Plasma membrane lipids are usually distributed asymmetrically, with phosphatidylserine (PS) confined to the inner leaflet. PS exposure at the outer leaflet occurs early in apoptosis, but it is also constitutive on some nonapoptotic cell populations where it plays a role in cell signaling. How PS is transported (“flopped”) to the cell surface is unknown. Contrary to previous reports that normal murine B lymphocytes lack lipid asymmetry, we show that PS is normally restricted to the inner leaflet of these cells. PS exposure on normal B cells did, however, occur spontaneously ex vivo. Consistent with the hypothesis that loss of PS asymmetry is regulated by CD45, PS is constitutively exposed on viable, CD45-deficient B cells. We show that calcium-stimulated PS exposure in B cells is strain variable, ABCA1 independent, and both preceded by and dependent on a decrease in lipid packing. This decrease in lipid packing is concomitant with cell shrinkage and consequent membrane distortion, both of which are potently inhibited by blockers of volume-regulatory K+ and Cl- ion channels. Thus, changes in plasma membrane organization precede PS translocation. The data suggest a model in which PS redistribution may occur by a translocase-independent mechanism at energetically favorable sites of membrane perturbation where lipid packing is decreased.
Introduction
The anionic phospholipid phosphatidylserine (PS) is largely confined to the inner leaflet of the plasma membrane of healthy cells, and its flopping to the outer leaflet and exposure to the extracellular face of the membrane is an early event in apoptosis, the safe removal of cellular debris, and initiation of the clotting cascade.1 The mechanisms by which PS distribution is altered are poorly understood but are generally assumed to involve proteins facilitating either inward or outward PS transport (“flippases” or “floppases,” respectively), and/or scramblases mediating bidirectional, headgroup-nonspecific phospholipid transport.2,3 The rate of PS translocation has, for example, been found to be sensitive to the altered expression of the ATP-binding cassette transporter ABCA1, suggesting that this protein might either directly transport PS or regulate another transporter.4 PS exposure in apoptosis is also accompanied by a decrease in the normal packing of plasma membrane phospholipids, which can be detected by increased binding of the lipophilic dye MC540.5 While it is not known whether the 2 phenomena are mechanistically related, exposure of PS (detected by binding of annexin V [AV]) and decreased lipid packing have been reported to identify equivalent populations of early apoptotic cells.6,7
Loss of PS asymmetry can also occur in the absence of apoptosis, for example, in T cells stimulated via the ATP receptor P2X7,8 and in a population of nonapoptotic CD4+CD45RBlo activated/memory T cells.9 PS has also been reported to be constitutively exposed on most or all viable murine B cells,10,11 with a level of surface PS of 10 to 100 times that of T cells that does not increase upon stimulation, indicating complete loss of lipid asymmetry.10,11 In contrast to these reports, we show that PS distribution on murine B lymphocytes, as for most cells, is asymmetric, although PS is rapidly exposed ex vivo in the absence of exogenous signal. PS exposure was accelerated by stimulation with a calcium ionophore and was preceded by and dependent on a decrease in plasma membrane lipid packing. This change in lipid packing could not be dissociated from cell shrinkage and membrane deformity, suggesting that loss of PS asymmetry may occur at sites of membrane-bending at which lipid packing is decreased in one leaflet.
Materials and methods
Mice
Friend virus B-type (FVB/n) and nonobese diabetic (NOD) mice were bred at the Biological Services Unit at Imperial College, Hammersmith Campus, London, United Kingdom. Mice lacking CD45 have been described elsewhere12 and were compared with age-matched nontransgenic mice from the same breeding program at The Babraham Institute, Babraham, Cambridge, United Kingdom. Mice lacking ABCA1 have been described elsewhere4 and were maintained on a DBA/1J background in a pathogenfree facility at Charles River Laboratories in Lyon, France. Where studied, ABCA1-deficient mice were compared with wild-type mice from the same breeding colony. All other strains were from Harlan-Olac (Bicester, United Kingdom). All home office and local ethical guidelines for the care of laboratory animals were followed.
PS is localized in the inner membrane leaflet of murine B cells and exposed on stimulation. (A) Lymphocytes from C57BL/10 mice were labeled with anti-CD4APC (T-cell-specific) and anti-CD19PERCP (B-cell-specific) antibodies and preincubated with AVFITC. (Ai) Density plots of the rate of PS exposure (binding of AV) by T cells (CD4+; left panel) and B cells (CD19+; right panel) following stimulation with 5 μM calcimycin (arrows). (Aii) PS exposure by T- and B-cell populations following stimulation. Data are from the experiment shown in panel Ai, plotted as histograms to compare AV binding by cell populations gated at t = 0 seconds (black line) and t = 500 seconds (gray line) in T cells (left panel) and B cells (right panel). (B) Lymphocytes from C57BL/10 mice were labeled with anti-CD19PERCP (B-cell-specific) antibodies, preincubated with AVFITC and stimulated with calcimycin at the doses shown. Density plots illustrate the rate and degree of PS exposure (AV binding). (C) Spontaneous PS exposure and cell death in B-lymphocyte populations ex vivo. Lymphocytes from C57BL/10 mice were labeled with anti-CD4CYCHROME and anti-CD19APC antibodies, to distinguish T and B cells, and incubated with AVFITC. (Ci) Histograms show PS exposure (AV binding) by T cells (CD4+) and B cells (CD19+)30 minutes (black lines) and 2 hours (gray lines) after animals were killed. (Cii) Line graph shows the proportion (± SD) of cells stained with anti-CD19 antibody (B cells) within “live cell” gates as determined by forward and side light scatter at times shown following the time the animals were killed.
PS is localized in the inner membrane leaflet of murine B cells and exposed on stimulation. (A) Lymphocytes from C57BL/10 mice were labeled with anti-CD4APC (T-cell-specific) and anti-CD19PERCP (B-cell-specific) antibodies and preincubated with AVFITC. (Ai) Density plots of the rate of PS exposure (binding of AV) by T cells (CD4+; left panel) and B cells (CD19+; right panel) following stimulation with 5 μM calcimycin (arrows). (Aii) PS exposure by T- and B-cell populations following stimulation. Data are from the experiment shown in panel Ai, plotted as histograms to compare AV binding by cell populations gated at t = 0 seconds (black line) and t = 500 seconds (gray line) in T cells (left panel) and B cells (right panel). (B) Lymphocytes from C57BL/10 mice were labeled with anti-CD19PERCP (B-cell-specific) antibodies, preincubated with AVFITC and stimulated with calcimycin at the doses shown. Density plots illustrate the rate and degree of PS exposure (AV binding). (C) Spontaneous PS exposure and cell death in B-lymphocyte populations ex vivo. Lymphocytes from C57BL/10 mice were labeled with anti-CD4CYCHROME and anti-CD19APC antibodies, to distinguish T and B cells, and incubated with AVFITC. (Ci) Histograms show PS exposure (AV binding) by T cells (CD4+) and B cells (CD19+)30 minutes (black lines) and 2 hours (gray lines) after animals were killed. (Cii) Line graph shows the proportion (± SD) of cells stained with anti-CD19 antibody (B cells) within “live cell” gates as determined by forward and side light scatter at times shown following the time the animals were killed.
Real-time flow cytometry
Mesenteric lymph node cells were prepared from adult mice. Cell suspensions in phenol red-free Dulbecco modified Eagle medium (DMEM; Sigma, Poole, United Kingdom) were stained with CD19PE, CD19PERCP, CD19APC, CD4APC, or CD4CYCHROME (Becton Dickinson, San Jose, CA) antibodies, as indicated. The use of differently labeled antibodies enabled B cells from 2 mouse strains to be detected differentially, and hence studied simultaneously in the same tube. Cells were washed and resuspended in DMEM and, where indicated, equilibrated with AVFITC or AVCY5 (AV; Becton Dickinson), or with 0.05 μM merocyanine 540 (MC540; Sigma) for 3 minutes. Where cells were stimulated, baseline fluorescence was established for 30 seconds to 1 minute prior to addition of 2 μMto5 μM calcium ionophore (calcimycin, A23 187; Sigma). Cells were monitored for PS exposure continuously in real time by flow cytometry on a FACScalibur machine and analyzed using CellQuest (Becton Dickinson) or FlowJo (Treestar.com, Ashland, OR) software. Forward light scatter (FSC) was used as a measure of the volume of spherical cells,13 its sensitivity being greatest when light is collected over an angle of less than 10 degrees14 as in the FACScalibur. Distortion of the plasma membrane was measured by an increase in side light scatter (SSC), which has previously been shown to be concomitant with cell shrinkage (decrease in FSC) early in apoptosis.14-16
DIDS (Sigma) was dissolved in water. Tamoxifen (Sigma) was dissolved in ethanol. Calcium ionophore, glybenclamide (Sigma), and the Ca2+ indicator Fluo-4AM (used at 0.25 μM; Molecular Probes, Leiden, The Netherlands) were dissolved in DMSO. All results are representative of at least 3 independent experiments.
Note on calcium ionophore concentrations
Because calcium ionophore (A23 187) is highly lipophilic, the concentration of ionophore required to induce PS flop (or MC540 uptake) is, in our experience, more dependent on the amount of cell membrane in the tube (and this is likely to include red cell contamination/debris/dead cells gated out during analysis) than on volume of the reaction mix. For this reason, responses of cells from different sources were compared in a single tube (to ensure identical ionophore to membrane concentrations in a given experiment). Changing ionophore and cell concentrations in a reaction mix have equivalent (but opposite) effects on responses.
Confocal imaging of murine lymphocytes stained with MC540 and AV
Murine mesenteric lymph node cells, freshly dissected, were adhered to glass cover slips coated with poly-l-lysine (Sigma). Cells were exposed for 1 minute to 10 μM calcimycin in the presence or absence of 1 μM MC540, successively fixed in a solution of 4% formaldehyde, 4% sucrose in phosphate-buffered saline (PBS), and, where indicated, stained with AV-Alexa Fluor 568 (Molecular Probes). All cells were also stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; 15 mg/mL; 1:10 000 dilution; Molecular Probes, Eugene, OR) for nuclear DNA. Cells were imaged with a Leica SP confocal microscope equipped with a 100×/1.4 PlanApoChromat oil-immersion objective lens (Leica, Wetzlar, Germany). Merocyanine 540 was excited with the 488-nm line of an argon laser and AV-Alexa Fluor 568 with the 561-nm line of a solid state laser; the emitted fluorescence collected through a triple-dichroic mirror (488/568/663) with a 498-nm to 700-nm bandpass filter and 575-nm to 700-nm bandpass filter, respectively. DAPI staining of nuclear DNA was excited with the 351-nm line of an ultraviolet (UV) laser, and emission fluorescence collected with a 396-nm to 508-nm bandpass filter. Stacks of confocal sections separated by 0.3-μm increments were taken and images analyzed with Metamorph 5.0v1 software (Universal Imaging, Downington, PA). Figures were prepared with Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA).
PS exposure by B cells is strain dependent. (A) Lymphocytes from NZW and DBA/2 mice were labeled with anti-CD19APC and anti-CD19PERCP antibodies, respectively (to allow B cells from each strain to be easily distinguished), mixed, and preincubated with AVFITC. Panels show density plots of the rate of PS exposure (binding of AV) by B cells from NZW (left panel) and from DBA/2 mice (center panel) measured in the same tube following stimulation with 3 μM calcimycin (arrows). The right panel shows an alternative representation of the same data, plotted as the percentage of cells with exposed PS as a function of time. (B) An equivalent experiment to that in panel A but with lymphocytes derived from NOD and C57BL/10 mice. (C) Cells were stained as in panel A to identify B-cell populations after mixing. Bars show the percentage (mean ± SD, n = 4) of B cells from DBA/2 and NZW (left panel), or NOD and C57BL/10 mice (right panel), with exposed PS approximately 7 minutes after stimulation with 3 μM calcimycin. (D) Cells from NZW and NOD mice were stained as in panel A to distinguish B-cell populations. Panels show density plots of the rate of PS exposure (binding of AV) by B cells from NZW (left panel) and NOD mice (center panel) following stimulation with 3 μM calcimycin (arrows). The rate of PS exposure was equivalent in the 2 strains. In similar experiments, the rate of PS exposure by B-cell populations from NOD and/or NZW mice was faster than that that by cells from 129, C3H, CBA, FVB/n, and NIH mice (not shown).
PS exposure by B cells is strain dependent. (A) Lymphocytes from NZW and DBA/2 mice were labeled with anti-CD19APC and anti-CD19PERCP antibodies, respectively (to allow B cells from each strain to be easily distinguished), mixed, and preincubated with AVFITC. Panels show density plots of the rate of PS exposure (binding of AV) by B cells from NZW (left panel) and from DBA/2 mice (center panel) measured in the same tube following stimulation with 3 μM calcimycin (arrows). The right panel shows an alternative representation of the same data, plotted as the percentage of cells with exposed PS as a function of time. (B) An equivalent experiment to that in panel A but with lymphocytes derived from NOD and C57BL/10 mice. (C) Cells were stained as in panel A to identify B-cell populations after mixing. Bars show the percentage (mean ± SD, n = 4) of B cells from DBA/2 and NZW (left panel), or NOD and C57BL/10 mice (right panel), with exposed PS approximately 7 minutes after stimulation with 3 μM calcimycin. (D) Cells from NZW and NOD mice were stained as in panel A to distinguish B-cell populations. Panels show density plots of the rate of PS exposure (binding of AV) by B cells from NZW (left panel) and NOD mice (center panel) following stimulation with 3 μM calcimycin (arrows). The rate of PS exposure was equivalent in the 2 strains. In similar experiments, the rate of PS exposure by B-cell populations from NOD and/or NZW mice was faster than that that by cells from 129, C3H, CBA, FVB/n, and NIH mice (not shown).
Calcium ionophore-stimulated PS exposure in murine B cells is ABCA1 independent. Lymphocytes from ABCA1-deficient mice and age-matched controls from the same colony were labeled with anti-CD19APC and anti-CD19PERCP antibodies, respectively, mixed and preincubated with AVFITC or MC540. Histograms show (A) the percentage of CD19+ B cells having exposed PS (indicated by binding of AV) following stimulation with 5 μM calcimycin (arrow), or (B) MC540 uptake by CD19+ B cells following stimulation with 2.5 μM calcimycin (arrow). ABCA1-/-, red line; parental, blue line.
Calcium ionophore-stimulated PS exposure in murine B cells is ABCA1 independent. Lymphocytes from ABCA1-deficient mice and age-matched controls from the same colony were labeled with anti-CD19APC and anti-CD19PERCP antibodies, respectively, mixed and preincubated with AVFITC or MC540. Histograms show (A) the percentage of CD19+ B cells having exposed PS (indicated by binding of AV) following stimulation with 5 μM calcimycin (arrow), or (B) MC540 uptake by CD19+ B cells following stimulation with 2.5 μM calcimycin (arrow). ABCA1-/-, red line; parental, blue line.
Results
PS exposure on unstimulated and stimulated B cells
It has been reported that PS is exposed at high levels on unstimulated murine B cells and that further exposure does not occur on stimulation.10,11 We assessed levels of cell-surface PS on rapidly isolated lymphocytes from C57BL/10 mice by real-time flow cytometry in the continuous presence of AVFITC. After basal binding of AVFITC was established, cells were treated with calcium ionophore (calcimycin, A23 187) to stimulate nonspecific uptake of Ca2+ and consequent PS exposure (Figure 1). Unstimulated B and T cells studied in the same tube exhibited comparably low-level binding of AV, indicating restriction of PS to the inner leaflet of the plasma membrane in both cell types. Stimulation with calcium ionophore resulted in marked PS exposure in both lymphocyte populations. Thus, as for most cell types and in contrast to previous reports,10,11 unstimulated B lymphocytes (like T lymphocytes) possess an asymmetric lipid bilayer with PS largely restricted to the inner leaflet. Stimulation resulted in PS “flopping” to the outer leaflet: the time at which PS exposure was detectable and the peak AV-binding per cell depended on the concentration of calcimycin added (Figure 1B).
To assess the possibility that mouse strain-dependent differences in B-cell distribution of PS account for differences between our data and those published previously, we studied lymphocytes from a variety of normal and autoimmune strains. To ensure direct comparison of responses, B cells from strains being studied were stained with differently labeled anti-CD19 antibodies (to allow subsequent discrimination during analysis) and mixed before stimulation. Responses were compared by real-time flow cytometry in a single tube. PS was restricted to the inner leaflet of B cells from all mice, although significant strain-dependent differences in sensitivity to stimulation were apparent. For example, responses of B cells from the autoimmune-prone New Zealand white (NZW) and NOD strains of mice were comparable (not shown), but responded more rapidly than those from C57BL/10, DBA/2 (Figure 2), and 6 other nonautoimmune prone strains (129/Ola, BALB/c, C3H, CBA/Ca, FVB/n, NIH; not shown). That responses were more pronounced in cells from autoimmune-prone mouse strains is consistent with our recent observation that calcium ionophore-stimulated lymphocytes from patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) externalize PS more readily than do control cells.17
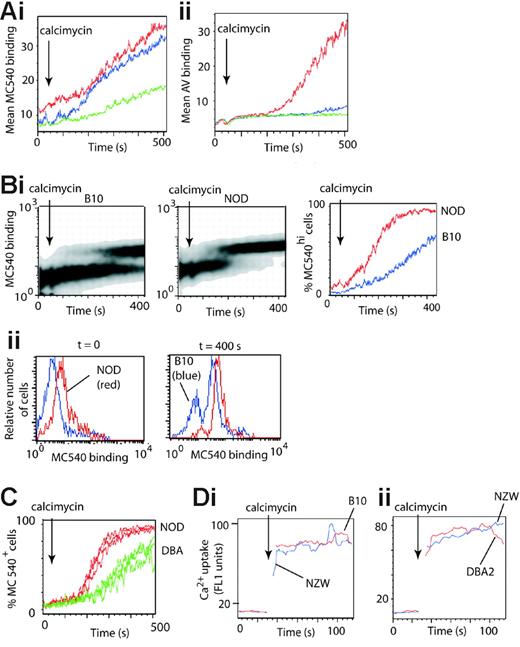
A decrease in lipid packing precedes PS exposure in B cells. (A) Lymphocytes from C57BL/10 mice were labeled with anti-CD19APC antibody and preincubated with either 0.05 μM MC540 or AVFITC. Histograms show (mean fluorescence units) the rate of (Ai) MC540 binding or (Aii) PS exposure (binding of AV) by CD19+ B cells following stimulation with 3 μM (red lines), 2 μM (blue lines), or 1 μM (green lines) calcimycin. (Bi) Lymphocytes from C57BL/10 and NOD mice were labeled with anti-CD19APC and anti-CD19PERCP antibodies, respectively, mixed, and preincubated with 0.05 μM MC540. Panels show density plots of the rate of decrease in lipid packing (increased uptake of MC540) by B cells from C57BL/10 (left panel) and from NOD mice (center panel) in the same tube following stimulation with 2 μM calcimycin (arrows). The right panel shows an alternative plot of the same data plotted as the percentage of cells binding high levels of MC540 as a function of time. Red lines, NOD B cells; blue lines, C57BL/10 B cells. (Bii) Histograms of MC540 binding by the B-cell populations shown in panel Bi, gated at t = 0 seconds and t = 400 seconds. Red lines, NOD B cells; blue lines, C57BL/10 B cells. (C) Results of 3 independent experiments equivalent to that in panel Bi but with lymph node cells derived from NOD (red lines) and DBA/2 (green lines) mice. (D) Calcium uptake in stimulated B cells. (Di) Lymphocytes from the C57BL/10 (red line) and NZW (blue line) mice were labeled with anti-CD19APC and anti-CD19PERCP antibodies respectively, mixed, incubated with 0.25 μM of the calcium-sensitive indicator Fluo-4AM for 10 minutes, washed, and stimulated with 0.2 μM calcimycin (arrow). Plots show Ca2+ uptake as a function of time. (Dii) Shows an equivalent experiment to that in panel Di but with lymph node cells derived from DBA/2 (red line) and NZW (blue line) mice.
A decrease in lipid packing precedes PS exposure in B cells. (A) Lymphocytes from C57BL/10 mice were labeled with anti-CD19APC antibody and preincubated with either 0.05 μM MC540 or AVFITC. Histograms show (mean fluorescence units) the rate of (Ai) MC540 binding or (Aii) PS exposure (binding of AV) by CD19+ B cells following stimulation with 3 μM (red lines), 2 μM (blue lines), or 1 μM (green lines) calcimycin. (Bi) Lymphocytes from C57BL/10 and NOD mice were labeled with anti-CD19APC and anti-CD19PERCP antibodies, respectively, mixed, and preincubated with 0.05 μM MC540. Panels show density plots of the rate of decrease in lipid packing (increased uptake of MC540) by B cells from C57BL/10 (left panel) and from NOD mice (center panel) in the same tube following stimulation with 2 μM calcimycin (arrows). The right panel shows an alternative plot of the same data plotted as the percentage of cells binding high levels of MC540 as a function of time. Red lines, NOD B cells; blue lines, C57BL/10 B cells. (Bii) Histograms of MC540 binding by the B-cell populations shown in panel Bi, gated at t = 0 seconds and t = 400 seconds. Red lines, NOD B cells; blue lines, C57BL/10 B cells. (C) Results of 3 independent experiments equivalent to that in panel Bi but with lymph node cells derived from NOD (red lines) and DBA/2 (green lines) mice. (D) Calcium uptake in stimulated B cells. (Di) Lymphocytes from the C57BL/10 (red line) and NZW (blue line) mice were labeled with anti-CD19APC and anti-CD19PERCP antibodies respectively, mixed, incubated with 0.25 μM of the calcium-sensitive indicator Fluo-4AM for 10 minutes, washed, and stimulated with 0.2 μM calcimycin (arrow). Plots show Ca2+ uptake as a function of time. (Dii) Shows an equivalent experiment to that in panel Di but with lymph node cells derived from DBA/2 (red line) and NZW (blue line) mice.
Decreased lipid packing is concomitant with and dependent on cell shrinkage. (A) Lymphocytes from NOD mice were stained with anti-CD19 antibody, preincubated with 0.05 μM MC540, and stimulated with 2 μM calcimycin (arrows) alone (red lines) or together with 10 μM clotrimazole (blue lines). Plots show (Ai) cell shrinkage (decrease in forward light scatter [FSC]), (Aii) percentage of MC540hi cells, and (Aiii) membrane buckling (side light scatter, [SSC]) plotted as a function of time. (B) Lymphocytes from NOD mice were stained with anti-CD19 antibody. (Bi) Cells were preincubated with AVFITC alone (left panel) or together with 1 μM tamoxifen (right panel) and stimulated with 2 μM calcimycin (arrows). Density plots show the change in AV binding as a function of time. (Bii) Cells were preincubated with 0.05 μM MC540 and stimulated with 2 μM calcimycin (arrows) alone (red lines) or in the presence of 1 μM tamoxifen (added immediately before baseline recording; blue lines). Plots show cell shrinkage (left panel; decrease in forward light scatter [FSC]); mean MC540 fluorescence units (center panel), membrane buckling (right panel; side light scatter [SSC]); all plotted as a function of time.
Decreased lipid packing is concomitant with and dependent on cell shrinkage. (A) Lymphocytes from NOD mice were stained with anti-CD19 antibody, preincubated with 0.05 μM MC540, and stimulated with 2 μM calcimycin (arrows) alone (red lines) or together with 10 μM clotrimazole (blue lines). Plots show (Ai) cell shrinkage (decrease in forward light scatter [FSC]), (Aii) percentage of MC540hi cells, and (Aiii) membrane buckling (side light scatter, [SSC]) plotted as a function of time. (B) Lymphocytes from NOD mice were stained with anti-CD19 antibody. (Bi) Cells were preincubated with AVFITC alone (left panel) or together with 1 μM tamoxifen (right panel) and stimulated with 2 μM calcimycin (arrows). Density plots show the change in AV binding as a function of time. (Bii) Cells were preincubated with 0.05 μM MC540 and stimulated with 2 μM calcimycin (arrows) alone (red lines) or in the presence of 1 μM tamoxifen (added immediately before baseline recording; blue lines). Plots show cell shrinkage (left panel; decrease in forward light scatter [FSC]); mean MC540 fluorescence units (center panel), membrane buckling (right panel; side light scatter [SSC]); all plotted as a function of time.
We suggest that the apparent discrepancy with previous reports arises from the fact that, in contrast to T cells, PS in B cells becomes exposed to the surface ex vivo within 2 hours even in the absence of exogenous stimulation (Figure 1C). Moreover, this exposure of PS on B cells was associated with their relatively rapid death ex vivo, as evidenced by a steadily falling proportion of CD19+ cells within the live gate population (Figure 1C). The disproportionate death of CD19+ B cells ex vivo is routinely observed in our hands, although the rate appears to be affected by factors such as temperature and serum concentration (data not shown). In previous studies,10,11 B lymphocytes appear to have been maintained for a relatively long period ex vivo as this population was enriched over magnetic bead columns prior to analysis. In our experiments, by contrast, B cells were not physically isolated, but instead gated electronically on a flow cytometer on the basis of fluorescent antibody binding.
PS translocation by B cells is independent of ABCA1
The rate of calcium ionophore-stimulated PS exposure has previously been reported to be decreased in erythrocytes and fibroblasts lacking the ABC transporter ABCA1, and increased in HeLa cells overexpressing ABCA1 following transfection,4 leading to the suggestion that ABCA1 might transport PS to the cell surface or regulate a heterologous PS transporter. However, we showed that the rates of PS exposure by ionophore-stimulated B lymphocytes from parental and from ABCA1-/- mice could not be distinguished (Figure 3), indicating that ABCA1 is not involved in calcium ionophore-stimulated PS transport in murine B cells.
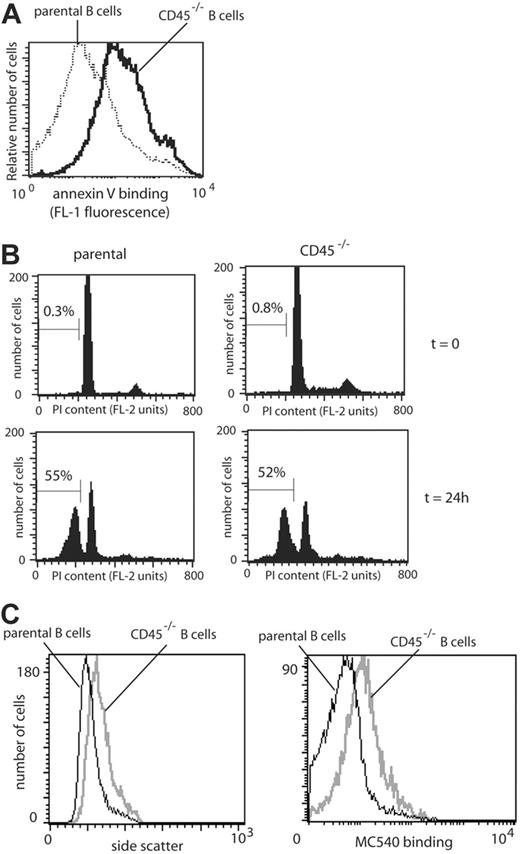
PS exposure on B cells from CD45-/- and parental mice. (A) Lymphocytes from CD45-/- (solid line) or parental (CD45+/+) mice (dotted line) were labeled with anti-CD19APC and anti-CD19PE antibodies, respectively, to discriminate between CD19+ B cells from each mouse after mixing. Cells were mixed and preincubated with AVFITC to assess PS exposure. (B) To compare the rate of cell death among spleen cells from parental (C57BL/6) and CD45-/- mice, the sub-G1 DNA content of lymphocytes at t = 0 and 24 hours was assessed through uptake of propidium iodide (PI) analyzed by flow cytometry. Percentages of cells containing sub-G1 levels of DNA are shown. (C) Lymphocytes from CD45-/- (gray line) or parental (CD45+/+) mice (black line) were labeled with anti-CD19APC and anti-CD19FITC antibodies, respectively, to discriminate between CD19+ B cells from each mouse, mixed, and incubated with MC540 to assess lipid packing.
PS exposure on B cells from CD45-/- and parental mice. (A) Lymphocytes from CD45-/- (solid line) or parental (CD45+/+) mice (dotted line) were labeled with anti-CD19APC and anti-CD19PE antibodies, respectively, to discriminate between CD19+ B cells from each mouse after mixing. Cells were mixed and preincubated with AVFITC to assess PS exposure. (B) To compare the rate of cell death among spleen cells from parental (C57BL/6) and CD45-/- mice, the sub-G1 DNA content of lymphocytes at t = 0 and 24 hours was assessed through uptake of propidium iodide (PI) analyzed by flow cytometry. Percentages of cells containing sub-G1 levels of DNA are shown. (C) Lymphocytes from CD45-/- (gray line) or parental (CD45+/+) mice (black line) were labeled with anti-CD19APC and anti-CD19FITC antibodies, respectively, to discriminate between CD19+ B cells from each mouse, mixed, and incubated with MC540 to assess lipid packing.
Cell shrinkage and MC540 uptake by murine B cells
MC540 is a fluorescent, lipophilic dye that binds preferentially to the outer leaflet of plasma membrane bilayers with relatively loosely packed lipids,5,18-20 including those of apoptotic cells.6,7 We compared the kinetic relationship between MC540-binding (to measure lipid packing) and AV-binding (to measure PS exposure). Following calcium ionophore-stimulation in the presence of MC540, dye uptake exhibited a rapid increase (Figure 4A). Increased MC540 uptake preceded loss of PS asymmetry and, indeed, occurred at concentrations of ionophore below that required to stimulate detectable PS exposure (Figure 4A). Hitherto, the only event (other than calcium uptake) shown to precede PS translocation following calcimycin-stimulation is cell shrinkage due to K+ efflux via KCa3.1 (formerly IKCa1).21 Cell shrinkage and consequent buckling of the cell membrane can be measured in flow cytometry by a decrease in forward light scatter (FSC) and concomitant increase in side light scatter (SSC).14-16 The rates of MC540 uptake (Figure 4B-C and Figure S1, which is available on the Blood website; see the Supplemental Figures link at the top of the online article) and cell shrinkage (Figure S1), as for PS externalization (AV-binding), were strain dependent with B cells from NOD and NZW mice exhibiting a relatively rapid response, suggesting that strain-dependent variation in rates of PS translocation reflects upstream differences in rates of cell shrinkage and decrease in lipid packing. Strain-dependent variations did not, however, appear to reflect differences in calcium uptake (Figure 4D). Decreased B-cell lipid packing (onset of MC540 uptake), shrinkage, and membrane buckling were concomitant (Figure 5A-B). Both membrane distortion and decreased lipid packing depended on cell shrinkage, as evidenced by their inhibition by clotrimazole, which block KCa3.1-dependent K+ efflux, and also by tamoxifen, which inhibits volume-regulated chloride channels.22,23 DIDS and glybenclamide, which are thought to inhibit loss of lipid asymmetry downstream of cell shrinkage, had no effect on MC540 uptake (not shown). Together, these data strongly suggest that decreased lipid packing requires cell shrinkage and membrane buckling, and that all precede PS exposure.
PS is constitutively exposed on CD45-deficient B lymphocytes
We have recently shown that PS translocation in T cells is negatively regulated by the tyrosine phosphatase CD45.9 Consistent with this finding (and in contrast to parental B cells) we found PS to be constitutively exposed on CD45-/- B cells (Figure 6). One possible explanation for the elevated exposure of PS on B cells from CD45-/- mice is that these cells might lack normal signaling pathways mediating survival, thereby leading to increased apoptosis. However, we have found no evidence for increased apoptosis in peripheral T or B cells from CD45-/- mice, assessed using 4 different assays (Figure 6, and Figure S2). It therefore seems unlikely that the high PS expression on more than 90% CD45-/- B cells could be significantly explained by apoptosis, particularly as B-cell numbers are higher in CD45-/- mice than the parentals.24 Thus, B cells from CD45-/- mice are viable but have exposed PS. Importantly, unstimulated CD45-/- B cells exhibit higher SSC and MC540 binding than do parental B cells stained in the same tube (Figure 6), consistent with the hypothesis that membrane curvature and decreased lipid packing promote PS exposure.
MC540 binding and PS exposure occur at sites of membrane blebbing
The finding that PS exposure depends on prior cell shrinkage, an increase in membrane buckling, and decreased lipid packing suggests a model whereby calcium ionophore-stimulated PS exposure might occur at energetically favorable sites on developing membrane blebs (see “Discussion”). We therefore analyzed MC540 binding and PS exposure by calcium ionophore-stimulated lymphocytes by confocal microscopy. As we have found MC540 to reduce the binding of AV per cell, the 2 stains were analyzed separately. Figure 7A shows a stack of confocal sections through calcium ionophore-stimulated lymphocytes stained with AV. Blebs are visible as small protrusions at the cell surface. Consistent with the hypothesis, binding of both AV and MC540 occur preferentially at sites of membrane blebbing (Figure 7). Bleb localization is somewhat more apparent for AV than MC540, consistent with the fact that MC540 exhibits greater background binding to unstimulated cells.
Merocyanine 540 and annexin V preferentially bind to membrane blebs on calcium ionophore-stimulated lymphocytes. Confocal sections of murine mesenteric lymph node cells are shown. Lymphocytes from C57BL/10 mice were stimulated with 10 μM calcimycin and stained with 1 μM MC540 (green) or annexin V AlexaFluor 568 (red). DAPI staining of nuclear DNA is in blue. Scale bars indicate 10 μm. (A) Stack of confocal sections separated by 0.3 μm increments showing membrane blebs. (B) Single confocal sections.
Merocyanine 540 and annexin V preferentially bind to membrane blebs on calcium ionophore-stimulated lymphocytes. Confocal sections of murine mesenteric lymph node cells are shown. Lymphocytes from C57BL/10 mice were stimulated with 10 μM calcimycin and stained with 1 μM MC540 (green) or annexin V AlexaFluor 568 (red). DAPI staining of nuclear DNA is in blue. Scale bars indicate 10 μm. (A) Stack of confocal sections separated by 0.3 μm increments showing membrane blebs. (B) Single confocal sections.
Discussion
In most cells, phospholipids are distributed asymmetrically in the plasma membrane, with the great majority of PS in the inner leaflet. PS translocation to the outer leaflet is required for the immunologically silent removal of apoptotic cells.25 PS exposure can also occur on nonapoptotic cells, for example on murine T lymphocytes following stimulation of the ATP receptor P2X7 (note: murine B cells do not express P2X7).8 Moreover, PS is constitutively exposed at the surface of many cells of the CD45RBlo subpopulation of viable CD4+ activated/memory lymphocytes. Importantly, PS distribution between inner and outer leaflets modulates the activity of several membrane proteins.9
Reports that viable murine B cells, in contrast to T cells, do not exhibit lipid asymmetry have been suggested to reflect a key aspect of their biology.10,11 Here, we show that, in contrast to published findings,10,16 PS in B cells, as in T cells, is in fact largely confined to the inner leaflet of the plasma membrane and surface exposure can readily be induced following stimulation with a calcium ionophore. A likely explanation for the previous report that PS distribution is not asymmetric is the observation that B cells spontaneously translocate PS to the cell surface relatively rapidly ex vivo. In contrast to previous studies, we did not physically isolate B cells, but instead gated cells electronically during analysis of flow cytometric data, thereby reducing time spent by cells ex vivo.
The rate of PS translocation in B cells, as measured by the proportion of responding cells at a given time, was found to be strain dependent. These strain-dependent events also correlated with differences in rates of MC540 uptake, supporting the hypothesis that decreased lipid packing is required for PS translocation. Significantly, in view of previous findings that lymphocytes from patients with rheumatoid arthritis and systemic lupus erythematosus have relatively high rates of PS translocation,17 the rate of PS exposure was highest in B cells from the autoimmune-prone strains NZW and NOD. Both NZW and NOD strains are also associated with susceptibility to lupuslike disease.26-28 In principle, a tendency toward PS exposure might promote susceptibility to SLE through increases in shedding of proinflammatory microvesicles,29,30 presentation of PS itself as an autoantigen,31 or subsequent increased release of other autoantigens in the form of apoptotic debris. As the increased rate of PS translocation in NOD and NZW lymphocytes is downstream of an increased rate of cell shrinkage and concomitant decrease in lipid packing, the data suggest that aberrant volume regulation may contribute toward susceptibility to lupuslike disease.
We have shown previously that ionophore-stimulated PS exposure is subsequent to and dependent on cell shrinkage caused by K+ efflux via KCa3.1.21 We show here that cell shrinkage results in a decrease in lipid packing, as evidenced by increased uptake of the dye MC540, presumably a consequence of membrane buckling. Thus, significant changes in the organization of the lipid bilayer appear to precede PS translocation. Uptake of MC540 and decreased lipid packing occur simultaneously and are both blocked by inhibitors of volume regulatory ion channels (along with cell shrinkage). Hence, PS translocation appears to depend on upstream cell shrinkage and concomitant decrease in lipid packing. Tamoxifen, a potent inhibitor of volume-regulated chloride channels, blocked PS exposure at concentrations as low as 1 μM, making it, to our knowledge, the most potent inhibitor of lipid scrambling described to date.
It is an apparent paradox that lipid packing decreases upon cell shrinkage, whereas an increase in packing might be anticipated. Nevertheless, as has been observed previously,14-16 distortion of the plasma membrane (measured by increase in SSC) is concomitant with cell shrinkage early in apoptosis. We suggest, therefore, that buckling of the plasma membrane as cells shrink distorts the lipid bilayer locally such that MC540 inserts into the plasma membrane at sites of decreased lipid binding at the apex of blebs (Figure 8). PS+ blebs/microvesicles are known to form and be shed following stimulation with calcium ionophore.2,32,33,35 Our observation that cell shrinkage, which is required for PS exposure, is strongly associated with decreased lipid packing provides a model for headgroup-nonspecific loss of lipid asymmetry (“scrambling”; Figure 8). In this model, bending of the membrane during cell shrinkage creates domains at the base and apex of blebs in which phospholipids in the outer leaflet are in relative excess or deficit, respectively, creating regions favorable for phospholipid flip-flop. Movement of inner leaflet phospholipids such as PS to the outer leaflet would be favored at the apex of blebs and that of PC to the inner leaflet favored at the base. This model predicts that phospholipid movement is bidirectional and headgroup nonspecific (consistent with the phenomenon of phospholipid “scrambling”), and that PS exposure is predominantly found on shed particles and is less apparent on the cell bodies that remain following shedding.32,33 Consistent with our hypothesis, MC540 binding and PS exposure on calcium ionophore-stimulated lymphocytes both localized to sites of membrane blebbing (Figure 7). The model also implies that a dedicated protein may not be required to mediate PS translocation. The search for a specific protein that mediates bidirectional transport has been a notable failure.36,37 While it has been shown that the rate of PS exposure is sensitive to altered expression of the ABC transporter, ABCA1, in human B cells,38 murine erythrocytes,4 and following ABCA1 transfection,4,38 the rate of PS exposure by murine ABCA1-/- and parental B cells could not be distinguished (Figure 3). Hence, whereas ABCA1 can modulate rates of PS exposure, it does not appear to be required. If ABCA1 is a floppase, therefore, its loss can be compensated for by another enzyme in genetically modified murine B lymphocytes. Alternatively, and perhaps more likely, ABCA1 is not itself a floppase but can modulate the rate of PS translocation indirectly.
Model for calcium-stimulated PS translocation. (A) In the absence of stimulation, the plasma membrane of most healthy cells is asymmetric, with PS and PE restricted to the inner leaflet and PC largely restricted to the outer leaflet. (B) Following stimulation with a calcium ionophore, K+ and Cl- ions leave the cell, water follows, and the cell shrinks.21 As a consequence of cell shrinkage, the plasma membrane buckles, prior to the shedding of microvesicles/blebs. At the apex of microvesicles/blebs the packing of phospholipids is relatively loose, reducing the energetic barrier to outward movement of PS and PE. A decrease in lipid packing in the outer leaflet allows the insertion of MC540. In the inner leaflet, PS and PE are tightly packed, increasing the energetic favorability of outward phospholipid “flop.” At the base of microvesicles/blebs, PC is tightly packed in the outer leaflet and PS/PE loosely packed in the inner leaflet, favoring inward phospholipid flip. (C) PS and PE flop out at the apex, and PC flips in at the base. Thus, averaged across the microvesicle/bleb, transport of phospholipids appears headgroup-nonspecific and bidirectional (“scrambling”). (D) As it is no longer tethered to cytoplasmic/cytoskeletal proteins, PS in the outer leaflet is relatively free to move laterally outside of the bleb (unfilled arrow). (E) Microvesicles/blebs are shed. Phospholipid balance is partially restored, with most PS/PE distributed in inner and outer leaflets. Consistent with the model above, both we32 and others33 have found that shed particles, but not cell remnants, exhibit high levels of exposed PS, though some movement of PS out of the bleb is likely and has been found on cell remnants in other systems.34
Model for calcium-stimulated PS translocation. (A) In the absence of stimulation, the plasma membrane of most healthy cells is asymmetric, with PS and PE restricted to the inner leaflet and PC largely restricted to the outer leaflet. (B) Following stimulation with a calcium ionophore, K+ and Cl- ions leave the cell, water follows, and the cell shrinks.21 As a consequence of cell shrinkage, the plasma membrane buckles, prior to the shedding of microvesicles/blebs. At the apex of microvesicles/blebs the packing of phospholipids is relatively loose, reducing the energetic barrier to outward movement of PS and PE. A decrease in lipid packing in the outer leaflet allows the insertion of MC540. In the inner leaflet, PS and PE are tightly packed, increasing the energetic favorability of outward phospholipid “flop.” At the base of microvesicles/blebs, PC is tightly packed in the outer leaflet and PS/PE loosely packed in the inner leaflet, favoring inward phospholipid flip. (C) PS and PE flop out at the apex, and PC flips in at the base. Thus, averaged across the microvesicle/bleb, transport of phospholipids appears headgroup-nonspecific and bidirectional (“scrambling”). (D) As it is no longer tethered to cytoplasmic/cytoskeletal proteins, PS in the outer leaflet is relatively free to move laterally outside of the bleb (unfilled arrow). (E) Microvesicles/blebs are shed. Phospholipid balance is partially restored, with most PS/PE distributed in inner and outer leaflets. Consistent with the model above, both we32 and others33 have found that shed particles, but not cell remnants, exhibit high levels of exposed PS, though some movement of PS out of the bleb is likely and has been found on cell remnants in other systems.34
Based on studies of bacterial membranes, it has recently been suggested that phospholipid flip-flop may be promoted by a wide range of α-helical membrane-spanning proteins that provide energetically favorable sites for charged headgroups to traverse the hydrophobic interior of bilayer.39 This model, which is complementary to our own, removes the need to posit (but does not disprove) the existence of a specific bidirectional phospholipid transporter.
Prepublished online as Blood First Edition Paper, May 9, 2006; DOI 10.1182/blood-2005-11-012328.
Supported by the Medical Research Council of Great Britain (J.I.E., A.S., and C.F.H.), institutional funding from Institut National de la Santé et de la Recherche Médicale (INSERM) and Centre National de la Recherche Scientifique (CNRS) (G.C. and S.D.), and specific funding from the European Union (G.C. and S.D.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




![Figure 5. Decreased lipid packing is concomitant with and dependent on cell shrinkage. (A) Lymphocytes from NOD mice were stained with anti-CD19 antibody, preincubated with 0.05 μM MC540, and stimulated with 2 μM calcimycin (arrows) alone (red lines) or together with 10 μM clotrimazole (blue lines). Plots show (Ai) cell shrinkage (decrease in forward light scatter [FSC]), (Aii) percentage of MC540hi cells, and (Aiii) membrane buckling (side light scatter, [SSC]) plotted as a function of time. (B) Lymphocytes from NOD mice were stained with anti-CD19 antibody. (Bi) Cells were preincubated with AVFITC alone (left panel) or together with 1 μM tamoxifen (right panel) and stimulated with 2 μM calcimycin (arrows). Density plots show the change in AV binding as a function of time. (Bii) Cells were preincubated with 0.05 μM MC540 and stimulated with 2 μM calcimycin (arrows) alone (red lines) or in the presence of 1 μM tamoxifen (added immediately before baseline recording; blue lines). Plots show cell shrinkage (left panel; decrease in forward light scatter [FSC]); mean MC540 fluorescence units (center panel), membrane buckling (right panel; side light scatter [SSC]); all plotted as a function of time.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/5/10.1182_blood-2005-11-012328/2/m_zh80170600330005.jpeg?Expires=1770417972&Signature=vXhCWLUYsUhhnC2vC13Adg4bPiHmoYtXxXIjyPid3iK0CvLECv2b5~khN6A5P9-TDELzZKwf1UcemDQiBoAceHahUliTumR~LOAiMR98VuEDarB1VO9ITOse~l~NBxZ0cRv1LyaKQsJZ6l5JvzdtYVv~veM9kIrPC5p7VVXux1dcPtL-K0j5drwZc2FzZLreJZMBvs-FwEPhUXkC5SaZC2Q~oYFgBNmPzGf0gGE4k9t8cTgU0SEJA8warkskd-OsN0vRlPJOczeE894iOH63T5Kk93Fl5dz7DzEsXntta2SRlT7q-X33Gw1VacG3ERD-KDE9a4CK2Gbcqbf1uTCXjA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal