Abstract
Imatinib mesylate (IM) therapy for chronic myeloid leukemia (CML) has transformed the treatment of this disease. However, the vast majority of patients, despite major responses, still harbor Philadelphia chromosome–positive (Ph+) cells. We have described a population of primitive Ph+ cells that are insensitive to IM and may be a source of IM resistance. Cell line studies have suggested that the drug transporter ABCG2 may be a mediator of IM resistance, however there is considerable debate about whether IM is an ABCG2 substrate or inhibitor. We demonstrate here that primitive CML CD34+ cells aberrantly overexpress functional ABCG2 but that cotreatment with IM and an ABCG2 inhibitor does not potentiate the effect of IM. We definitively show that IM is an inhibitor of, but not a substrate for, ABCG2 and that, therefore, ABCG2 does not modulate intracellular concentrations of IM in this clinically relevant cell population.
Introduction
The introduction of imatinib mesylate (IM) has transformed the treatment of chronic myeloid leukemia (CML), however a significant minority of patients does not respond and others lose response during treatment. Such states reflect IM resistance in the whole cell population, however suboptimal responses may represent the persistence of a subpopulation of insensitive leukemic stem cells (LSCs).1-4 One possible mechanism of LSC persistence is the limitation of intracellular drug concentration due to the activity of ATP binding cassette (ABC) drug-resistance proteins as seen previously with conventional chemotherapeutics.5 One such transporter, ABCG2, is highly expressed on normal stem cell populations6 and as such may also be expected to be expressed on LSCs.
There have been a number of convincing but contradictory reports on the interaction of IM with ABCG2. Houghton et al7 found IM to be a potent inhibitor of ABCG2, but not a substrate; Burger et al8 found IM to be a substrate; and importantly, Nakanishi et al9 showed that Bcr-Abl itself may regulate ABCG2 protein expression via AKT activity and suggest that ABCG2 may be a mediator of IM resistance. However, these studies all used cell lines that had been engineered to express ABCG2.
It was therefore, critical to look at this potential transporter for IM in primary CML cells and particularly within the LSC compartment. We report here our investigation of the expression and function of ABCG2 on primary CD34+ CML cells, and the effect of this transporter on cellular IM levels and their response to this drug.
Study design
Primary cell samples and cell lines
Leukapheresis products from chronic-phase CML patients at diagnosis prior to IM treatment were obtained with informed consent in accordance with the Declaration of Helsinki, and with the approval of the Glasgow Royal Infirmary Ethics Committee, and enriched for CD34+ cells using CLINIMACS (Miltenyi Biotec, Bisley, United Kingdom). CD34+ cells were confirmed to be Bcr-Abl+ by fluorescence in situ hybridization (FISH) and cultured in growth factor medium (5GF) as previously published.1 Normal CD34+ cells were purchased from Cambrex Bioscience (Wokingham, United Kingdom). AML6.2 (ABCG2 high) and OCI-AML3 (parental ABCG2 negative) cell lines10 were from Dr Brian Sorrentino (St Jude Children's Research Hospital, Memphis, TN).
Efflux studies
The efflux protocol was a modification of that previously described11 using the ABCG2-specific inhibitor fumitremorgin C (FTC; Dr R Robey, Center for Cancer Research, NIH, Bethesda, MD). Cells were incubated with ± 10 μM FTC ± 5 μM IM (Novartis Pharma, Basel, Switzerland) for 15 minutes to block pump activity before the addition of 200 nM BODIPY-Prazosin (B-P; Molecular Probes, Eugene, OR) and a further 30-minute incubation. Cells were then washed and incubated ± 10 μM FTC/5 μM IM for 60 minutes to allow efflux before being washed in ice-cold medium and analyzed.
Determination of cellular drug levels and effect of ABCG2 inhibition
ABCG2 activity was preinhibited as described under “Efflux studies” before the addition of 14C-IM (84 μCi [3.1 MBq]/mg; Novartis) or 3H-mitoxantrone (3 Ci [111 GBq]/mmol; Moravek Biochemicals, Brae, CA) ± 10 μM FTC. After a further 2 hours, cells were lysed as previously published12 before scintillation counting. To test the effect of IM on 3H-mitoxantrone accumulation, 5 μM IM replaced FTC.
Results and discussion
ABCG2 is overexpressed in CD34+ CML cells
Using quantitative polymerase chain reaction (PCR) and previously published primers and probes,13 we determined that normal CD34+ samples resulted in a mean ΔCT value of 15.2 ± 0.8 compared with 12.5 ± 0.5 in CML CD34+ cells (P < .01). Consequently, the calculated 2-ΔΔCT value determined that CML CD34+ cells expressed 6.8-fold more ABCG2 than their normal counterparts.
Flow analysis of cells stained with the antibody BXP2114,15 demonstrated that ABCG2 protein could be detected on transduced AML6.2 cells (isotype geometric mean [GM] = 4.3 vs BXP21 GM = 12.7) and that parental AML3 cells10 were negative. Analysis of 7 CML CD34+ samples revealed a significant amount of detectable ABCG2 protein on these cells (isotype GM = 3.8 ± 0.2 vs BXP21 GM 7.8 ± 0.8 [P < .002; n = 7]). All 7 samples contained ABCG2-positive cells with 25% (range, 25.3%-79.0%) of cells positive in 5 of 7 cases. Thus, a significant proportion of CML CD34+ cells express ABCG2 protein. Many previous studies have shown that normal human hematopoietic cells expressing functional ABCG2 (side population or SP cells) are CD34–.16-18 Thus, our data suggest that ABCG2 is aberrantly expressed in an increased proportion of CD34+ cells than in the equivalent normal cell population.
ABCG2 is overexpressed on CML CD34+cells, is functional, and interacts with IM. (A) Cell lines were tested for their capacity to efflux B-P. Unstained cells (filled curve), ABCG2-positive AML6.2 cells with B-P (light gray shaded curve; labeled B-P alone), and AML6.2 with B-P + 10 μM FTC (dark gray shaded curve; labeled FTC) and with increasing concentrations of IM added (open curve; labeled 0.1 IM, 0.5 IM, 2.5 IM, 5 IM). (B) Normal CD34+ cells were tested in the same manner. Normal CD34+ cells with no B-P (unstained control [filled curve]) and incubated with B-P alone (light gray shaded curve; labeled B-P alone), B-P + 10 μM FTC (dark gray shaded curve; labeled FTC), and B-P + 5 μM IM (open curve; labeled 5 IM). Representative data shown. (C) CML CD34+ cells assayed similarly. CML CD34+ cells with no B-P (unstained control [filled curve]) and incubated with B-P alone (light gray shaded curve; labeled B-P alone), B-P + 10 μM FTC (dark gray shaded curve; labeled FTC), and B-P + 5 μM IM (open curve; labeled IM). Plots are one representative plot from n = 6 samples assayed in duplicate. (D) Mean data from CML cells. Mean data (n = 6) normalized to the maximum B-P retention as determined by incubation of CML CD34+ cells in B-P + 10 μM FTC (dark gray bar), cells with B-P alone and capable of efflux (light gray bar), and cells with B-P and 5 μM IM (open bar). Statistical analysis was by paired Student t test. All data are mean ± SEM.
ABCG2 is overexpressed on CML CD34+cells, is functional, and interacts with IM. (A) Cell lines were tested for their capacity to efflux B-P. Unstained cells (filled curve), ABCG2-positive AML6.2 cells with B-P (light gray shaded curve; labeled B-P alone), and AML6.2 with B-P + 10 μM FTC (dark gray shaded curve; labeled FTC) and with increasing concentrations of IM added (open curve; labeled 0.1 IM, 0.5 IM, 2.5 IM, 5 IM). (B) Normal CD34+ cells were tested in the same manner. Normal CD34+ cells with no B-P (unstained control [filled curve]) and incubated with B-P alone (light gray shaded curve; labeled B-P alone), B-P + 10 μM FTC (dark gray shaded curve; labeled FTC), and B-P + 5 μM IM (open curve; labeled 5 IM). Representative data shown. (C) CML CD34+ cells assayed similarly. CML CD34+ cells with no B-P (unstained control [filled curve]) and incubated with B-P alone (light gray shaded curve; labeled B-P alone), B-P + 10 μM FTC (dark gray shaded curve; labeled FTC), and B-P + 5 μM IM (open curve; labeled IM). Plots are one representative plot from n = 6 samples assayed in duplicate. (D) Mean data from CML cells. Mean data (n = 6) normalized to the maximum B-P retention as determined by incubation of CML CD34+ cells in B-P + 10 μM FTC (dark gray bar), cells with B-P alone and capable of efflux (light gray bar), and cells with B-P and 5 μM IM (open bar). Statistical analysis was by paired Student t test. All data are mean ± SEM.
Overexpressed ABCG2 is functional and is inhibited by IM
To determine whether the ABCG2 expressed on CML cells was capable of efflux, we used the specific substrate B-P. Using AML6.2 and AML3, we established that B-P was maximally effluxed after 60 minutes and that efflux was inhibited by 10 μM FTC (Figure 1A). Of importance, IM resulted in a dose-dependent decrease in efflux, with inhibition equivalent to 10 μM FTC seen at 0.5 μM or more IM.
We examined the ABCG2-mediated efflux capacity and interaction with IM in 6 individual CML CD34+ samples. To reduce interexperimental variation, the GM of cells maximally loaded with B-P + FTC was used as an internal control and considered as 100% B-P retention. In the absence of FTC, there was significant efflux from CML CD34+ cells (retention = 54.4% ± 19.3% of control; P = .04). As in cell lines, this efflux was also completely inhibited by the addition of therapeutic concentrations (≥ 0.5 μM) of IM, with 5 μM IM resulting in retention of 223.5% ± 66.4% of control (P = .03 to B-P alone, P = NS to B-P + FTC; Figure 1C-D). The combination of FTC and IM had no further effect. In contrast, when we looked at 2 samples of normal CD34+ cells we did not observe any B-P efflux (Figure 1B), again supporting the hypothesis that ABCG2 is expressed on a greater proportion of CD34+ cells in CML. This finding supports the findings of Nakanishi et al9 who demonstrated that Bcr-Abl up-regulates ABCG2 protein via AKT activity in cell lines.
ABCG2 inhibition does not alter the response to IM
Thus, overexpression of ABCG2 on CML CD34+ cells results in increased efflux activity that can be inhibited by IM. However, these data do not definitively discriminate between the activity of IM as inhibitor rather than a competitive substrate. As this was the main point of debate in previous reports,7-9 we looked directly at cellular levels of drug in primary primitive CML cells and the effect of ABCG2 inhibition on the response to IM.
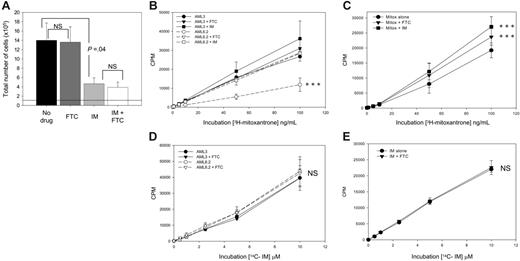
IM is an inhibitor of, not a substrate for, ABCG2. (A) ABCG2 inhibition does not potentiate IM. CD34+ CML cells were seeded at 1 × 105 cells at day 0 (indicated by dotted line), and the number of total viable cells was analyzed after 72 hours in culture with 5GF alone (filled bar; no drug), + 10 μM FTC alone (dark gray bar), + 5 μMIM (light gray bar), or both drugs (open bar). All data are mean of duplicate analyses of n = 6 samples. (B-E) To determine whether IM is a substrate or inhibitor of ABCG2, the cellular concentration of the known substrate mitoxantrone or IM and the effect of the ABCG2 inhibitor FTC or IM on drug accumulation was tested in cell lines and CML CD34+ cells. (B) Mitoxantrone accumulation in cell lines. The cellular concentration of 3H-mitoxantrone, a known ABCG2 substrate, was determined over a range of extracellular concentrations of the drug for both ABCG2-negative AML3 cells (solid lines, black symbols) and ABCG2-overexpressing AML6.2 cells (dashed lines, open symbols). The effect of the ABCG2 inhibitor FTC (10 μM; ▾, ▿) or 5 μM IM (□, ▪) was also determined. ***P < .01 compared with all other treatments. (C) Mitoxantrone accumulation in CML CD34+ cells. CD34+ CML cells were incubated with 3H-mitoxantrone similarly (3H-mitoxantrone alone •, + 10 μM FTC ▾, + 5 μM IM ▪). ***P < .01 compared with mitoxantrone alone. (D) IM accumulation in cell lines. The cellular concentration of 14C-IM and the effect of ABCG2 inhibition was determined over a range of extracellular concentrations of the drug for both AML3 cells (solid lines, black symbols) and AML6.2 cells (dashed lines, open symbols) in the presence (▾, ▿) and absence (•, ○) of the ABCG2 inhibitor FTC (10 μM). (E) IM accumulation in CML CD34+ cells. The cellular concentration of 14C IM accumulated by CD34+ CML cells was determined similarly (14C-IM alone •, + 10 μM FTC ▾). All data are the mean standard error from at least 3 individual patients for CML cells and 3 separate experiments for cell lines; all were analyzed in triplicate. All statistical analyses were by paired Student t test; NS indicates not significant.
IM is an inhibitor of, not a substrate for, ABCG2. (A) ABCG2 inhibition does not potentiate IM. CD34+ CML cells were seeded at 1 × 105 cells at day 0 (indicated by dotted line), and the number of total viable cells was analyzed after 72 hours in culture with 5GF alone (filled bar; no drug), + 10 μM FTC alone (dark gray bar), + 5 μMIM (light gray bar), or both drugs (open bar). All data are mean of duplicate analyses of n = 6 samples. (B-E) To determine whether IM is a substrate or inhibitor of ABCG2, the cellular concentration of the known substrate mitoxantrone or IM and the effect of the ABCG2 inhibitor FTC or IM on drug accumulation was tested in cell lines and CML CD34+ cells. (B) Mitoxantrone accumulation in cell lines. The cellular concentration of 3H-mitoxantrone, a known ABCG2 substrate, was determined over a range of extracellular concentrations of the drug for both ABCG2-negative AML3 cells (solid lines, black symbols) and ABCG2-overexpressing AML6.2 cells (dashed lines, open symbols). The effect of the ABCG2 inhibitor FTC (10 μM; ▾, ▿) or 5 μM IM (□, ▪) was also determined. ***P < .01 compared with all other treatments. (C) Mitoxantrone accumulation in CML CD34+ cells. CD34+ CML cells were incubated with 3H-mitoxantrone similarly (3H-mitoxantrone alone •, + 10 μM FTC ▾, + 5 μM IM ▪). ***P < .01 compared with mitoxantrone alone. (D) IM accumulation in cell lines. The cellular concentration of 14C-IM and the effect of ABCG2 inhibition was determined over a range of extracellular concentrations of the drug for both AML3 cells (solid lines, black symbols) and AML6.2 cells (dashed lines, open symbols) in the presence (▾, ▿) and absence (•, ○) of the ABCG2 inhibitor FTC (10 μM). (E) IM accumulation in CML CD34+ cells. The cellular concentration of 14C IM accumulated by CD34+ CML cells was determined similarly (14C-IM alone •, + 10 μM FTC ▾). All data are the mean standard error from at least 3 individual patients for CML cells and 3 separate experiments for cell lines; all were analyzed in triplicate. All statistical analyses were by paired Student t test; NS indicates not significant.
If IM were a substrate of ABCG2, then cotreatment with FTC should enhance the effect of IM. Cells cultured with 5GF alone and seeded at 1 × 105 cells at day 0 generated 14.0 ± 3.7 × 105 cells after 72 hours. FTC alone had no effect on the total output of viable cells (13.6 ± 3.3 × 105). IM (5 μM) reduced the total output of cells per culture to 4.65 ± 1.3 × 105, however, the combination of IM + FTC did not result in any further significant decrease (3.9 ± 1.1 × 105) (Figure 2A). These data were consistent with IM being an inhibitor, but not a substrate, for ABCG2 and contrast with those of Nakanishi et al9 who, using ABCG2-transduced K562 cells, saw a slight additive effect of IM and FTC and concluded that IM may be a substrate for this transporter.
IM is an inhibitor of ABCG2 in CML CD34+ cells
We established cellular drug concentration assays using AML3, AML6.2 (Figure 2B,D), and CD34+ CML (Figure 2C,E) cells. CML CD34+ cells accumulated 3H-mitoxantrone, a known substrate of ABCG2, in a concentration-dependent manner (Figure 2C). This accumulation was significantly increased by the addition of 10 μM FTC, confirming ABCG2-mediated efflux. Replacing FTC with 5 μM IM resulted in a similar increase in 3H-mitoxantrone accumulation, confirming the activity of IM as an ABCG2 inhibitor. In contrast, when the cells were incubated with 14C-IM, there was no difference between the accumulation in AML3 (ABCG2–) or AML6.2 (ABCG2+) cells and FTC had no effect on the accumulation in either cell line or CML cells (Figure 2D-E). Hence IM is not an ABCG2 substrate. These assays also allowed us to determine the cellular concentration of IM in these cells. The 14C-IM measured in CML CD34+ cells was equivalent to 180 ng or 306 pmol per 5 × 105 cells, which was unaffected by treatment with FTC.
Together these data show for the first time that ABCG2 is up-regulated in CD34+ CML cells. These results complement those of Nakanishi et al9 with regard to Bcr-Abl regulating ABCG2 expression. However, of more importance from a clinical perspective, we have direct and conclusive evidence that IM is an inhibitor of, but not a substrate for, ABCG2 in primary cells. Therefore, the aberrant ABCG2 expression will neither affect intracellular IM concentration nor mediate resistance in this population.
Prepublished online as Blood First Edition Paper, April 20, 2006; DOI 10.1182/blood-2006-02-003145.
Supported by grants from the Leukaemia Research Fund, UK (04/034) and the Scottish National Blood Transfusion Service. H.G.J. is the recipient of the William Thyne Centenary Fellowship.
J.C.M., H.G.J., and T.L.H. participated in designing the research; N.E.J. and J.C.M. performed the research; J.C.M., H.G.J., and T.L.H. analyzed the data; J.C.M., H.G.J., and T.L.H. wrote the paper; and all authors checked the final version of the paper.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Diane Gilmour and Siobhan Macmillan for their technical help with these studies.

![Figure 1. ABCG2 is overexpressed on CML CD34+ cells, is functional, and interacts with IM. (A) Cell lines were tested for their capacity to efflux B-P. Unstained cells (filled curve), ABCG2-positive AML6.2 cells with B-P (light gray shaded curve; labeled B-P alone), and AML6.2 with B-P + 10 μM FTC (dark gray shaded curve; labeled FTC) and with increasing concentrations of IM added (open curve; labeled 0.1 IM, 0.5 IM, 2.5 IM, 5 IM). (B) Normal CD34+ cells were tested in the same manner. Normal CD34+ cells with no B-P (unstained control [filled curve]) and incubated with B-P alone (light gray shaded curve; labeled B-P alone), B-P + 10 μM FTC (dark gray shaded curve; labeled FTC), and B-P + 5 μM IM (open curve; labeled 5 IM). Representative data shown. (C) CML CD34+ cells assayed similarly. CML CD34+ cells with no B-P (unstained control [filled curve]) and incubated with B-P alone (light gray shaded curve; labeled B-P alone), B-P + 10 μM FTC (dark gray shaded curve; labeled FTC), and B-P + 5 μM IM (open curve; labeled IM). Plots are one representative plot from n = 6 samples assayed in duplicate. (D) Mean data from CML cells. Mean data (n = 6) normalized to the maximum B-P retention as determined by incubation of CML CD34+ cells in B-P + 10 μM FTC (dark gray bar), cells with B-P alone and capable of efflux (light gray bar), and cells with B-P and 5 μM IM (open bar). Statistical analysis was by paired Student t test. All data are mean ± SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/4/10.1182_blood-2006-02-003145/3/m_zh80160699890001.jpeg?Expires=1767759904&Signature=wEo2Skj~N2z99rXdHLnwCDK63LlJzgyVabKwAjXSPY~d0OzbkS5IGHpEkrbvOTUfpiuYSka1gFqm9jdChBZfp-sI7hCXd-3xNDaD-SxK-82ZuSDvNw5xeGHfoF6fz3uF9ag5qBophgZHJ1D2s77fhikSYMuCT42uBrKWfiXq3kkWfahBW6-NW9oxbcyNFssuvhGbyMNb1iPr~G1028iMAVU7-Mzap8sDmRkVcsswi0CbrBwalBkSjyG7PBZaXSSuRMysRcOZdQFfJ554jvwLLoOSAzXP9kRle2CYjpR3u6anKZgQ0iuQG7fIp1SjRI~qOLbNIV7tnc1L1QMyPFEQvw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal