Abstract
Newborns are prone to microbial infection and have poor memory responses to multiple antigens. We have previously shown that human neonatal blood monocytes exhibit impaired TNF-α responses to most known TLR agonists, including the pure TLR7 agonist imiquimod. Surprisingly, however, neonatal TNF-α responses to the imiquimod congener R-848 (TLR 7/8) were fully intact. We now show that TLR8 agonists, including R-848 (TLR7/8), the imidazoquinoline congeners 3M-003 (TLR7/8) and 3M-002 (TLR8), as well as single-stranded viral RNAs (TLR8) induced robust production of the Th1-polarizing cytokines TNF-α and IL-12 from neonatal antigen-presenting cells (APCs) that substantially exceeds responses induced by TLR-2, -4, or -7 (alone) agonists. TLR8 agonists also effectively induced up-regulation of the costimulatory molecule CD40 on neonatal and adult myeloid dendritic cells (DCs). The strong activity of TLR8 agonists correlates with their induction of p38 MAP kinase phosphorylation and with degradation of IκB-α in both neonatal and adult monocytes. We conclude that TLR8 agonists are uniquely efficacious in activating costimulatory responses in neonatal APCs and suggest that these agents are promising candidate adjuvants for enhancing immune responses in human newborns.
Introduction
Human newborns suffer a high frequency of microbial infection resulting in morbidity and mortality.1,2 Neonatal immunization is desirable because newborns are susceptible to multiple bacterial and viral pathogens, strategies involving immunization of the mother pose substantial logistic and medicolegal challenges, and birth represents a reliable point of health care contact and therefore newborn immunization results in a substantially higher rate of vaccination coverage.3 Unfortunately, many vaccines are ineffective in newborns, thereby frustrating immunization efforts in this susceptible population.4 Thus, there is an unmet medical need to develop improved vaccination approaches to prevent neonatal infection.
The poor responses of newborns to multiple vaccines have been traditionally ascribed to “immaturity” of the neonatal immune system. A more nuanced view is emerging because it is increasingly recognized that expression of T helper cell type 1 (Th1) immunity is impaired at birth, and there is a strong T helper cell type 2 (Th2) bias to many aspects of the neonatal immune response.5 Th2 polarization of the neonatal immune response is thought to protect the fetus from potentially harmful inflammatory reactions that could trigger premature delivery and its attendant complications.6,7 However, such Th2 polarization biases against the Th1-type immune responses required for effective cell-mediated immunity and for effective immune responses to some vaccines. Some complex stimuli such as bacillus Calmette-Guérin (BCG) effectively induce Th1-type immune responses in neonates,8 but the molecular rules governing why a particular stimulus does or does not stimulate neonatal Th1 immunity are not well understood. Efforts to prevent and treat neonatal infections are therefore limited by incomplete understanding of the neonatal immune response, and, in particular, mechanisms by which the neonatal adaptive immune system may be instructed to generate Th1-type responses.
Generation of Th1-type immune responses, including memory responses to many vaccines, is dependent on appropriate activation of antigen-presenting cells (APCs), including macrophages and dendritic cells (DCs) that function at the interface of innate and adaptive immunity.9 APCs take up foreign antigens, digest them, and display antigen fragments on the cell surface in the context of major histocompatibility complex (MHC) molecules, thereby presenting them to antigen-specific T cells. Among the APCs, DCs are the most efficient activators of naive T cells both in vitro and in vivo. In particular, myeloid DCs (mDCs) capture antigens in the periphery, then migrate to the lymphoid organs to initiate immunity10 and are mobilized to sites of viral entry during viral infection.11 Effective APC-induced activation of T cells requires the expression by APCs of costimulatory molecules, including (1) IL-12, a soluble heterodimer (p40-p35) that activates antigen-specific CD4 lymphocytes,12 and (2) CD40, a cell surface receptor that binds CD40 ligand on T cells, resulting in activation of kinase cascades culminating in NF-κB activation and consequent regulation of DC activity as well as T-cell priming and differentiation.13,14 In general, the functional responses of neonatal APCs, including synthesis of IL-1215 and up-regulation of CD4016 are limited.
APCs express TLRs that act as homodimers or heterodimers to recognize and respond to a wide variety of microbial components.17 TLR-mediated activation of APCs triggers expression of costimulatory molecules necessary for effective T-cell activation and subsequent memory responses.18 Of note, TLR7 and TLR8 are members of a genetically and structurally related subfamily of TLRs19,20 that have evolved to recognize unmodified RNAs.21 Guanosine- and uridine-rich single-stranded RNAs (ssRNAs) derived from HIV-1 activate murine cells via TLR7 and human cells via TLR8.22 In addition, small synthetic compounds known as imidazoquinolines, including imiquimod and R-848, activate cells of galliform birds23 and of mice24 via TLR7, whereas R-848 activates human cells via TLR8, as well.24-26 Mirroring the TLR specificity of ssRNAs and imidazoquinolines, wild-type influenza virus triggers innate immune responses in mice via TLR727 and coxsackie B virus activates human cardiac inflammatory responses via TLR8.28 TLRs 7 and 8 are localized to endosomes and transduce signals via MyD8824 and Bruton tyrosine kinase,29 that activate kinase cascades culminating in phosphorylation and degradation of IκB-α, nuclear translocation of NF-κB, and consequent activation of innate immune response genes. In humans, TLR7 is expressed in plasmacytoid DCs and B cells,30 whereas TLR8 is abundant in monocytes, monocyte-derived DCs, and mDCs.26
The ability of TLR agonists to activate APC costimulatory pathways is the basis for the adjuvant activity of several microbial products, including monophosphoryl lipid A (MPL), a congener of LPS that activates cells via TLR4, is a potent adjuvant in adults, and is currently used as an adjuvant component of some vaccines.31 Given the importance of TLR function in activation of APCs and the inability of many stimuli to adequately activate neonatal APCs, characterization of the function of the neonatal TLR system is of great interest. We have recently discovered a marked 2- to 3-log impairment in synthesis of the proinflammatory and Th1-polarizing cytokine TNF-α from human neonatal blood monocytes in response to an array of TLR agonists.32 Our previous study revealed that despite normal basal expression of TLRs and related signaling components, responses of newborn cord blood monocytes to multiple TLR agonists, including agonists of TLR2 (bacterial lipopeptides [BLPs]), TLR4 (LPS), and TLR7 (imiquimod), are impaired such that little of the Th1-polarizing proinflammatory cytokine TNF-α is synthesized.32 Impaired neonatal monocyte responses to multiple TLR agonists were due to differences in soluble components of neonatal blood plasma as compared to that of adults. In marked contrast, however, among the array of TLR agonists assessed in that study, only R-848, an imidazoquinoline compound that activates cells via TLR 7/8, fully activated TNF-α production from neonatal monocytes.
Based on the efficacy of the TLR 7/8 agonist R-848, but not the TLR7 agonist imiquimod, to activate neonatal monocytes to produce the Th1-polarizing cytokine TNF-α,32 we posited that TLR8 agonists may have unique efficacy in activating neonatal APCs. We therefore studied the effects of TLR7, TLR7/8, and TLR8 agonists in activating neonatal APCs with respect to both expression of Th1-polarizing cytokines and costimulatory molecules as well as their activation of intracellular signaling cascades. We find that TLR8 agonists have unique efficacy in activating expression of Th1-polarizing cytokines and costimulatory molecules in neonatal APCs, which mirrors their efficacy in activating TLR-triggered intracellular signaling cascades. These properties suggest that TLR8 agonists may fulfill a unique role as potential adjuvants for neonatal vaccines.
Materials and methods
Blood
Peripheral blood was collected from healthy adult volunteers (n = 33; mean age, 27 years) and newborn cord blood (n = 33; mean gestational age, 38.4 weeks) collected immediately after cesarean section delivery (epidural anesthesia) of the placenta. Births at which antibiotics were administered during labor or delivery and births to HIV+ mothers were excluded. Human experimentation guidelines of the US Department of Health and Human Services, the Brigham and Women's Hospital, and Children's Hospital Boston were observed, following protocols approved by the local institutional review boards. Volunteer blood donors provided informed consent in accordance with the Declaration of Helsinki. Blood was anticoagulated with 109 mM sodium citrate (BD Vacutainer, Becton Dickinson, Franklin Lakes, NJ) or 15 U/mL heparin sodium (American Pharmaceutical Partners, Schaumberg, IL).
Isolation of mononuclear cells and monocytes
Heparinized blood was layered onto Ficoll-Hypaque gradients (Sigma-Aldrich, St Louis, MO), the peripheral blood mononuclear cell (PBMC) layer collected, and subjected to hypotonic lysis to remove red blood cells as previously described.32 Monocytes were isolated from PBMCs by negative selection using a cocktail of biotin-conjugated monoclonal antibodies (mAbs) directed against CD3, CD7, CD16, CD19, CD56, CD123, and glycophorin A as well as antibiotin-conjugated magnetic microbeads according to the manufacturer's instructions (Monocyte Isolation Kit II; Miltenyi Biotec, Auburn, CA). This procedure routinely yielded monocyte preparations with purity of about 90% as assessed by measuring CD14 staining by flow cytometry as previously described.32
TLR agonists
TLR agonists used in this study are listed in Table 1. The synthetic diacylated BLP macrophage-activating lipopeptide-2 (MALP; S-(2,3-bisAcyloxypropyl)-cysteine-GNNDESNISFKEK; Alexis Biochemicals, Lausen, Switzerland) is based on a BLP of Mycoplasma fermentans. Ultrapure Re 595 LPS from Salmonella minnesota was from List Biologicals (Campbell, CA). Imidazoquinolines included imiquimod (TLR7; Sequoia Research Products, Oxford, United Kingdom), R-848 (TLR7/8; InvivoGen, Carlsbad, CA), as well as 3M-002 (TLR8), 3M-003 (TLR7/8), and 3M-013 (TLR7; 3M Pharmaceuticals, St Paul, MN) and were prepared in dimethyl sulfoxide (MP Biomedicals, Aurora, OH) or 20% ethanol (EM Science, Bibbstown, NJ). DMSO and ethanol controls were included for each type of experiment to verify that at the final concentrations present in the assay, these solvents did not affect TLR-induced cell activation. Single-stranded RNAs included single-stranded poly-uridine (ssPolyU) and ssRNA40 (20-mer phosphothioate-protected, containing a GU-rich sequence) both commercially prepared as cationic lipid complexes to facilitate cellular uptake (InvivoGen). Specificity of imidazoquinolone congeners, including the novel TLR7 agonist 3M-013, was determined using HEK293 cells and an NF-κB-luciferase reporter (BD Clontech, Palo Alto, CA) as previously described.26
TLR agonists used in this study
Agonist . | TLR . | Derivation . | Source . | Comment . | Reference . |
|---|---|---|---|---|---|
| MALP | TLR2/6 | Mycoplasma fermentans | Alexis Biochemicals | — | Galanos et al33 |
| LPS | TLR4 | Salmonella minnesota R595 (Re) | List Biological Laboratories | Ultrapure | Hoshino et al34 |
| Imiquimod | TLR7 | Imidazoquinoline | Sequoia | Aldara antiviral cream | Hemmi et al24 |
| 3M-013 | TLR7 | Imidazoquinoline | 3M Pharmaceuticals | Novel compound | — |
| 3M-003 | TLR7/8 | Imidazoquinoline | 3M Pharmaceuticals | — | Gorden et al26 |
| R-848 | TLR7/8 | Imidazoquinoline | InvivoGen | Resiquimod | Jurk et al35 |
| 3M-002 | TLR8 | Imidazoquinoline | 3M Pharmaceuticals | — | Gorden et al26 |
| ssRNA polyU | TLR8 | Synthetic polyuridine sequence | InvivoGen | Complexed to cationic lipid to facilitate uptake | Hell et al22 |
| ssRNA40 | TLR8 | Synthetic GU-rich sequence based on U5 region of HIV | InvivoGen | Complexed to cationic lipid to facilitate uptake | Heil et al22 |
Agonist . | TLR . | Derivation . | Source . | Comment . | Reference . |
|---|---|---|---|---|---|
| MALP | TLR2/6 | Mycoplasma fermentans | Alexis Biochemicals | — | Galanos et al33 |
| LPS | TLR4 | Salmonella minnesota R595 (Re) | List Biological Laboratories | Ultrapure | Hoshino et al34 |
| Imiquimod | TLR7 | Imidazoquinoline | Sequoia | Aldara antiviral cream | Hemmi et al24 |
| 3M-013 | TLR7 | Imidazoquinoline | 3M Pharmaceuticals | Novel compound | — |
| 3M-003 | TLR7/8 | Imidazoquinoline | 3M Pharmaceuticals | — | Gorden et al26 |
| R-848 | TLR7/8 | Imidazoquinoline | InvivoGen | Resiquimod | Jurk et al35 |
| 3M-002 | TLR8 | Imidazoquinoline | 3M Pharmaceuticals | — | Gorden et al26 |
| ssRNA polyU | TLR8 | Synthetic polyuridine sequence | InvivoGen | Complexed to cationic lipid to facilitate uptake | Hell et al22 |
| ssRNA40 | TLR8 | Synthetic GU-rich sequence based on U5 region of HIV | InvivoGen | Complexed to cationic lipid to facilitate uptake | Heil et al22 |
— indicates none.
For study of TLR-induced responses in suspension, cells (PBMCs at 106/mL or monocytes at 3 × 106/mL) were cultured in 100% fresh autologous serum and stimulated with end-over-end rotation at 37°C. For study of TLR-induced IL-12 production, monocytes were prepared by allowing PBMCs to adhere onto 24-well plates for 1 hour at 37°C/5% CO2. Nonadherent cells were removed with 2 washes of minimal essential medium (MEM; Gibco BRL, Carlsbad, CA) and adherent monocytes incubated in the presence of 10% autologous serum in MEM for 48 hours.
Cytokine measurement
Concentrations of TNF-α and IL-12 were determined by dilution of samples with 5 or 2 volumes, respectively, of ice-cold RPMI medium (Gibco BRL) and centrifugation at 1000g at 4°C for 5 minutes. The supernatant was recovered and stored at –20°C until enzyme-linked immunosorbent assay (ELISA) for TNF-α (R&D Systems, Minneapolis, MN) or IL-12 (p40/p70; GE Healthcare, Piscataway, NJ).
Identification of mDCs and measurement of CD40 expression
To assess the up-regulation of CD40 expression on mDCs, whole blood was anticoagulated with heparin (15 U/mL) and incubated for 19 hours in the presence of TLR agonists, then stained with lineage cocktail 1 (FITC-conjugated mAbs to CD3, CD14, CD16, CD19, CD20, and CD56), HLA-DR (PerCP), CD11c (APC), and CD40 (PE) or isotype (all antibodies, BD Biosciences, San Jose, CA) and incubated for 30 minutes. Cells were treated with FACS Lyse (BD Biosciences) per the manufacturer's instructions, washed, and filtered for flow cytometric analysis using a MoFlo cytometer (DakoCytomation, Fort Collins, CO) with 488-nm and 635-nm lasers. Fifty thousand events (excluding debris) were recorded per sample. Data were analyzed with Summit version 3.1 software (DakoCytomation). mDCs were located by gating on lin1–/HLA-DR+/CD11c+ cells.
Measurement of TLR8 expression
For measurement of TLR8 expression by flow cytometry, whole blood was processed as for CD40 measurement, using markers for monocytes and for mDCs as described. APCs were stained for TLR8 (PE; Imgenex, San Diego, CA) after permeabilizing with Perm2 Buffer (BD Biosciences). A TLR8 expression index was calculated within cell populations as a product of (median fluorescent intensity of mDCs) × (% of mDCs positive for TLR8).
Phosphorylation of p38 MAPK
Following incubation of heparinized whole blood with TLR agonists for 10 minutes at 37°C, cells were fixed using PhosFlow Lyse/Fix Buffer (BD Biosciences), then washed and stained with CD14 (FITC) or isotype to identify monocytes. After washing, cells were permeabilized (PhosFlow Perm Buffer 2; BD Biosciences), washed (PhosFlow Stain Buffer; BD Biosciences), then stained with a PE-conjugated phospho-specific mAb against pp38 (BD Biosciences) or isotype control. The amount of pp38 was calculated as an expression index equal to the product of median fluorescent intensity and the percentage of cells positive for pp38.
IκB-α Western blot
Neonatal and adult monocytes were cultured in 100% fresh autologous serum (2.5 × 106 cells/mL) and stimulated in a total volume of 200 μL with 50 μM 3M-013 (TLR7) or 3M-002 (TLR8) for 10, 30, or 60 minutes at 37°C, washed, and lysed in NuPAGE LDS sample buffer containing NuPAGE reducing agent (Invitrogen, Carlsbad, CA), then boiled for 5 minutes and centrifuged at 1000g. Samples were separated on Novex Bis-Tris gels (Invitrogen) and transferred onto PVDF membranes (Invitrogen) as directed. Membranes were blocked in buffer containing 3% BSA, then probed with 1° antibody IκBα/MAD-3 (BD Biosciences Pharmingen, San Jose, CA) and a peroxidase-conjugated 2° antibody (AffiniPure goat anti–mouse IgG; Jackson ImmunoResearch, West Grove, PA). Quantitative analysis was performed using the software program Image 1.33u (National Institutes of Health, Bethesda, MD). After creating a profile of image density for the blot area corresponding to the protein size of interest, IκB-α levels were determined by measuring the area under each band peak. As a loading control, actin was stained using an anti–human actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis
Data were analyzed using Prism 4 for MacIntosh version 4.0a (GraphPad Software, San Diego, CA). Data in figures represent means ± SEM. The Mann-Whitney test was used for statistical analysis for all paired comparisons and the Spearman test was used to determine the significance of the correlation of p38 phosphorylation with TNF-α production. P < .05 was considered significant.
Results
TLR8 agonists preferentially induce robust TNF-α production in both neonatal and adult blood
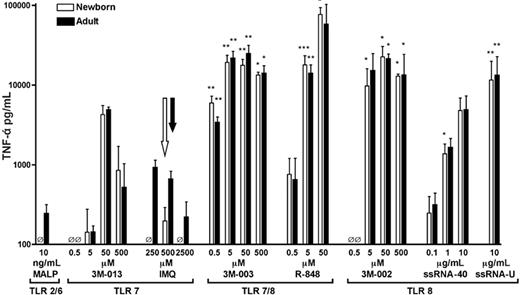
We have previously shown that R-848 (TLR7/8) effectively induced TNF-α production from neonatal monocytes, whereas imiquimod (TLR7) did not,32 raising the possibility that agents that signal via TLR8 possess unique efficacy in inducing TNF-α from neonatal monocytes. To assess the specificity of TLR agonists in neonatal TNF-α induction, we compared the ability of a panel of TLR agonists, including those that activate via TLR7, TLR 7/8, or TLR8 only, to induce TNF-α production in citrated peripheral adult blood or neonatal cord blood (Figure 1). Agonists of TLR2/6 (MALP) and of TLR7 (3M-013 and imiquimod) induced limited amounts of TNF-α (< 10 000 pg/mL). In contrast, 3M-003 and R-848, both agonists of TLR7 and TLR8, induced substantial TNF-α production reaching 20 000 to 80 000 pg/mL. Similarly, agonists that signal via TLR8 alone, including 3M-002, ssRNA40, and ssRNA polyU, induced substantial TNF-α production that was equivalent in neonatal and adult blood. These results suggest that signaling via TLR8 (with or without TLR7) results in generally greater TNF-α induction and a relatively greater activation of neonatal cells than activation of other TLRs.
Preferential induction of TNF-α by TLR8 agonists. TNF-α production from newborn cord blood (□) and adult peripheral blood (▪) was measured after a 5-hour incubation with TLR agonists. Extracellular medium was collected for TNF-α ELISA. Data represent means ± SEM. Statistical comparisons of TLR agonists were made within each group (ie, newborns analyzed separately from adults) in relation to responses to 500 μM imiquimod (TLR7; open arrow, newborns; filled arrow, adults). n = 2-9; *P < .05, **P < .01, ***P < .001. For comparisons of TNF-α production induced by TLR8 or TLR7/8 agonists with that induced by each TLR7 agonist at every dose, see Table S1 (available at the Blood website; see the Supplemental Tables link at the top of the online article).
Preferential induction of TNF-α by TLR8 agonists. TNF-α production from newborn cord blood (□) and adult peripheral blood (▪) was measured after a 5-hour incubation with TLR agonists. Extracellular medium was collected for TNF-α ELISA. Data represent means ± SEM. Statistical comparisons of TLR agonists were made within each group (ie, newborns analyzed separately from adults) in relation to responses to 500 μM imiquimod (TLR7; open arrow, newborns; filled arrow, adults). n = 2-9; *P < .05, **P < .01, ***P < .001. For comparisons of TNF-α production induced by TLR8 or TLR7/8 agonists with that induced by each TLR7 agonist at every dose, see Table S1 (available at the Blood website; see the Supplemental Tables link at the top of the online article).
A similar pattern of neonatal and adult TNF-α production, with relatively high TLR8-induced TNF-α production, was noted using heparin (instead of citrate) as anticoagulant, indicating that the pattern is independent of the means of anticoagulation (not shown).
TLR8 agonists fully activate neonatal and adult mononuclear cells
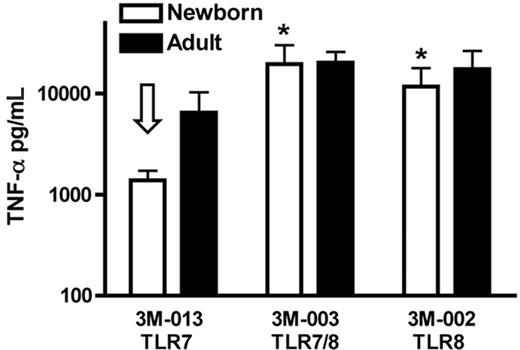
To determine whether the preferential efficacy of TLR8 agonists noted in whole blood extended to assays with isolated mononuclear cells, we compared the ability of TLR 7, TLR 7/8, and TLR8 agonists to activate neonatal and adult mononuclear cells in 100% autologous serum (Figure 2). Whereas the TLR7 agonist 3M-013 induced lower TNF-α in newborn than adult cells, 3M-003 (TLR7/8) and 3M-002 (TLR8) induced robust TNF-α in both newborn and adult PBMCs. In addition to confirming the overall pattern of TLR responses noted in whole blood, these data also indicate that the pattern of preferential responsiveness of neonatal PBMCs to TLR8 agonists is independent of the presence of other blood cells (eg, red blood cells and neutrophils) and is manifest in serum as well as plasma.
TLR8 agonists effectively induce IL-12 synthesis by adherent neonatal and adult monocytes
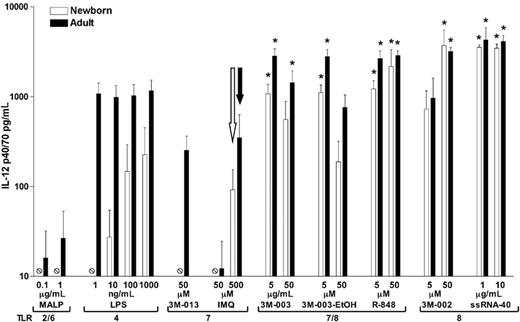
IL-12 is a heterodimeric cytokine that is crucial for driving Th1-type responses and cell-mediated immunity.12 To determine whether the efficacy of TLR8 agonists toward neonatal APCs extends to induction of IL-12, we compared the ability of MALP (TLR2/6) and LPS (TLR4) with that of TLR7, TLR 7/8, and TLR8 agonists to induce IL-12 p40/70 production from adherent monocytes derived from neonates and adults (Figure 3). MALP failed to induce detectable neonatal IL-12 synthesis, and LPS preferentially induced IL-12 in adult cells. Similarly, TLR7 agonists (3M-013 and imiquimod) induced modest IL-12 production (≤ 350 pg/mL) in adults and were even less active in newborns. In marked contrast, agonists of TLR 7/8 (3M-003 and R-848) as well as TLR8 (3M-002, ssRNA polyU, and ssRNA40) induced robust peak values of IL-12 production (> 1000 pg/mL) from both newborn and adult monocytes.
TLR8 agonists, but not a TLR7 agonist, induce robust TNF-α production from neonatal PBMCs. TLR agonists (50 μM) were added to PBMCs cultured in 100% fresh autologous serum (106 cells/mL) and incubated for 5 hours prior to measurement of TNF-α by ELISA. Data represent means ± SEM. Comparison is made to 3M-013 (TLR7)–induced neonatal TNF-α production (open arrow). n = 3; *P < .05.
TLR8 agonists, but not a TLR7 agonist, induce robust TNF-α production from neonatal PBMCs. TLR agonists (50 μM) were added to PBMCs cultured in 100% fresh autologous serum (106 cells/mL) and incubated for 5 hours prior to measurement of TNF-α by ELISA. Data represent means ± SEM. Comparison is made to 3M-013 (TLR7)–induced neonatal TNF-α production (open arrow). n = 3; *P < .05.
TLR8 agonists induce robust IL-12 p40/70 production from adherent monocytes. PBMCs were allowed to adhere onto plastic wells (1.5 × 106 PBMCs/well) and cultured in 10% fresh autologous serum prior to addition of TLR agonists and incubation at 37°C/5% CO2 for 48 hours. IL-12 p40/70 production was measured in culture supernatants by ELISA. Data represent means ± SEM. Statistical comparisons were made within each group in relation to 500 μM of the TLR7 agonist imiquimod (open arrow, newborns; filled arrow, adults). n = 4; *P < .05.
TLR8 agonists induce robust IL-12 p40/70 production from adherent monocytes. PBMCs were allowed to adhere onto plastic wells (1.5 × 106 PBMCs/well) and cultured in 10% fresh autologous serum prior to addition of TLR agonists and incubation at 37°C/5% CO2 for 48 hours. IL-12 p40/70 production was measured in culture supernatants by ELISA. Data represent means ± SEM. Statistical comparisons were made within each group in relation to 500 μM of the TLR7 agonist imiquimod (open arrow, newborns; filled arrow, adults). n = 4; *P < .05.
TLR8 agonists preferentially induce CD40 expression by neonatal and adult mDCs
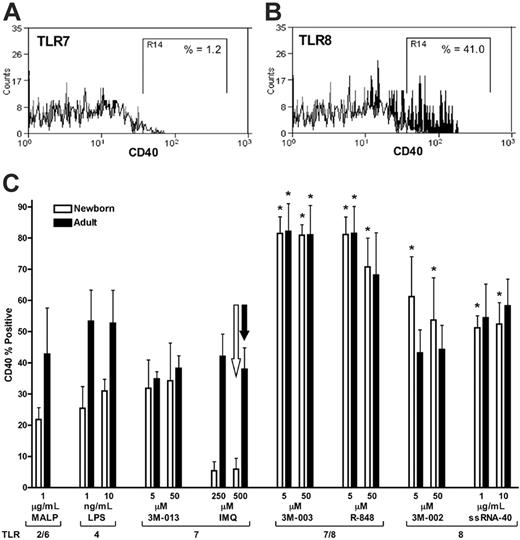
The ability of TLR8 agonists to effectively induce production of the Th1-polarizing cytokines TNF-α and IL-12 from neonatal mononuclear cells raises the possibility that these agents may have unique efficacy in activating neonatal APCs. To explore the ability of TLR8 agonists to activate neonatal APCs, we measured the ability of TLR8 agonists to induce up-regulation of the surface costimulatory molecule CD40 on neonatal and adult mDCs using flow cytometry (Figure 4). Agonists of TLR2/6 (MALP) and TLR4 (LPS) induced modest up-regulation of CD40 on neonatal mDCs (∼5%-35% CD40+). Similarly, the TLR7 agonists imiquimod and 3M-013 induced 35% or less CD40 positivity in neonatal (or adult) cells. In contrast, TLR8 agonists, including the TLR 7/8 agonists R-848 and 3M-003 as well as the pure TLR8 agonists 3M-002 and ssRNA40 effectively induced greater than 50% to 80% CD40 positivity in neonatal mDCs.
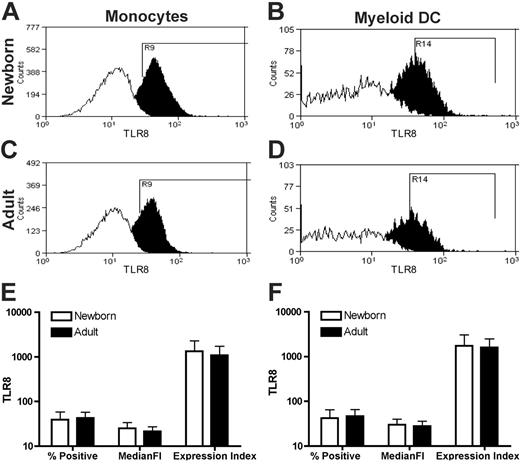
Neonatal monocytes and mDCs express equivalent amounts of TLR8 protein as do their adult counterparts
To begin assessing the mechanism by which TLR8 agonists maintain full activity toward neonatal APCs, we characterized basal expression of TLR8 protein in neonatal and adult APCs. We have previously reported that neonatal monocytes exhibit normal basal expression of TLR8 mRNA,32 but to date there is no published information regarding the expression of TLR8 protein in human neonatal cells. We therefore measured TLR8 expression in neonatal monocytes and mDCs, in comparison to that in adults, using flow cytometry and a PE-conjugated mAb directed at human TLR8 (Figure 5). Permeabilized neonatal and adult monocytes (Figure 5A and C, respectively) and mDCs (Figure 5B and D, respectively) demonstrated similar patterns of TLR8 staining. Comparison of TLR8 staining by percent positivity, median fluorescent intensity, or expression index (percent positive × median fluorescent intensity) demonstrated equivalent TLR8 protein expression in neonatal and adult monocytes (Figure 5E) as well as in neonatal and adult mDCs (Figure 5F). These data indicate that, on a cellular basis, neonatal monocytes and mDCs express similar amounts of TLR8 protein as their adult counterparts.
TLR8 agonists efficiently induce CD40 expression on neonatal and adult mDCs. Whole blood was stimulated with TLR agonists for 19 hours prior to measurement of CD40 expression on mDCs (lin1–/HLA-DR+/CD11c+ cells) by flow cytometry using a PE-conjugated anti-CD40 mAb as described in “Materials and methods.” Representative examples are provided of imiquimod (TLR7)–induced (A) and of 3M-002 (TLR8)–induced (B) CD40 expression on neonatal mDCs with isotype control (white), buffer control (gray), and TLR-stimulated (black) conditions indicated. The “R14” gate defines CD40+ events in the examples provided, 1.2% for the TLR7 (A) and 41% for the TLR8 agonist (B). (C) Composite analysis of such data demonstrating the percentage of mDCs positive for CD40 in relation to the indicated concentrations of TLR agonists. Data represent means ± SEM. Statistical comparison is made in relation to the TLR7 agonist imiquimod at 500 μM (open arrow, newborns; filled arrow, adults). n = 3-4, *P < .05. Table S2 presents comparisons of CD40 expression induced by TLR8 and TLR7/8 agonists with that induced by each TLR7 agonist at every dose.
TLR8 agonists efficiently induce CD40 expression on neonatal and adult mDCs. Whole blood was stimulated with TLR agonists for 19 hours prior to measurement of CD40 expression on mDCs (lin1–/HLA-DR+/CD11c+ cells) by flow cytometry using a PE-conjugated anti-CD40 mAb as described in “Materials and methods.” Representative examples are provided of imiquimod (TLR7)–induced (A) and of 3M-002 (TLR8)–induced (B) CD40 expression on neonatal mDCs with isotype control (white), buffer control (gray), and TLR-stimulated (black) conditions indicated. The “R14” gate defines CD40+ events in the examples provided, 1.2% for the TLR7 (A) and 41% for the TLR8 agonist (B). (C) Composite analysis of such data demonstrating the percentage of mDCs positive for CD40 in relation to the indicated concentrations of TLR agonists. Data represent means ± SEM. Statistical comparison is made in relation to the TLR7 agonist imiquimod at 500 μM (open arrow, newborns; filled arrow, adults). n = 3-4, *P < .05. Table S2 presents comparisons of CD40 expression induced by TLR8 and TLR7/8 agonists with that induced by each TLR7 agonist at every dose.
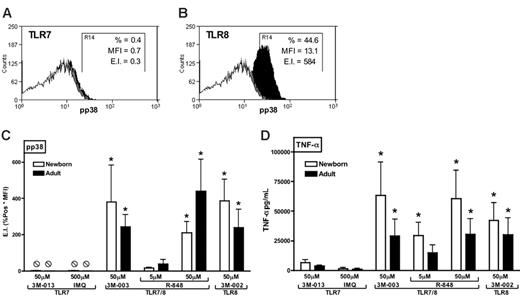
TLR8 agonists effectively and preferentially induce phosphorylation of p38 MAPK in neonatal and adult monocytes
The preferential response of neonatal APCs to TLR8 agonists raises the question of the mechanism by which TLR8 agonists are so efficacious at activating Th1-polarizing responses. To begin assessing this issue, we used flow cytometry to measure the ability of TLR agonists to induce phosphorylation of p38 MAPK in neonatal and adult monocytes (Figure 6). Whereas 3M-013 and imiquimod induced little or no detectable phosphorylation of p38 MAPK (representative experiment in Figure 6A), the TLR7/8 agonists 3M-003 and R-848 as well as the pure TLR8 agonist 3M-002 induced robust p38 phosphorylation in both neonatal and adult monocytes (representative experiment in Figure 6B). Composite analysis of such data revealed that TLR8 and TLR7/8 agonists induced significantly more phosphorylation of p38 than did TLR7 agonists (Figure 6C). Similarly, under these same assay conditions, TLR8 and TLR7/8 agonists induced substantially greater TNF-α production than did agonists of TLR7 alone (Figure 6D). Spearman analysis indicated that TLR-induced p38 phosphorylation and TLR-induced TNF-α production were significantly correlated (adults: r = 0.7973, r2 = 0.6357, P = .003; newborns: r = 0.8322, r2 = 0.6926; P < .01).
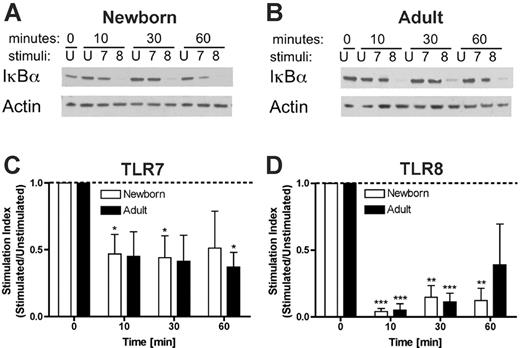
Preferential degradation of IκB-α by a TLR8 agonist
To further characterize the mechanism of activation of neonatal and adult monocytes by TLR8 agonists, we compared TLR7- and TLR8-mediated disappearance of IκB-α. Neonatal and adult monocytes were stimulated with the pure TLR7 agonist 3M-013 or the pure TLR8 agonist 3M-002 for 10, 30, or 60 min prior to assay for IκB-α by Western blotting (Figure 7). As shown in a representative experiment, newborn (Figure 7A) and adult (Figure 7B) monocytes stimulated via TLR7 (3M-013) demonstrated a modest reduction in IκB-α. In contrast, TLR8 stimulation (3M-002) rapidly induced profound disappearance of IκB-α. Such data were analyzed by densitometric scanning and plotted as a stimulation index (stimulated/unstimulated; Figure 7C-D). TLR7 stimulation induced a transient and modest (∼50%) reduction in IκB-α levels that for neonatal cells lost significance by 60 minutes (Figure 7C). In contrast, TLR8 stimulation induced a profound (∼90%) reduction in IκB-α levels that remained significant in neonates at 60 minutes (Figure 7D). These results suggest that activation of TLR8 results in more profound and prolonged degradation of IκB-α that may contribute to the efficacy of TLR8 agonists in activating neonatal and adult APCs.
Discussion
Multiple studies have documented impaired neonatal Th1-type immune responses to a variety of stimuli.16,32,36-38 Building on our prior study that indicated that a TLR 7/8 agonist (R-848) had preserved TNF-α–inducing activity in neonatal monocytes,32 we have assessed the ability of TLR8 agonists to activate neonatal APCs. We have discovered that agents that activate cells via TLR8 have a unique ability to induce production of Th1-responses in neonatal APCs, revealing a unique preservation of the TLR8-mediated activation pathway in human newborns.
In contrast to agonists of TLR2/6, TLR4, or TLR7 (only), agonists of TLR8 (or TLR 7/8) effectively induced production of the proinflammatory and Th1-polarizing cytokine TNF-α from mononuclear cells in a range of relatively physiologic assay formats, including whole blood (Figure 1) and PBMCs cultured in fresh autologous serum (Figure 2). TLR8 agonists also effectively induced production of IL-12, a cytokine that enhances cell-mediated immunity,12 from adherent monocytes cultured in fresh autologous serum (Figure 3). In addition, TLR8 agonists preferentially induced expression of the costimulatory molecule CD40 on neonatal mDCs (Figure 4), a population of mononuclear cells known to express TLR826 and that play important roles in antigen presentation.39 We demonstrate that on a cellular basis, neonatal monocytes and mDCs express TLR8 protein to a similar extent as adult monocytes and mDCs (Figure 5).
Equivalent expression of TLR8 by neonatal and adult APCs. Neonatal cord or adult peripheral blood was stained with a PE-conjugated anti-TLR8 mAb prior to flow cytometry. Monocytes (CD14+) and mDCs (lin1–/HLA-DR+/CD11c+) were identified as described in “Materials and methods.” Representative histograms (open, isotype control; filled, PE-conjugated anti–human TLR8 mAb) depicting newborn monocytes (A) and mDCs (B) as well as adult monocytes (C) and mDCs (D) are shown. The expression index for TLR8 was calculated as the product of median fluorescent intensity and percent positivity. A composite analysis for expression of TLR8 in monocytes (E) and mDCs (F) reveals equivalent expression in neonatal and adult cells. Data represent means ± SEM. Statistical analysis indicates no significant differences in TLR8 expression; n = 3.
Equivalent expression of TLR8 by neonatal and adult APCs. Neonatal cord or adult peripheral blood was stained with a PE-conjugated anti-TLR8 mAb prior to flow cytometry. Monocytes (CD14+) and mDCs (lin1–/HLA-DR+/CD11c+) were identified as described in “Materials and methods.” Representative histograms (open, isotype control; filled, PE-conjugated anti–human TLR8 mAb) depicting newborn monocytes (A) and mDCs (B) as well as adult monocytes (C) and mDCs (D) are shown. The expression index for TLR8 was calculated as the product of median fluorescent intensity and percent positivity. A composite analysis for expression of TLR8 in monocytes (E) and mDCs (F) reveals equivalent expression in neonatal and adult cells. Data represent means ± SEM. Statistical analysis indicates no significant differences in TLR8 expression; n = 3.
TLR8 agonists induce robust phosphorylation of p38 MAP kinase in both neonatal and adult blood monocytes. Blood was stimulated with TLR agonists for 10 minutes prior to red cell lysis, fixation, and permeabilization. Representative examples of imiquimod (TLR7)–induced (A) and 3M-002 (TLR8)–induced (B) phosphorylation of p38 in newborn monocytes are provided with isotype (open), buffer control (gray), and TLR-stimulated (black) conditions indicated. Phosphorylation of p38 MAPK in CD14+ monocytes was detected by flow cytometry using a PE-conjugated anti–phospho-p38 mAb and depicted as an expression index (EI) calculated as a product of percent positive and mean fluorescent intensity (MFI). In these examples, TLR7 activation (A) induced a pp38 expression index (EI) of 0.3, whereas TLR8 activation (B) induced an EI of 584. (C) Composite analysis of such data reveals that TLR8 and TLR7/8 agonists induce substantially and significantly greater p38 phosphorylation than do TLR7 agonists. (D) Additional blood samples were allowed to incubate for 5 hours to measure TNF-α production in parallel. Data represent means ± SEM. Statistical comparisons were made within groups. n = 3-4, *P < .05 in comparison to both of the TLR7 agonists tested. Spearman analysis indicates that TLR-induced p38 phosphorylation and TNF-α production were significantly correlated (adults: r = 0.7973, r 2 = 0.6357, P < .001; newborns: r = 0.8322, r 2 = 0.6926; P <.001).
TLR8 agonists induce robust phosphorylation of p38 MAP kinase in both neonatal and adult blood monocytes. Blood was stimulated with TLR agonists for 10 minutes prior to red cell lysis, fixation, and permeabilization. Representative examples of imiquimod (TLR7)–induced (A) and 3M-002 (TLR8)–induced (B) phosphorylation of p38 in newborn monocytes are provided with isotype (open), buffer control (gray), and TLR-stimulated (black) conditions indicated. Phosphorylation of p38 MAPK in CD14+ monocytes was detected by flow cytometry using a PE-conjugated anti–phospho-p38 mAb and depicted as an expression index (EI) calculated as a product of percent positive and mean fluorescent intensity (MFI). In these examples, TLR7 activation (A) induced a pp38 expression index (EI) of 0.3, whereas TLR8 activation (B) induced an EI of 584. (C) Composite analysis of such data reveals that TLR8 and TLR7/8 agonists induce substantially and significantly greater p38 phosphorylation than do TLR7 agonists. (D) Additional blood samples were allowed to incubate for 5 hours to measure TNF-α production in parallel. Data represent means ± SEM. Statistical comparisons were made within groups. n = 3-4, *P < .05 in comparison to both of the TLR7 agonists tested. Spearman analysis indicates that TLR-induced p38 phosphorylation and TNF-α production were significantly correlated (adults: r = 0.7973, r 2 = 0.6357, P < .001; newborns: r = 0.8322, r 2 = 0.6926; P <.001).
TLR8 agonists induce profound and prolonged degradation of IκB-α. Purified neonatal or adult monocytes were cultured in 100% autologous serum and incubated for the indicated number of minutes with buffer control (ie, unstimulated, U) or with 50 μM of the pure TLR7 agonist 3M-013 (7) or the pure TLR8 agonist 3M-002 (8) prior to lysis in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer, electrophoresis, and Western blotting for IκB-α. Autoradiograms were scanned and digital intensity analyzed as described in “Materials and methods.” Representative autoradiograms are shown in panels A and B. Composite densitometric analysis expressed as a stimulation index (ie, density of IκB-α in the stimulated condition divided by that in the unstimulated condition) demonstrates that whereas TLR7 agonist-induced degradation of IκB-α in neonatal monocytes was of modest extent (∼50%) and duration (< 60 minutes; C), TLR8 activation was associated with profound (∼90%) and prolonged (≥ 60 minutes) disappearance of IκB-α (D). Statistical comparisons are made within groups (□, newborns; ▪, adults) in relation to the unstimulated condition (defined as 1.0). n = 4, *P < .05; **P < .01, ***P < .001.
TLR8 agonists induce profound and prolonged degradation of IκB-α. Purified neonatal or adult monocytes were cultured in 100% autologous serum and incubated for the indicated number of minutes with buffer control (ie, unstimulated, U) or with 50 μM of the pure TLR7 agonist 3M-013 (7) or the pure TLR8 agonist 3M-002 (8) prior to lysis in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer, electrophoresis, and Western blotting for IκB-α. Autoradiograms were scanned and digital intensity analyzed as described in “Materials and methods.” Representative autoradiograms are shown in panels A and B. Composite densitometric analysis expressed as a stimulation index (ie, density of IκB-α in the stimulated condition divided by that in the unstimulated condition) demonstrates that whereas TLR7 agonist-induced degradation of IκB-α in neonatal monocytes was of modest extent (∼50%) and duration (< 60 minutes; C), TLR8 activation was associated with profound (∼90%) and prolonged (≥ 60 minutes) disappearance of IκB-α (D). Statistical comparisons are made within groups (□, newborns; ▪, adults) in relation to the unstimulated condition (defined as 1.0). n = 4, *P < .05; **P < .01, ***P < .001.
The overall efficacy of TLR8 agonists in activating neonatal APCs generally correlated with their higher efficacy, in comparison to agonists of other TLRs, in inducing Th1 responses from adult mononuclear cells as well. Such a pattern suggests that the efficacy of TLR8 agonists in newborns may reflect their greater stimulatory activity. Indeed, the efficacy of TLR8 agonists in activating neonatal APCs correlated with their ability to effectively induce phosphorylation of p38 MAPK (Figure 6) as well as their ability to induce prolonged degradation of IκB-α (Figure 7). In aggregate, these results suggest that the unique potency of TLR8 agonists relates to the ability of the TLR8 pathway to strongly induce TLR-mediated intracellular signaling via the p38 MAPK and NF-κB pathways that are known to trigger expression of costimulatory molecules including TNF-α,40,41 IL-12,42 and CD40.43
Given the abundant evidence that most stimuli, including most TLR agonists,32,37,44-47 fail to effectively induce Th1-type inflammatory responses in neonatal mononuclear cells, the identification of a TLR8-mediated molecular pathway that is uniquely preserved in human neonatal APCs represents a significant advance in neonatal immunology. Thus TLR8 agonists, including ssRNAs and imidazoquinoline compounds that activate cells via TLR8 or TLR 7/826 and that have recently been shown to act as effective vaccine adjuvants in vivo,45 may play important roles in treating or preventing diseases by enhancing innate and acquired responses in human newborns. Because there continues to be a major unmet medical need for more effective vaccination strategies for neonates,2 further study of the potential of TLR8 and TLR 7/8 agonists both in vitro and in animal models in vivo is warranted.
Prepublished online as Blood First Edition Paper, April 25, 2006; DOI 10.1182/blood-2005-12-4821.
Supported in part by National Institutes of Health grant KO8AI50583 and a grant from the Patterson Trust (O.L.).
One of the authors (R.L.M.) is employed by a company (3M Pharmaceuticals) whose products were studied in the present work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Raif Geha, Holden Maecker, and Jordan S. Orange for expert technical advice and helpful discussions. Melissa Coughlin, Mandana Farhadi, Emily Babendreier, and Camilo Chao provided expert technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal