Comment on Jordanides et al, page 1370
The relationship between a drug and its transporter proteins is a crucial determinant of the drug's efficacy. Can imatinib mesylate “outsmart” an important efflux protein that could potentially pump it out of the leukemic cell?
In this issue of Blood, Jordanides and colleagues bring up a new chapter in the polemic story of resistance to imatinib mesylate. This exquisitely effective ATP competitor, which inhibits the deregulated tyrosine kinase encoded by the BCR-ABL gene, has revolutionized the treatment of chronic myeloid leukemia (CML). Maybe even more important, this drug and its mechanism of action have become the paradigm for the development of similar small molecules to target oncoproteins responsible for the origin of other leukemias and solid tumors. Unfortunately, as with any other drug, imatinib does not fully control the disease in every patient. Furthermore, a proportion of those patients who exhibit a significant response to imatinib relapse during the course of treatment. The main mechanisms of such secondary resistance are related to the selection of leukemic subclones carrying additional copies of the BCR-ABL gene, thus producing larger amounts of the target Bcr-Abl protein or, more frequently, clones with a mutant Bcr-Abl where amino acid residues essential for a correct “fit” of the inhibitor molecule to the tyrosine kinase pocket are changed by DNA point mutations. However, neither of these 2 Bcr-Abl–dependent mechanisms underlies the phenomenon of primary resistance, which is a failure to achieve defined levels of hematologic, cytogenetic, or molecular responses within prescribed periods of time after initiation of treatment.1 It appears that biologic abnormalities other than those dictated by the direct effects of the oncogenic fusion protein prevent a complete elimination of the leukemic progenitors by imatinib.FIG1
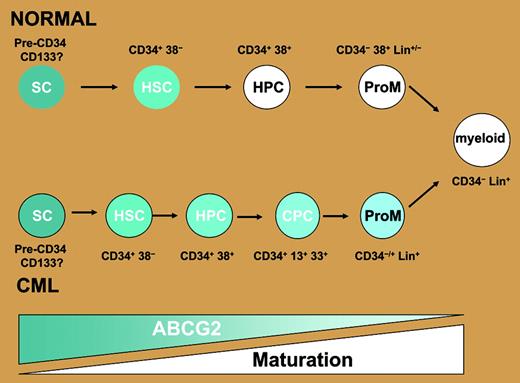
ABCG2 is overexpressed in CML cells. ABCG2 is normally highly expressed in CD34+ CD38– human hematopoietic stem cells and switched off in most maturing lineages including granulocyte/monocytes. The data from Jordanides et al's paper suggest that in CML, the expression of this transporter persists further down the maturation spectrum, such that it may be found on the majority of CML CD34+ cells, including those beyond the CD34+ CD38– stage, and maybe even those that coexpress promyeloid markers including CD13 and CD33. Thus their findings are consistent with the possibility that a greater proportion of cells have the active transporter rather than the per-cell level of ABCG2 being elevated. SC indicates multipotent stem cell/hemangioblast; HSC, hematopoietic stem cell; HPC, hematopoietic progenitor; CPC, CML progenitor; and ProM, promyeloid. Blue shading indicates ABCG2 expression levels. Diagram kindly provided by Dr J. Mountford, Royal Infirmary, Glasgow, United Kingdom.
ABCG2 is overexpressed in CML cells. ABCG2 is normally highly expressed in CD34+ CD38– human hematopoietic stem cells and switched off in most maturing lineages including granulocyte/monocytes. The data from Jordanides et al's paper suggest that in CML, the expression of this transporter persists further down the maturation spectrum, such that it may be found on the majority of CML CD34+ cells, including those beyond the CD34+ CD38– stage, and maybe even those that coexpress promyeloid markers including CD13 and CD33. Thus their findings are consistent with the possibility that a greater proportion of cells have the active transporter rather than the per-cell level of ABCG2 being elevated. SC indicates multipotent stem cell/hemangioblast; HSC, hematopoietic stem cell; HPC, hematopoietic progenitor; CPC, CML progenitor; and ProM, promyeloid. Blue shading indicates ABCG2 expression levels. Diagram kindly provided by Dr J. Mountford, Royal Infirmary, Glasgow, United Kingdom.
The search for the causes of primary resistance to imatinib has therefore intensified over the past few years, with a special focus on the possible role of drug transporters. These proteins are key determinants of intracellular drug concentrations, as they actively regulate the traffic of small molecules across the cell membrane. Thus, a cell may be “resistant” to a drug because not much of this drug remains inside it for a significant effect to be achieved. The problem may be due to either defective uptake or influx and/or, conversely, excessive and rapid pumping out or efflux. Resistance to imatinib has been linked to both mechanisms. Thus, the human organic cation transporter 1 (hOCT1) actively transports imatinib into cells, and low expression of hOCT1 has been detected before treatment in cells from patients who failed to achieve a good cytogenetic response.2 Likewise, imatinib is also a substrate of the ABCB1 pump glycoprotein encoded by the MDR1 gene, the overexpression of which leads to excessive efflux and cellular resistance to imatinib.3,4 The relationship between imatinib and ABCG2 (BRCP), another efflux protein of the ATP binding cassette family, is, however, less clear, and the source of much controversy lately. At least 4 independent groups have investigated this issue, reaching somewhat different conclusions. Thus, Houghton et al5 reported that imatinib is an inhibitor but not a substrate of ABCG2, whereas Burger et al6 concluded otherwise when demonstrating that ABCG2-overexpressing cell lines are resistant to imatinib. Recently, Nakanishi et al7 showed that the interaction between ABCG2 and imatinib in BCR-ABL–expressing cells is in fact rather complex, with ABCG2-mediated resistance to imatinib being counteracted by the inhibitor's capacity to down-modulate ABCG2 expression via the AKT signaling pathway. A crucial limitation of all 3 studies is the fact that they are based on cell lines engineered to overexpress ABCG2, and do not therefore represent the true clinical targets of imatinib (ie, primary cells from CML patients). Jordanides and colleagues now address this specific question. They show that a large proportion of CD34+ cells from patients at diagnosis of CML express significant amounts of ABCG2 protein, unlike their normal counterparts (see figure). Furthermore, by measuring the accumulation of imatinib in these CML progenitor CD34+ cells, they provide evidence that, as suggested by Houghton et al,5 imatinib is an inhibitor but not a substrate of ABCG2, and does not therefore mediate resistance in these cells. Although rather compelling in their own right, the data still leave room for some questions. Thus, the use of 5 μM imatinib for inhibiting ABCG2-mediated substrate efflux in CML cells may not be clinically relevant. Likewise, such a high dose may be “saturating,” blocking any possible additional effect of fumitremorgin C, a specific ABCG2 inhibitor, and obscuring therefore the possibility that imatinib could be also a substrate of ABCG2. The recent emergence of second-generation Abl kinase inhibitors such as nilotinib (AMN107) and dasatinib (BMS345825) will no doubt fuel the interest in drug transporters and their role in controlling the cellular bioavailability of these small molecules in the targeted therapy of CML. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal