consecutively. Of the 206 children who qualified for the study, 130 (60 boys and 70 girls) consented for enrollment and were randomly assigned to either receive transfusions to maintain hemoglobin S (Hb S) concentrations of less than 30% (transfusion [TX] group, 63 patients), or remain on standard supportive care with transfusion only when clinically indicated (standard care [SC] group, 67 patients). Blood transfusions were given as either simple or exchange transfusion, or a combination of both. The transfusion protocol required the use of leukocyte-reduced, sickle-negative packed red blood cells that were matched for ABO, C, D, E, and Kell antigens. At the time of enrollment, magnetic resonance imaging (MRI) of the brain, and in some cases, magnetic resonance angiography (MRA) was performed at baseline. TCD was performed annually during the study. The primary endpoint was clinical stroke as determined by a panel of neurologists blinded to the patient's treatment assignment who reviewed available clinical and imaging data.
The second interim analysis of the trial demonstrated a significantly increased rate of stroke in the SC group compared with the TX group, resulting in premature closure of the study 16 months before schedule.
Posttrial follow-up phase
After termination of the randomized trial, patients who had not developed stroke were notified of the results of the study and given the option to start, resume, or continue transfusion. Patients elected their choice of treatment between September and December of 1997. All patients were asked to participate in a posttrial follow-up study, with 3 exceptions: (1) a patient from the SC group who was lost to follow-up; (2) a patient from the SC group who crossed over to transfusion because of leg ulcer; and (3) a patient from the TX group who was initially randomized but later considered ineligible for the trial (only 1 abnormal TCD result). Approval of this study was obtained from the institutional review boards of the 14 STOP trial centers. Informed consent was obtained in accordance with the Declaration of Helsinki. Thus, 127 previously randomized patients were enrolled in the posttrial follow-up study from January 1998 to June 2000. Quarterly visits included medical history and progress reports, physical examinations, and blood examinations, including complete blood count (CBC) with differential and reticulocyte count, liver function tests, quantitative hemoglobin analyses, and serum ferritin measurements. Blood examinations for infection with Hepatitis B and C, and HIV and human T-cell leukemia virus 1 (HTLV-1) viruses were added at study exit. TCD examinations were offered every 6 months, and MRI and MRA studies were performed at study exit. A panel of experts who were unaware of the patients' treatment and condition interpreted the TCD, MRI, and MRA. Information on transfusion status with data of each transfusion visit, including method of transfusion, pretransfusion hemoglobin (Hb), hematocrit (Hct), percentage of Hb S, red-cell antibody identification, and adverse reactions related to transfusion were collected. Adherence to the blood product and delivery specifications required during the STOP trial was recommended. Any neurologic event was adjudicated by a panel of neurologists in a manner identical to that used during the STOP trial.
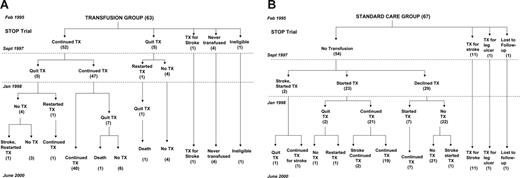
Treatment history. (A) Transfusion group. (B) Standard care group. Dates indicate study time points: February 1995 being the start of STOP Trial and September 1997, the end; January 1998 is the start of post-trial followup, and June 2000, its end. Numbers in parentheses represent the number of patients.
Treatment history. (A) Transfusion group. (B) Standard care group. Dates indicate study time points: February 1995 being the start of STOP Trial and September 1997, the end; January 1998 is the start of post-trial followup, and June 2000, its end. Numbers in parentheses represent the number of patients.
Statistical analysis
The probability of remaining stroke-free was analyzed by the Kaplan-Meier method based on the original treatment assignment and measured from the time of randomization to the earliest of stroke, change in treatment, or end of the follow-up study. Stroke risks in the 2 groups were compared by the log-rank test. Baseline characteristics and follow-up TCD of the remaining 25 patients on standard care who did not have stroke were compared with the 14 patients on standard care who had stroke by using Student t test for continuous variables and Fisher exact test for sex and presence or absence of MRI lesions. The same comparisons were made between these 25 patients and the rest of the STOP cohort (104 patients, after excluding the ineligible patient). Comparison of TCD velocity changes between the transfused and nontransfused groups was assessed using Student t test (for baseline TCD velocities) and Fisher exact test (for TCD classifications at last testing). Analyses of the transfusion data were made using percentages and mean values and standard deviations.
Results
Treatment history
Figure 1A (TX group) and 1B (SC group) illustrate the treatment received by the STOP cohort with subsequent changes during the follow-up study.
At the end of the clinical trial, 12 patients (1 from TX, 11 from SC) were on transfusion for stroke. The remaining 115 patients who had not developed stroke were offered transfusion.
From the TX group, 52 patients continuously received transfusion for primary stroke prevention during the STOP trial. Most (47 of 52) elected to continue transfusion after the trial, and 40 remained on transfusion throughout the follow-up phase. Two patients who had quit transfusion subsequently died of sepsis.
From the SC group, 2 patients developed stroke soon after closure of the trial and started transfusion (1 at 2 weeks and another at 10 weeks after the trial). Of 52 patients who were stroke-free after completion of the trial, initially 23 and subsequently another 7 began transfusion.
Stroke outcome
In addition to the 12 stroke events in the STOP trial, 6 patients developed stroke during the posttrial follow-up (5 from the SC group and 1 from the TX group). From the SC group, aside from the 2 patients who had stroke in the immediate posttrial period prior to choosing treatment, 1 patient who refused transfusion and 2 who started transfusion during the posttrial follow-up developed stroke. Of these 2 patients who went on transfusion, 1 was transfused suboptimally for 30 months with an average pretransfusion Hb S level of 45%. The other was transfused for 28 months with Hb S levels maintained at less than 30%. The TCD velocities while on transfusion remained abnormal in both cases. The only patient from the TX group who developed stroke had discontinued transfusion after the trial. Her TCD was normal while on transfusion but reverted to abnormal 6 months after stopping transfusion and 8 months before the stroke. All stroke events were ischemic infarctions. There were no deaths related to stroke. Table 1 describes the characteristics of these 6 patients who developed stroke during the posttrial follow-up.
Characteristics of the 6 patients who developed stroke during the posttrial follow-up
Pt no. . | Age, y . | Sex . | Trial SC/TX . | Posttrial TX/no TX . | Time from study entry to stroke, mo . | Maximum baseline TCD,*cm/s . | Last TCD before stroke, cm/s . | Baseline MRI . | Posttrial MRI . | Posttrial MRA . | Status after discharge . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | F | SC | No TX | 23 | 248 | 343; 1 mo BS | Positive | Worse | Normal | No symptoms |
| 2 | 8 | M | SC | No TX | 28 | 221 | 202; 1 mo BS | Negative | Negative | Not done | Unknown |
| 3 | 7 | M | SC | No TX | 31 | 252 | 202; 2 mo BS | Positive | Worse | Normal | Mild-moderate physical disability |
| 4 | 10 | F | TX | Stopped TX | 42 | 220 | 158 on TX; 221 8 mo BS | Positive | Worse | Normal | Symptoms but no physical disability |
| 5 | 10 | F | SC | TX | 42 | 293 | 254 27 mo BS; inadequate 11 mo BS | Positive | Worse | Worse | Symptoms but no physical disability |
| 6 | 11 | M | SC | TX | 43 | 304 | 222 14 mo BS; inadequate 9 mo BS | Positive | Worse | Worse | Symptoms but no physical disability |
Pt no. . | Age, y . | Sex . | Trial SC/TX . | Posttrial TX/no TX . | Time from study entry to stroke, mo . | Maximum baseline TCD,*cm/s . | Last TCD before stroke, cm/s . | Baseline MRI . | Posttrial MRI . | Posttrial MRA . | Status after discharge . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 9 | F | SC | No TX | 23 | 248 | 343; 1 mo BS | Positive | Worse | Normal | No symptoms |
| 2 | 8 | M | SC | No TX | 28 | 221 | 202; 1 mo BS | Negative | Negative | Not done | Unknown |
| 3 | 7 | M | SC | No TX | 31 | 252 | 202; 2 mo BS | Positive | Worse | Normal | Mild-moderate physical disability |
| 4 | 10 | F | TX | Stopped TX | 42 | 220 | 158 on TX; 221 8 mo BS | Positive | Worse | Normal | Symptoms but no physical disability |
| 5 | 10 | F | SC | TX | 42 | 293 | 254 27 mo BS; inadequate 11 mo BS | Positive | Worse | Worse | Symptoms but no physical disability |
| 6 | 11 | M | SC | TX | 43 | 304 | 222 14 mo BS; inadequate 9 mo BS | Positive | Worse | Worse | Symptoms but no physical disability |
BS indicates before stroke.
Values represent the highest time-averaged mean velocity in the middle cerebral or internal carotid artery during the confirmatory TCD before randomization.
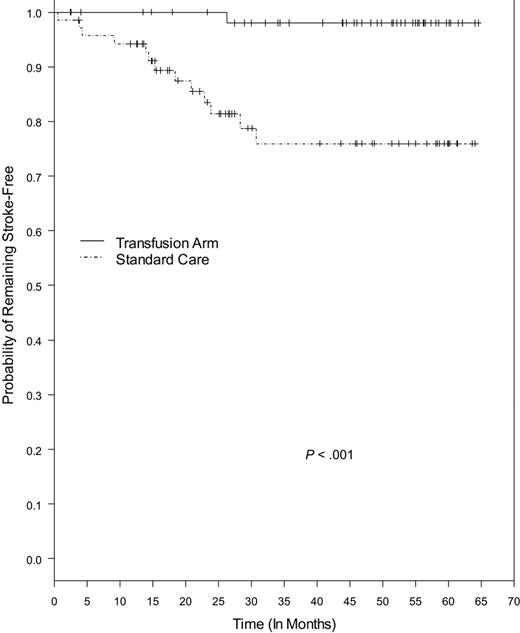
Kaplan-Meier estimates of remaining stroke-free in the STOP cohort with continued follow-up of about 65 months were significantly different between the SC and TX groups (P < .001), with stroke risk in the TX group 93% lower (Figure 2). Four stroke events were not included in this analysis. One was an intracerebral hemorrhage noted on baseline MRI that occurred before the patient's randomization to standard care, and 3 were stroke events that occurred after change in treatment during the posttrial follow-up (2 from SC started transfusion, 1 from TX stopped transfusion).
Stroke-free survival in the SC group appeared to plateau after about 30 months, with 25 patients remaining under observation. Most (17 of 25) had converted to normal or conditional TCD velocities, while 7 remained abnormal and 1 inadequate. The mean age of these patients at the end of follow-up was 13.3 years ± 2.82 years (median, 13.10 years; range, 8.38-20.41 years). Twelve patients received episodic transfusions over the course of observation for other clinical indications, such as severe anemia and presurgical preparation (average of 2 transfusions per patient; range, 1-4 transfusions).
Comparison of variables including age at randomization, sex, baseline laboratory values (CBC, reticulocyte count, hemoglobin F, ferritin, lactate dehydrogenase, and indirect bilirubin), and baseline MRI between these 25 patients and the 14 patients in the SC group who developed stroke did not differ significantly. The only variable that was different between the 2 groups was the TCD velocity, both at baseline (confirmatory TCD, P = .02; average of the 2 abnormal TCDs, P = .03; average of all TCDs within 12 months before randomization, P = .03) and at last examination (P < .001), with the velocity higher in the stroke group. However, analysis of the same variables did not identify any difference between these 25 and the rest of the STOP cohort.
Kaplan-Meier estimates of the probability of remaining stroke-free among patients receiving transfusion and patients on standard care. The P value was calculated by log-rank test. Tick marks indicate the lengths of observation of patients who did not have a stroke. Four patients were not included in this analysis: 1 patient from the standard care group who experienced intracerebral hematoma as noted on baseline MRI, and 3 patients who developed stroke after change in treatment during the posttrial follow-up (2 from the standard care and 1 from the transfusion group).
Kaplan-Meier estimates of the probability of remaining stroke-free among patients receiving transfusion and patients on standard care. The P value was calculated by log-rank test. Tick marks indicate the lengths of observation of patients who did not have a stroke. Four patients were not included in this analysis: 1 patient from the standard care group who experienced intracerebral hematoma as noted on baseline MRI, and 3 patients who developed stroke after change in treatment during the posttrial follow-up (2 from the standard care and 1 from the transfusion group).
TCD velocity changes
All 6 patients who developed stroke during the posttrial follow-up had a last interpretable TCD that was abnormal prior to stroke regardless of transfusion status.
Among 109 patients who did not have stroke, 108 had at least 1 TCD examination during the posttrial follow-up. For this analysis, only the result of the last TCD was considered when multiple TCD studies were available in a patient. Without regard to transfusion status at the time of TCD examination, results were normal in 48 (44.4%) patients, conditional in 29 (26.9%) patients, abnormal in 24 (22.2%) patients, and inadequate in 7 (6.5%) patients.
To determine whether transfusion induced the changes in TCD velocities, TCD results in 40 patients who received transfusions throughout the study were compared with 25 patients who were not on chronic transfusion. Average baseline TCD velocities in both groups were similar (transfused, 217.4 ± 24.9 cm/sec; nontransfused, 212.2 × 23.85 cm/sec; P = .42), as were the durations of observation (transfused, 54.58 ± 5.45 months; nontransfused, 55.44 ± 5.64 months). Results of the last TCD examinations were significantly different between the 2 groups (P = .008). Patients on transfusion were more likely to have normal velocities.
The results of the TCD examinations at last testing during the posttrial follow-up are shown in Table 2.
Results of TCD examinations at last testing during the posttrial follow-up study
. | . | No stroke . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| TCD . | Stroke . | All patients . | Transfused . | Not transfused . | Partially transfused . | |||
| Normal | 0 | 48 | 23 | 7 | 18 | |||
| Conditional | 0 | 29 | 10 | 10 | 9 | |||
| Abnormal | 4 | 24 | 2 | 7 | 15 | |||
| Inadequate | 2 | 7 | 5 | 1 | 1 | |||
| Total | 6 | 108 | 40 | 25 | 43 | |||
. | . | No stroke . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| TCD . | Stroke . | All patients . | Transfused . | Not transfused . | Partially transfused . | |||
| Normal | 0 | 48 | 23 | 7 | 18 | |||
| Conditional | 0 | 29 | 10 | 10 | 9 | |||
| Abnormal | 4 | 24 | 2 | 7 | 15 | |||
| Inadequate | 2 | 7 | 5 | 1 | 1 | |||
| Total | 6 | 108 | 40 | 25 | 43 | |||
Transfusion data
A number of patients (79) received transfusion, entirely or partially, for primary prevention of stroke during the posttrial follow-up. Transfusion data on 78 of these 79 patients were available for analysis and compared with the data from the STOP trial4,9 (Table 3). The overall transfusion practice in the posttrial period was similar to that in the clinical trial, except that more pretransfusion Hb S values exceeded the goal of less than 30% in the posttrial follow-up. However, the average percentage of Hb S per patient was still within the goal (27.5% ± 12.4%). Complications from transfusion were also similar except that iron overload progressed, with more patients on iron chelation therapy in the posttrial follow-up.
Comparison of the transfusion data from the STOP clinical trial and posttrial follow-up
Variable . | STOP trial* . | Posttrial follow-up . |
|---|---|---|
| No. of patients | 61 | 78 |
| No. of transfusions | 1521 | 2354 |
| No. of PRBC units | 1830 | 3512 |
| Methods of transfusion, % of total | ||
| Simple | 63 | 63 |
| Exchange, full or partial | 12 | 9 |
| Combination of both | 25 | 28 |
| Mean interval of transfusion, d | 25 ± 8 | 28 ± 10 |
| Pretransfusion Hb S > 30% | ||
| Total no. of episodes (%) | 143 (9) | 721 (31) |
| Hb S 30-34.9 (%) | 70 (4) | 287 (12) |
| Hb S 35-39.9 (%) | 31 (2) | 165 (7) |
| Hb S at least 40 (%) | 42 (3) | 269 (12) |
| Ferritin, mean ng/mL ± SD | ||
| Baseline | 164 ± 155 | 190 ± 284 |
| 12 mo | 1804 ± 773 | 1762 ± 786 |
| 24 mo | 2509 ± 974 | 2797 ± 1500 |
| 36 mo | — | 2955 ± 128 |
| 48 mo | — | 3089 ± 771 |
| No. patients on iron chelation | 8 | 43 |
| Immunization rate, % (no. patients with new antibody/unit of exposure) | 0.5 (10/1830) | 0.5 (18/3512) |
| No. patients with antibodies to C, D, E, Kell | 4 (E, Kell only) | 6 |
| No. patients with antibodies to other antigens | 3 | 6 |
| No. patients with warm autoantibodies | 3 | 6 |
| Other transfusion-adverse events | 19 | 13 |
| Exposure to infectious pathogens | ||
| Hepatitis B | None | None |
| Hepatitis C | None | None |
| HIV and HTLV-1 | None | None |
Variable . | STOP trial* . | Posttrial follow-up . |
|---|---|---|
| No. of patients | 61 | 78 |
| No. of transfusions | 1521 | 2354 |
| No. of PRBC units | 1830 | 3512 |
| Methods of transfusion, % of total | ||
| Simple | 63 | 63 |
| Exchange, full or partial | 12 | 9 |
| Combination of both | 25 | 28 |
| Mean interval of transfusion, d | 25 ± 8 | 28 ± 10 |
| Pretransfusion Hb S > 30% | ||
| Total no. of episodes (%) | 143 (9) | 721 (31) |
| Hb S 30-34.9 (%) | 70 (4) | 287 (12) |
| Hb S 35-39.9 (%) | 31 (2) | 165 (7) |
| Hb S at least 40 (%) | 42 (3) | 269 (12) |
| Ferritin, mean ng/mL ± SD | ||
| Baseline | 164 ± 155 | 190 ± 284 |
| 12 mo | 1804 ± 773 | 1762 ± 786 |
| 24 mo | 2509 ± 974 | 2797 ± 1500 |
| 36 mo | — | 2955 ± 128 |
| 48 mo | — | 3089 ± 771 |
| No. patients on iron chelation | 8 | 43 |
| Immunization rate, % (no. patients with new antibody/unit of exposure) | 0.5 (10/1830) | 0.5 (18/3512) |
| No. patients with antibodies to C, D, E, Kell | 4 (E, Kell only) | 6 |
| No. patients with antibodies to other antigens | 3 | 6 |
| No. patients with warm autoantibodies | 3 | 6 |
| Other transfusion-adverse events | 19 | 13 |
| Exposure to infectious pathogens | ||
| Hepatitis B | None | None |
| Hepatitis C | None | None |
| HIV and HTLV-1 | None | None |
Discussion
This extended analysis of the STOP trial further confirmed the reliability of TCD in identifying children with SCD at high risk for stroke and the efficacy of transfusion in reducing this risk. All 6 stroke events in the posttrial follow-up were predicted by abnormal TCD results, regardless of treatment status. In contrast, none of the patients with normal and conditional TCD results on follow-up developed stroke. Although the survival analyses could be underestimated because of exclusion of 4 stroke events, the difference in stroke risk between those who were and were not transfused was still highly significant, with markedly lower risk in the transfused group similar to the findings in the randomized trial.4
An interesting observation is the flattening of the stroke-free survival curve in the SC group after 30 months, suggesting that in a subgroup of patients the risk of stroke diminishes after some time even without transfusion. The 25 patients remaining in the SC group who did not develop stroke had lower baseline and follow-up TCD velocities compared with the group who developed stroke. Most patients had converted to normal or conditional TCD results at last examination during follow-up, perhaps explaining the decline in stroke risk. Stroke risk may vary among children with SCD who have abnormal TCD results. Those at higher risk could have stroke if not transfused, while certain patients at lower risk may remain stroke-free without transfusion, even in a few with prolonged abnormal TCD findings.
A variation in stroke risk was also observed in STOP II.6 A fraction (16) of 41 patients randomized to discontinue transfusion did not tolerate stopping transfusion and reverted to abnormal TCD results, with 2 patients developing stroke. Nine patients resumed transfusion, leaving 16 patients remaining with normal TCD results and without stroke off transfusion. The only indicator of the risk of reversion to abnormal TCD results or stroke was the baseline TCD result, similar to our data from STOP comparing patients in the SC group who did and did not develop stroke. The data confirm that stroke risk increases with TCD velocity.10 Unfortunately, our data from STOP and STOP II did not allow us to prospectively identify those patients whose screening Doppler studies were above the defined abnormal velocity of 200 cm/sec but were not destined to have stroke.
This follow-up study allowed an opportunity to observe longitudinal changes in TCD velocities. In a preliminary analysis of TCD velocity changes of the STOP cohort with a mean follow-up time of 13.3 ± 1.9 months, Olivieri et al11 reported that 60.5% of 38 patients on transfusion reached normal velocities, compared with only 4.7% of 42 patients in the SC group. In the posttrial follow-up, patients were followed over an extended period of about 55 months and a similar proportion (57.5% of 40 patients) normalized while on transfusion. Of the 67 patients in the SC group, only 25 remained on observation without transfusion and 7 converted to normal TCD results (10% of the SC group), suggesting that some patients may have only transiently abnormal TCD. Whether this reflects the poorly understood gradual decrease in stroke risk as age progresses into young adulthood is unclear. Our data may have overestimated the likelihood of TCD conversion to normal results without transfusion due to selection after those who had stroke were removed and those at highest risk were continued on transfusion. Some patients may have also been started on hydroxyurea, perhaps accounting for some decline in TCD velocities.12 Unfortunately, data on hydroxyurea therapy were not available.
Elevated cerebral blood-flow velocities in SCD are related to severe anemia, vessel stenosis, and cerebral vasodilatation caused by tissue hypoxia. Transfusion reduces cerebral blood flow velocities by correcting these abnormalities to some degree, and may explain the reduction in stroke risk.13,14 TCD provides a simple, inexpensive, and noninvasive means of monitoring these changes, and may aid in making therapeutic decisions regarding transfusion for stroke prevention in patients with sickle-cell anemia. Studies have reported using TCD monitoring during chronic transfusion for both primary and secondary stroke prevention and found that TCD results correlated well with MRA findings.15,16 Similar findings were observed by Abboud et al8 in the STOP cohort. Patients whose TCD results normalized during transfusion therapy were noted to have normal MRAs, while most of the patients with persistently abnormal TCDs had abnormal MRAs. STOP II was based on the hypothesis that the former patients were potential candidates for stopping transfusion; unfortunately, this did not prove to be the case. Discontinuation of transfusion after at least 30 months in patients who have converted to normal velocities was associated with high risk of reversion to abnormal and chance of stroke.6 Whether these patients might benefit from other therapy substituted for transfusion is unknown and worthy of study. The single institution experience of secondary stroke prevention using hydroxyurea and phlebotomy,17 if confirmed to be effective, may be an attractive alternative to chronic transfusion in the primary prevention of stroke; this approach has been explored in France.16 Those with persistently abnormal TCD results despite transfusion often have severe vasculopathy and are at much higher risk for stroke. Our report demonstrated in 2 patients that persistently abnormal TCD results while on transfusion is ominous and may predict the uncommon stroke that occurs on transfusion therapy.18 Such patients may potentially require alternative and more aggressive therapies such as surgical revascularization procedure19 or stem-cell transplantation.16,20
Although the efficacy of the STOP strategy for primary stroke prevention has been proven in a randomized clinical trial,4 and its effectiveness in reducing stroke incidence suggested in a statewide stroke incidence survey,21 the potential morbidity from long-term transfusion, particularly iron overload, has deterred its universal acceptance among clinicians and patients.22 Infectious complication was nonexistent, and alloimmunization from blood transfusion was prevented to a certain degree in the STOP trial and posttrial experience. Overall, only about 70% of patients from the original TX group and 45% from the SC group were on transfusion for primary prevention of stroke in the follow-up study. Some consider the 10% annual stroke risk apparent from STOP adequate to justify prolonged transfusion, while others do not. This issue remains controversial and is a matter of clinical judgment and patient preference. The extended data of the STOP study we present, coupled with the results of STOP II, provide additional valuable information for medical decision making and in counseling patients and families regarding treatment choices for primary stroke prevention.
Given the difficulties and risks of prolonged transfusion, alternative therapies of primary stroke prevention in SCD are desirable. The recent availability of an oral iron chelator may render prolonged transfusion more acceptable.23,24 Further improvement in risk stratification using TCD and/or other imaging modalities, or perhaps other clinical or genetic factors, could optimize future preventive therapies for stroke in SCD.25,26
Appendix
STOP Trial investigators and key contributors include the following: Miguel Abboud (Medical University of South Carolina, Charleston, SC), Robert J. Adams (Medical College of Georgia, Augusta, GA), Brian Berman (Rainbow Babies and Children's Hospital, Cleveland, OH), Duane R. Bonds (National Heart, Lung, and Blood Institute, Bethesda, MD), Donald Brambilla (New England Research Institutes, Watertown, MA), Elizabeth Carl (Medical College of Georgia, Augusta, GA), Catherine Driscoll (Children's National Medical Center, Washington, DC), Beatrice Files (East Carolina University School of Medicine, Greenville, NC), Dianne Gallagher (New England Research Institutes, Watertown, MA), Lewis Hsu (Emory University School of Medicine, Atlanta, GA), Ann Hurlet-Jensen (Columbia Presbyterian Medical Center, New York, NY), Anne Jones (Medical College of Georgia, Augusta, GA), Abdullah Kutlar (Medical College of Georgia, Augusta, GA), Virgil McKie (Medical College of Georgia, Augusta, GA), Scott Miller (SUNY-Downstate Medical Center/Kings County Hospital Center, Brooklyn, NY), Nancy Olivieri (The Hospital for Sick Children, Toronto, ON, Canada), Charles Pegelow (University of Miami School of Medicine, Miami, FL), E. Steve Roach (University of Southwestern Medical Center, Dallas, TX), Charles Scher (Tulane University Medical School, New Orleans, LA), Elliott Vichinsky (Children's Hospital Oakland, Oakland, CA), Myron Waclawiw (National Heart, Lung, and Blood Institute, Bethesda, MD), Winfred Wang (St Jude Children's Research Hospital, Memphis, TN), Gerald Woods (Children's Mercy Hospital, Kansas City, MO), Elizabeth Wright (New England Research Institutes, Watertown, MA), and Robert Zimmerman (Children's Hospital of Philadelphia, Philadelphia, PA).
Prepublished online as Blood First Edition Paper, April 6, 2006; DOI 10.1182/blood-2005-10-009506.
A complete list of the members of the STOPstudy group appears in the “Appendix.”
Supported by Cooperative Agreements (U10 HL 52193 and U10 HL 52016) with the National Heart, Lung, and Blood Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the entire STOP Investigative Team (members listed in “Appendix”), research coordinators, patients, and their families for their valuable contributions to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal