Programmed cell death is vital for a number of pathophysiologic settings. Apoptotic cells are rapidly engulfed by phagocytes (ie, macrophages), which in turn acquire an anti-inflammatory phenotype known as alternative activation or the M2-type. Here we show that interaction of apoptotic cells with macrophages attenuates cell death pathways in the latter. Protection of human macrophages required phosphoinositide 3-kinase (PI3K), extracellular signal-regulated kinase 1/2 (ERK1/2), and Ca2+ signaling, and correlated with Bcl-XL and Bcl-2 up-regulation as well as Ser136-Bad phosphorylation. Unexpectedly, neither phagocytosis nor binding of apoptotic debris to the phagocyte was necessary to induce protection. Surprisingly, apoptotic cells released sphingosine-1-phosphate (S1P), mainly derived from sphingosine kinase 2, as a survival messenger. This points to an active role of apoptotic cells in preventing cell destruction in their neighborhood, with implications for innate immunity and inflammation.
Introduction
Multicellular life needs to maintain cellular homeostasis by balancing proliferation versus death. Physiologic cell death, known as apoptosis, therefore plays an important role in a number of physiologic as well as pathophysiologic settings, including cancer and inflammation.1,2 Apoptotic cell death avoids an inflammatory response because apoptotic debris is rapidly cleared by professional phagocytes such as macrophages. In addition, one finds a shift from a proinflammatory macrophage phenotype toward an anti-inflammatory one.3,4 Phagocytosis of apoptotic cells (ACs) thus resembles a mode of alternative macrophage activation (M2 phenotype) in contrast to the classical, proinflammatory activation (M1 phenotype) in response to bacteria or T-cell-derived interferon-γ (IFN-γ).5,6 Along this phenotype switch, a proinflammatory mediator profile (eg, tumor necrosis factor-α [TNF-α] or nitric oxide) changes to an immunosuppressive one, characterized by, for example, interleukin-10 (IL-10), transforming growth-β1 (TGF-β1), or prostaglandin E2.7-9 Recent studies showed protection from apoptosis induced by cytokine withdrawal in macrophages that had ingested apoptotic cells or protection from apoptosis after phagocytosis of apoptotic cells in endothelial cells.10,11 Changes in the macrophage phenotype in response to phagocytosis of apoptotic cells are thought to be either dependent on recognition of the apoptotic cell by the phagocyte via receptor-ligand interactions or on signals derived from phagocytosis itself. More recently, the secretion of an apoptotic cell-derived phagocyte attraction factor has been identified as the bioactive phospholipid lysophosphatidylcholine (LPC). LPC is produced by apoptotic cells in response to caspase-3-dependent activation of a calcium-independent phospholipase A2.12 Besides, TGF-β is released from apoptotic cells and participates in coordinating anti-inflammatory responses.13 We present evidence that apoptotic cells affect the survival response of phagocytes by releasing the bioactive lipid sphingosine-1-phosphate (S1P), mainly derived from sphingosine kinase 2 (Sphk2). S1P provoked activation of PI3K-, ERK1/2-, and Ca2+-dependent survival pathways in human macrophages, which are established downstream signaling circuits of S1P receptors.14 Indeed, S1P-receptor type 1 (S1P1) expression on macrophages conveyed protection, at least in part. S1P from ACs increased expression of the antiapoptotic proteins Bcl-2 and Bcl-XL, as well as inactivation of proapoptotic Bad by phosphorylation at Ser136. Our findings support the notion that ACs release S1P, thus affecting macrophage survival. This might have implications for the pathogenesis of a variety of human diseases such as inflammation or cancer.
Materials and methods
Cell culture and reagents
RAW 264.7 mouse monocytes/macrophages, THP-1 human monocytic cells, Jurkat T cells, and MCF-7 breast carcinoma cells were maintained in RPMI 1640. HEK293 embryonal kidney and RKO colon carcinoma cells were cultured in DMEM high glucose, each supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated FCS. LY29004 and PD98059 were purchased from Alexis (Lausen, Switzerland). The Ca2+-scavenger Bapta-AM was from Molecular Probes (Leiden, The Netherlands). LPA, S1P, PAF, and LPC were from Avanti (Alabaster, AL).
Human monocyte isolation and culture
Human monocytes were isolated as described.15 In brief, monocytes were isolated from buffy coats (DRK-Blutspendedienst Baden-WürttembergHessen, Institut für Transfusionsmedizin und Immunhämatologie Frankfurt am Main, Frankfurt, Germany) using Ficoll-Hypaque gradients (PAA Laboratories, Cölbe, Germany). Peripheral blood mononuclear cells (PBMCs) were washed twice with PBS and were allowed to adhere to culture dishes (Primaria 3072; Becton Dickinson, Heidelberg, Germany) for 1 hour at 37°C. Nonadherent cells were removed. Monocytes were then differentiated into macrophages with RPMI 1640 containing 10% AB-positive human serum (PAA Laboratories) for 7 days.
Generation of apoptotic and necrotic cells
For the generation of apoptotic cells (ACs), Jurkat cells were cultured in RPMI 1640 without FCS. Apoptosis was induced with 0.5 μg/mL staurosporine (Sigma, St Louis, MO) for 3 hours or 0.05 ng/mL anti-Fas (Immunotools, Friesoythe, Germany) for 5 hours.16 ACs were washed twice prior to the addition to macrophages. Necrosis was induced by heating Jurkat cells for 30 minutes at 56°C.16 Apoptosis in RKO, MCF-7, and HEK293 was induced as described in Table 1. Cell death was quantified by fluorescence-activated cell sorting (FACS) after staining with annexin V and propidium iodide (Immunotech, Miami, FL). Agonist concentrations and incubation times have been chosen to provoke roughly 80% apoptotic cell death.
Macrophage protection by ACM from different cell lines and stimuli
Cell line and stimulus . | Protection, %* . | SD, n ≥ 3 . |
|---|---|---|
| Jurkat | ||
| Staurosporine, 0.5 μg/mL, 3 h | 100 | ± 0 |
| Anti-Fas, 0.05 ng/mL, 5 h | 91.1 | ± 18.6 |
| RKO | ||
| Staurosporine, 0.5 μg/mL, 5 h | 103.3 | ± 23.6 |
| MCF-7 | ||
| Staurosporine, 0.5 μg/mL, 4 h | 91.3 | ± 12.9 |
| HEK293 | ||
| Staurosporine, 0.5 μg/mL, 8 h | 80.5 | ± 19.7 |
| TNF-α/CHX, 50 ng/mL/10 μg/mL, 24 h | 106.7 | ± 27.5 |
Cell line and stimulus . | Protection, %* . | SD, n ≥ 3 . |
|---|---|---|
| Jurkat | ||
| Staurosporine, 0.5 μg/mL, 3 h | 100 | ± 0 |
| Anti-Fas, 0.05 ng/mL, 5 h | 91.1 | ± 18.6 |
| RKO | ||
| Staurosporine, 0.5 μg/mL, 5 h | 103.3 | ± 23.6 |
| MCF-7 | ||
| Staurosporine, 0.5 μg/mL, 4 h | 91.3 | ± 12.9 |
| HEK293 | ||
| Staurosporine, 0.5 μg/mL, 8 h | 80.5 | ± 19.7 |
| TNF-α/CHX, 50 ng/mL/10 μg/mL, 24 h | 106.7 | ± 27.5 |
100% protection is set as the difference in caspase-3 activity (fold of control) between untreated THP-1 macrophages and those treated with ACM (16 hours) from Jurkat cells killed with staurosporine, followed by induction of apoptosis in macrophages with 250 μM etoposide for 8 hours. Caspase-3 activity in whole-cell lysates was quantified as described in “Materials and methods.” Differences in caspase-3 activity between untreated and ACM-treated macrophages were significant (P < .05) for each cell line and stimulus indicated
Production and treatments of conditioned media
To produce apoptotic cell-conditioned medium (ACM), cells were washed twice with PBS subsequent to apoptosis induction, followed by an incubation for another 2 hours in RPMI 1640 with 10% FCS. ACM was harvested by centrifugation (13 000g, 5 minutes), and filtration through 0.2-μm pore filters removes apoptotic bodies.12,17 Conditioned media from necrotic (NCM) or viable (VCM) cells were prepared accordingly. For heat inactivation, media were heated at 100°C for 1 hour. Enzyme digestion was carried out with 50 μg/mL proteinase K or 0.1% trypsin at 37°C for 1 hour. Afterward, enzymes were inactivated by heat treatment and/or the use of excess soybean trypsin inhibitor. Chloroform extraction was performed with 1 volume of chloroform and was repeated at least twice.12 Specific extraction of S1P was performed essentially as described.18 In brief, we used a 2-step partitioning protocol with chloroform/methanol/HCL (100:200:1), first in alkaline conditions followed by re-extraction under acidic conditions. The acidic organic phase was evaporated to dryness and the lipid extract was resolved in PBS containing 0.4% fatty acid-free BSA for cell culture or ethanol for quantitative analysis for 30 minutes at 37°C followed by sonification. Cells were generally exposed to conditioned media for 16 hours. Inhibitors were added 2 hours prior to conditioned medium. In one set of experiments, S1P secretion into the medium was blocked with 20 μM dimethylsphingosine (Biomol, Plymouth Meeting, PA) during incubations of Jurkat cells with staurosporine.
Coculture experiments
THP-1 cells were differentiated to macrophages at a density of 2 × 105 cells/mL, treated with 50 nM tetradecanoyl-phorbol-13-acetat (TPA) (Sigma) for 24 hours, washed, and cultured for another 24 hours without TPA. Apoptotic, necrotic, or viable cells were added at a ratio of 1:5, and cocultures were performed for 16 hours at 37°C. Afterward, residual Jurkat cells were washed off. Conditioned media from cocultures were produced as described. In one set of experiments, we used transwell inserts to ensure spatial separation between macrophages and apoptotic cells.19
Induction and measurement of apoptosis
Apoptosis in THP-1 and RAW 264.7 macrophages was induced with 250 μM etoposide (Alexis) for 8 hours.20 Primary human macrophages were subjected to apoptosis using 10 ng/mL human recombinant TNF-α (Biomol) and 10 μg/mL CHX. Caspase-3 activity was quantified following the cleavage of Ac-DEVD-AMC (Alexis) as described.21 Phosphatidylserine exposure, membrane integrity, and mitochondrial membrane depolarization (ΔΨ) were visualized with annexin V, propidium iodide, and JC-1 (Biomol),22 by flow cytometry.
Western blot analysis
Western blot analysis was performed as described.23 A monoclonal antibody directed against Bcl-2 (BD Transduction Laboratories, Lexington, KY) and polyclonal antibodies toward phospho-Ser136-Bad (Cell Signalling, Beverly, MA), Bcl-XL (BD Transduction Laboratories), Sphk1, Sphk2 (Exalpha Biologicals, Watertown, MA), and S1P1 (Orbigen, San Diego, CA) were used. Western blots were quantified with AIDA software (raytest, Straubenhardt, Germany).
Down-regulation of S1P1
Internalization of S1P1 was induced as described.24 Briefly, primary human macrophages were incubated with 100 nM FTY720 (Cayman Chemical, Ann Arbor, MI) for 1 hour. Afterward, cells were washed twice with PBS and cultured for another 48 hours. S1P1 down-regulation was analyzed by Western blotting compared with unstimulated controls.
siRNA treatments
Sphk1-specific siRNA and Sphk2 predesigned Hs_SPHK2_3 siRNA (Qiagen, Hilden, Germany) were nucleofected into Jurkat cells using Nucleofector technology (Amaxa, Gaithersburg, MD) according to the manufacturer's instructions. Nucleofection efficiency, analyzed by FACS after nucleofection of Jurkat cells with pmaxGFP (Amaxa), was around 80% (data not shown). Additionally, Sphk1 and Sphk2 knock-down were controlled by Western blot analysis in comparison with siCONTROL nontargeting Duplex #1 (Dharmacon, Lafayette, CO).
S1P quantification in cell culture supernatants
For S1P quantification using liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, lipids were extracted from cell culture supernatants of 5 × 106 cells cultured in medium devoid of FCS with chloroform/HCl (50:1) after addition of the internal standard C17-sphingosine-1-phosphate (C17-S1P). Extraction was repeated 3 times to achieve maximum recovery. The combined organic phases were evaporated to dryness and resolved in ethanol for quantification. After liquid-liquid extraction, concentrations of S1P and the internal standard were determined by liquid chromatography coupled to tandem mass spectrometry. High-performance liquid chromatography (HPLC) analysis was done under gradient conditions using a Luna C18 column (150 cm × 2 mm ID, 5-μm particle size, and 10-nm pore size; Phenomenex, Aschaffenburg, Germany). MS/MS analyses were performed on a 4000 Q TRAP triple quadrupole mass spectrometer with a TurboIonSpray source (Applied Biosystems, Darmstadt, Germany). Precursor-to-product ion transitions of m/z 380→82 for S1P and 366→250 for C17-S1P were used for the Multiple Reaction Monitoring (MRM). Concentrations of the calibration standards, quality controls, and unknowns were evaluated by Analyst software 1.4 (Applied Biosystems). Linearity of the calibration curve was proven from 1 to 250 ng/mL. The coefficient of correlation for all measured sequences was at least 0.99. Variations in accuracy and intraday and interday precision (n = 6 for each concentration, respectively) were less than 15% over the range of calibration.
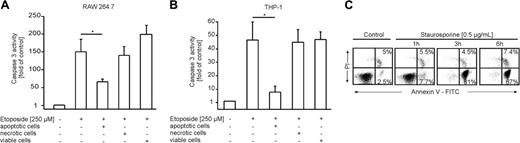
Apoptotic cells attenuate caspase-3 activation in murine and human macrophages. Caspase-3 activity in (A) murine RAW 264.7 macrophages and (B) human monocyte-derived THP-1 macrophages normalized to untreated control cells. Macrophages were pretreated with AC, NC, or VC or remained as controls for 16 hours. Cell death was induced for 8 hours with 250 μM etoposide. Data are presented as the mean ± SD from 3 independent experiments. Differences between untreated and AC-treated cells are statistically significant and marked with an asterisk (P < .05). (C) Apoptosis in Jurkat cells induced with 0.5 μg/mL staurosporine followed by annexin V/PI staining after 1, 3, and 6 hours. Parameters were assessed by flow cytometry.
Apoptotic cells attenuate caspase-3 activation in murine and human macrophages. Caspase-3 activity in (A) murine RAW 264.7 macrophages and (B) human monocyte-derived THP-1 macrophages normalized to untreated control cells. Macrophages were pretreated with AC, NC, or VC or remained as controls for 16 hours. Cell death was induced for 8 hours with 250 μM etoposide. Data are presented as the mean ± SD from 3 independent experiments. Differences between untreated and AC-treated cells are statistically significant and marked with an asterisk (P < .05). (C) Apoptosis in Jurkat cells induced with 0.5 μg/mL staurosporine followed by annexin V/PI staining after 1, 3, and 6 hours. Parameters were assessed by flow cytometry.
Statistical analysis
P values were calculated using the paired Student t test and considered significant at P values less than .05, unless indicated otherwise.
Results
Protection from apoptosis by apoptotic cells
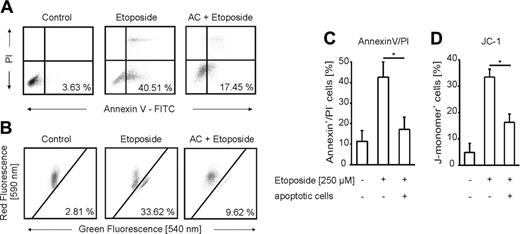
To determine the impact of apoptotic cells in protection from apoptosis, we incubated RAW 264.7 mouse macrophages (Figure 1A) and THP-1 human monocyte-derived macrophages (Figure 1B) with apoptotic Jurkat cells (ACs) and subsequently induced apoptosis with etoposide (Figure 1). The amount of apoptosis in Jurkat cells was around 80% after 3-hour treatments with 0.5 μg/mL staurosporine, while necrosis started to increase only from 6 hours onward (Figure 1C). In contrast to necrotic (NC) or viable (VC) Jurkat cells, cocultures with apoptotic cells attenuated caspase-3 activation in RAW 264.7 as well as THP-1 cells compared with controls. Similar results were obtained with alternative apoptosis-inducing stimuli such as nitric oxide (NO) or TNF-α in combination with cycloheximide (CHX) (data not shown). Looking for phosphatidylserine exposure and mitochondrial membrane integrity as alternative apoptotic markers confirmed that ACs indeed attenuate etoposide-induced apoptosis (Figure 2A-D). THP-1 cells preincubated with ACs showed significantly less annexin V binding following treatment with etoposide compared with controls (Figure 2A,C). Macrophage staining with the fluorescent dye JC-1, which differentiates between metabolically active (red fluorescence) and defective (green fluorescence) mitochondria, further verified prevention of cell death (Figure 2B,D). In macrophages, the presence of apoptotic cells preserved mitochondrial integrity that otherwise would be lost upon etoposide treatment.
Apoptotic cells attenuate macrophage apoptosis. (A-D) THP-1 cells were untreated or pretreated with ACs for 16 hours. Apoptosis was induced for 8 hours with 250 μM etoposide. Viability was assessed by (A,C) annexin V/PI staining, (B,D) JC-1 staining, and subsequent analysis by flow cytometry. (C-D) Data are presented as the mean ± SD from 4 independent experiments. Differences between untreated and AC-treated cells are statistically significant and are marked with an asterisk (P < .05) for both parameters shown.
Apoptotic cells attenuate macrophage apoptosis. (A-D) THP-1 cells were untreated or pretreated with ACs for 16 hours. Apoptosis was induced for 8 hours with 250 μM etoposide. Viability was assessed by (A,C) annexin V/PI staining, (B,D) JC-1 staining, and subsequent analysis by flow cytometry. (C-D) Data are presented as the mean ± SD from 4 independent experiments. Differences between untreated and AC-treated cells are statistically significant and are marked with an asterisk (P < .05) for both parameters shown.
Survival is transmitted by a soluble factor
We questioned the type of interaction between ACs and macrophages that was required to attenuate etoposide-evoked cell death. First, we investigated the contribution of phagocytosis. Therefore cytochalasine D, an inhibitor of phagocytosis, was added to differentiated THP-1 cells prior to incubations with ACs (Figure 3A). Upon stimulation with etoposide, there was no difference in the ability of ACs to protect from cell death, irrespective of the presence or absence of cytochalasine D. This excludes a contribution of phagocytosis in conveying protection.
Next, we collected conditioned medium from macrophage/apoptotic cell cocultures (ACCM) to check whether a soluble factor might be involved. For control reasons, only conditioned medium of macrophage/necrotic cell cocultures (NCCM) and conditioned medium from apoptotic cells (ACM) were used (Figure 3B). Of interest, not only ACCM but also ACM protected THP-1 cells from etoposide-induced apoptosis, while NCCM did not (Figure 3B). This finding argued for a factor released from apoptotic cells. For further validation, we performed transwell experiments separating macrophages and apoptotic cells by a permeable membrane to exclude direct cell-cell contacts. Spatial separation of macrophages and apoptotic cells still conveyed protection (Figure 3C). These results argue for a soluble, apoptotic cell-derived factor to communicate protection toward etoposide in macrophages.
Macrophage protection caused by an apoptotic cell-derived soluble factor. (A-C) Caspase-3 activity in etoposide-treated (8 hours, 250 μM) THP-1 macrophages normalized to untreated controls. Cells and media were preincubated for 16 hours. Data are presented as the mean ± SD from 3 independent experiments. Statistical differences between samples are marked with an asterisk (P < .05). (A) Cells remained untreated or were treated with apoptotic cells alone or in combination with 2 μM cytochalasine D. (B) Cells remained untreated or were treated with conditioned media from macrophages cocultured with apoptotic cells (ACCM), necrotic cells (NCCM), or conditioned medium from apoptotic cells (ACM). (C) Cells remained untreated or were pre-exposed to apoptotic cells in transwell inserts for 16 hours prior to stimulation with etoposide.
Macrophage protection caused by an apoptotic cell-derived soluble factor. (A-C) Caspase-3 activity in etoposide-treated (8 hours, 250 μM) THP-1 macrophages normalized to untreated controls. Cells and media were preincubated for 16 hours. Data are presented as the mean ± SD from 3 independent experiments. Statistical differences between samples are marked with an asterisk (P < .05). (A) Cells remained untreated or were treated with apoptotic cells alone or in combination with 2 μM cytochalasine D. (B) Cells remained untreated or were treated with conditioned media from macrophages cocultured with apoptotic cells (ACCM), necrotic cells (NCCM), or conditioned medium from apoptotic cells (ACM). (C) Cells remained untreated or were pre-exposed to apoptotic cells in transwell inserts for 16 hours prior to stimulation with etoposide.
Survival is induced by apoptotic cells of different origin and proapoptotic stimuli
To analyze whether the production of an antiapoptotic factor by ACs was an inherent feature of apoptotic cell death, we induced apoptosis in different cell lines by activating intrinsic (staurosporine) as well as extrinsic (TNF-α, anti-Fas) pathways of apoptosis. Conditioned medium from apoptotic Jurkat, RKO, HEK293, and MCF-7 cells elicited protection in etoposide-induced THP-1 macrophage apoptosis (Table 1). These results imply that macrophage protection by ACs resembles a general principle, because it was reproduced by using distinct cell lines treated either with extrinsic or intrinsic apoptotic stimuli. Moreover, by using caspase-3-negative MCF-7 cells, we were able to rule out caspase-3 activation being involved in the production of the protective factor.12
Characterization of the protective factor
With the intention to characterize the nature of the protective factor present in ACM, we noticed that neither heating conditioned medium at 100°C nor digestion with proteinase K or trypsin abrogated the protective principle (Table 2), thus ruling out a protein factor. However, extracting lipids from ACM with chloroform eliminated protection (Table 2). These experiments argue for a lipid factor as the messenger in question.
Macrophage protection by ACM
ACM treatment . | Protection, %* . | SD . | P, n ≥ 3† . |
|---|---|---|---|
| None | 100 | ± 0 | ND |
| Heat inactivation, 100°C | 87.4 | ± 2.4 | .4 |
| Proteinase K digestion, 50 μM | 98.5 | ± 8.2 | .39 |
| Trypsin digestion, 0.1% | 99.2 | ± 12 | .46 |
| Chloroform extraction | –5.2 | ± 17.5 | .007 |
ACM treatment . | Protection, %* . | SD . | P, n ≥ 3† . |
|---|---|---|---|
| None | 100 | ± 0 | ND |
| Heat inactivation, 100°C | 87.4 | ± 2.4 | .4 |
| Proteinase K digestion, 50 μM | 98.5 | ± 8.2 | .39 |
| Trypsin digestion, 0.1% | 99.2 | ± 12 | .46 |
| Chloroform extraction | –5.2 | ± 17.5 | .007 |
ND indicates not determined
100% protection is set as the difference in caspase-3 activity (fold of control) between untreated and ACM-treated (16 hours) THP-1 macrophages after induction of apoptosis with 250 μM etoposide for 8 hours. Caspase-3 activity in whole-cell lysates was quantified as described in “Materials and methods”
Statistical analysis was performed using the paired Student t test
Characterization of the protective principle
To establish the time dependency for protection, we incubated THP-1 cells with ACM for 1 to 6 hours prior to stimulation with etoposide. Significant protection was first detected after 6 hours, with the further notion that protection, once established, lasted for 48 hours (data not shown). Additional examinations confirmed protection by ACM in human monocyte-derived macrophages, as determined by annexin V/propidium iodide staining and caspase-3 activity measurements (Figure 4A). To characterize protective signaling pathways, we used pharmacologic inhibitors known to interfere with antiapoptotic pathways. LY29004 (an inhibitor of PI3K signaling), PD98059 (an inhibitor of ERK1/2), and Bapta-AM (a calcium chelator) restored caspase-3 activation in human primary macrophages upon preincubation with ACM. Sulfasalazine, a nuclear factor-κB (NF-κB) inhibitor, showed no effect (data not shown). Inhibitor studies imply that preferentially a PI3K-sensitive pathway transmits protection in response to ACM, while Ca2+ as well as ERK1/2 signaling contributes to protection to a lower extent (Figure 4B). Supportive argumentation comes from Bcl-2 expression and Ser136-Bad phosphorylation. Treatment of human primary macrophages with TNF-α and CHX decreased Bcl-2 levels and Bad phosphorylation compared with controls, with the notion that these changes were restored by ACM. In analogy to caspase activity, PD98059 reduced Bcl-2 stabilization, while Bad phosphorylation was decreased to control levels by LY29004 and Bapta-AM (Figure 4B). In order to investigate the contribution of other Bcl-2 family members toward protection, expression of Mcl-1 and Bcl-XL, known to be relevant for macrophage survival,25,26 was analyzed in response to ACM. Although Mcl-1 was not significantly induced by ACM in macrophages, we observed up-regulation of Bcl-XL (Figure 5B). We conclude that the ACM-protective principle is reflected at the level of pro- and/or antiapoptotic Bcl-2 family members. This is further supported by JC-1 experiments (Figure 2B-C), which imply the involvement of mitochondria.
Contribution of sphingosine-1-phosphate toward protection
Results so far pointed to a lipid mediator in protection toward apoptotic cell death. We tested candidates such as platelet-activating factor (PAF), lysophosphatidic acid (LPA), lysophosphatidylcholine (LPC), or sphingosine-1-phosphate (S1P), known to manipulate apoptosis in other test systems. Increasing concentrations of each compound, compared with ACM, were added to primary macrophages in combination with TNF-α/CHX to provoke apoptosis (Table 3). Only S1P induced significant protection at a rather low concentration of 1 μM (Table 3). LPC attenuated cell death at higher concentrations only, and PAF as well as LPA increased rather than decreased death.
Bioactive lipids and human macrophage survival
Treatment (prior to apoptosis induction) . | Caspase-3 activity, fold of control* . | SD . | P, n ≥ 3† . |
|---|---|---|---|
| None | 30.6 | ± 4 | ND |
| ACM | 5.3 | ± 3.6 | .02 |
| LPA | |||
| 1 μM | 30.1 | ± 12.8 | .35 |
| 10 μM | 33.8 | ± 19.7 | .49 |
| 100 μM | 39.5 | ± 20.8 | .35 |
| SIP | |||
| 1 μM | 13.2 | ± 9.2 | .05 |
| 10 μM | 10 | ± 6.3 | .02 |
| 100 μM | 6.1 | ± 4 | .006 |
| PAF | |||
| 1 μM | 40.5 | ± 5.1 | .4 |
| 10 μM | 61.1 | ± 41.3 | .15 |
| 100 μM | 97.2 | ± 59.6 | .09 |
| LPC | |||
| 1 μM | 54.2 | ± 27.8 | .25 |
| 10 μM | 32.1 | ± 18.4 | .44 |
| 100 μM | 20 | ± 5.2 | .2 |
Treatment (prior to apoptosis induction) . | Caspase-3 activity, fold of control* . | SD . | P, n ≥ 3† . |
|---|---|---|---|
| None | 30.6 | ± 4 | ND |
| ACM | 5.3 | ± 3.6 | .02 |
| LPA | |||
| 1 μM | 30.1 | ± 12.8 | .35 |
| 10 μM | 33.8 | ± 19.7 | .49 |
| 100 μM | 39.5 | ± 20.8 | .35 |
| SIP | |||
| 1 μM | 13.2 | ± 9.2 | .05 |
| 10 μM | 10 | ± 6.3 | .02 |
| 100 μM | 6.1 | ± 4 | .006 |
| PAF | |||
| 1 μM | 40.5 | ± 5.1 | .4 |
| 10 μM | 61.1 | ± 41.3 | .15 |
| 100 μM | 97.2 | ± 59.6 | .09 |
| LPC | |||
| 1 μM | 54.2 | ± 27.8 | .25 |
| 10 μM | 32.1 | ± 18.4 | .44 |
| 100 μM | 20 | ± 5.2 | .2 |
ND indicates not determined
Human primary macrophages were exposed to ACM, LPA, S1P, PAF, or LPC at the indicated concentrations for 16 hours. Apoptosis was induced with 10 ng/mL TNF-α and 10 μg/mL CHX for 8 hours. Caspase-3 activity in whole-cell lysates was quantified as described in “Material and methods”
Statistical analysis was performed using the paired Student t test
Protection of primary human macrophages by ACM is PI3K-, Ca2+-, and ERK1/2-dependent. (A) Human primary monocyte-derived macrophages remained untreated or were exposed to ACM for 16 hours. Following a medium change, apoptosis was induced for 8 hours with 10 ng/mL TNF-α and 10 μg/mL CHX. Viability was assessed by annexin V/PI staining and FACS analysis. (i) Data in histograms are presented as the mean ± SD from 3 independent experiments. Statistical differences are marked with an asterisk (P < .05). (ii) Typical FACS traces. (B) Human primary macrophages were not treated or were exposed to ACM for 16 hours in the presence or absence of LY92004, PD98059, or Bapta-AM at the indicated concentrations. Apoptosis was induced for 8 hours with 10 ng/mL TNF-α and 10 μg/mL CHX. (i) Caspase-3 activity. Data are presented as the mean ± SD from 6 independent experiments. Significant differences between samples are marked with an asterisk (P < .05). Western analysis shows expression of Bcl-2 and phospho-Ser136-Bad. One representative experiment of 3 is displayed. (ii) Quantification of Western data normalized to actin by using AIDA software. Data are presented as the mean ± SEM from 4 independent experiments.
Protection of primary human macrophages by ACM is PI3K-, Ca2+-, and ERK1/2-dependent. (A) Human primary monocyte-derived macrophages remained untreated or were exposed to ACM for 16 hours. Following a medium change, apoptosis was induced for 8 hours with 10 ng/mL TNF-α and 10 μg/mL CHX. Viability was assessed by annexin V/PI staining and FACS analysis. (i) Data in histograms are presented as the mean ± SD from 3 independent experiments. Statistical differences are marked with an asterisk (P < .05). (ii) Typical FACS traces. (B) Human primary macrophages were not treated or were exposed to ACM for 16 hours in the presence or absence of LY92004, PD98059, or Bapta-AM at the indicated concentrations. Apoptosis was induced for 8 hours with 10 ng/mL TNF-α and 10 μg/mL CHX. (i) Caspase-3 activity. Data are presented as the mean ± SD from 6 independent experiments. Significant differences between samples are marked with an asterisk (P < .05). Western analysis shows expression of Bcl-2 and phospho-Ser136-Bad. One representative experiment of 3 is displayed. (ii) Quantification of Western data normalized to actin by using AIDA software. Data are presented as the mean ± SEM from 4 independent experiments.
Considering a potential impact of S1P, we analyzed the effect of ACM-extracted lipids, the ACM aqueous phase, as well as conditioned medium derived from necrotic cells (NCM) in modulating death in primary human macrophages (Figure 5A). TNF-α/CHX-evoked caspase-3 activation was attenuated by the lipid but neither by the aqueous phase of ACM nor by NCM. Protection conveyed by ACM as well as S1P was reflected at the level of Bcl-2, Bcl-XL, and phospho-Bad (Figure 5B). ACM and S1P increased Bcl-2 as well as Bcl-XL expression and provoked Bad phosphorylation in analogy to results shown in Figure 4B. To strengthen a protective role of S1P, we applied dimethylsphingosine (DMS), an inhibitor of sphingosine kinase, to Jurkat cells 3 hours prior to initiation of cell death. ACM derived from DMS-treated Jurkat cells did not protect macrophages from TNF-α/CHX-induced apoptosis (Figure 5A). To further substantiate a role of S1P in protection, we used an extraction procedure designed to be specific for S1P.18 S1P extracted from ACM reproduced protection (Figure 5A), which underscores the assumption that indeed S1P is released from apoptotic cells to protect macrophages from entering pathways of cell demise.
Sphingosine-1-phosphate conveys protection. (A-C) Apoptosis in human primary macrophages was induced for 8 hours with 10 ng/mL TNF-α and 10 μg/mL CHX. (A) Cells remained untreated or were treated with ACM, the ACM-lipid fraction, the ACM-aqueous fraction, ACM derived from Jurkat cells exposed to DMS prior to initiation of apoptosis (ACM-DMS), or the ACM-lipid fraction specific for S1P extraction (S1P-extraction), 16 hours prior to induction of cell death. Caspase-3 activity was quantified as described in “Materials and methods.” Data are presented as the mean ± SD from 4 independent experiments. Significant differences between samples are marked with an asterisk (P < .01). (B) Cells were not treated, exposed to the ACM, or stimulated with 10 μM S1P for 16 hours. Western analysis shows expression of Bcl-2, Bcl-XL, and phosphorylated Ser136-Bad. One representative experiment of 3 is displayed. Histograms show quantification of Western data normalized to actin by using AIDA software. Data are presented as the mean ± SEM from 3 independent experiments. (C) Cells remained untreated or were exposed for 16 hours to ACM alone or to ACM after pretreatment with 100 nM FTY720 for 1 hour followed by further incubations of 48 hours. Afterward, TNF-α/CHX treatments lasted for 8 hours. Data are presented as the mean ± SD from 4 independent experiments. Significant differences between samples are marked with an asterisk (P < .05).
Sphingosine-1-phosphate conveys protection. (A-C) Apoptosis in human primary macrophages was induced for 8 hours with 10 ng/mL TNF-α and 10 μg/mL CHX. (A) Cells remained untreated or were treated with ACM, the ACM-lipid fraction, the ACM-aqueous fraction, ACM derived from Jurkat cells exposed to DMS prior to initiation of apoptosis (ACM-DMS), or the ACM-lipid fraction specific for S1P extraction (S1P-extraction), 16 hours prior to induction of cell death. Caspase-3 activity was quantified as described in “Materials and methods.” Data are presented as the mean ± SD from 4 independent experiments. Significant differences between samples are marked with an asterisk (P < .01). (B) Cells were not treated, exposed to the ACM, or stimulated with 10 μM S1P for 16 hours. Western analysis shows expression of Bcl-2, Bcl-XL, and phosphorylated Ser136-Bad. One representative experiment of 3 is displayed. Histograms show quantification of Western data normalized to actin by using AIDA software. Data are presented as the mean ± SEM from 3 independent experiments. (C) Cells remained untreated or were exposed for 16 hours to ACM alone or to ACM after pretreatment with 100 nM FTY720 for 1 hour followed by further incubations of 48 hours. Afterward, TNF-α/CHX treatments lasted for 8 hours. Data are presented as the mean ± SD from 4 independent experiments. Significant differences between samples are marked with an asterisk (P < .05).
Next, we addressed the question on the subtype of S1P-receptor being expressed on human macrophages and participating in protection. Primary human macrophages were prestimulated with 100 nM of the S1P-receptor agonist FTY720, and S1P1 expression was analyzed after 48 hours.24 Western blot analysis revealed a strong down-regulation of S1P1 following prestimulation with FTY720 compared with controls (Figure 5C). When ACM was added to cells with S1P1 being down-regulated, protection was significantly but not completely diminished compared with controls incubated only with ACM (Figure 5C). Since prestimulation of cells with 100 nM FTY720 was shown to specifically induce internalization of S1P-receptor subtypes 1 and 5,24 we concluded that S1P1 was at least partially responsible for macrophage protection by S1P because macrophages do not express S1P5.27,28
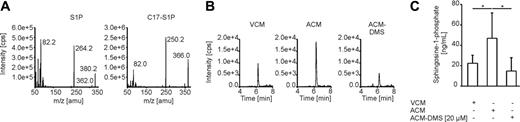
We then analyzed conditioned medium from control cells, ACM, and ACM from DMS-treated Jurkat cells for S1P amounts using liquid chromatography-tandem mass spectrometry analysis (Figure 6). Induction of Jurkat cell apoptosis with staurosporine increased the release of S1P into the cell supernatant compared with nonstimulated cells (Figure 6B-C). Moreover, S1P release from apoptotic cells was blocked by the sphingosine kinase inhibitor DMS (Figure 6B-C). These data corroborate release of the antiapoptotic molecule S1P from apoptotic cells.
Sphingosine kinase 2 contributes to S1P production in apoptotic cells
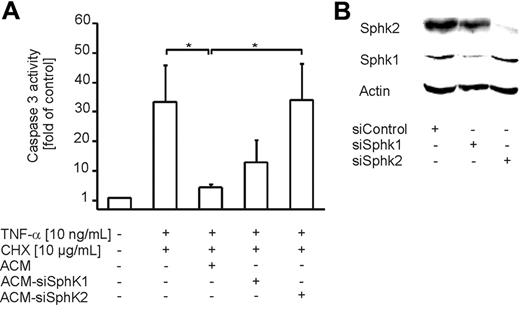
Since 2 isoforms of sphingosine kinase (Sphk) are known to date and DMS attenuates the activity of both enzymes, we used siRNA technology to determine which Sphk isoform contributes to S1P production in ACs. Therefore, we nucleofected Jurkat cells with siRNA for both Sphk isoforms. Nucleofected cells were subsequently used for the preparation of ACM. Induction of apoptosis with staurosporine in Jurkat cells was not affected by knock-down of Sphk1 or Sphk2 (data not shown). Western analysis revealed strong down-regulation of Sphk1 and Sphk2 in Jurkat cells after nucleofection of corresponding siRNA compared with control siRNA (Figure 7B). Incubation of human primary macrophages with ACM-siSphk2 significantly reduced protection compared with the efficacy of ACM, whereas ACM-siSphk1 was only marginally effective (Figure 7A). These data strongly suggest a role of Sphk2 in the production of S1P in apoptotic cells.
Measurement of S1P secretion by apoptotic cells. (A) Fragment spectra of S1P and the internal standard C17-S1P by using product ion scans in the positive ionization mode. Product ion scans were obtained by infusion of 100 to 1000 ng/mL of the respective analyte in methanol with a flow of 10 μL/min. (B-C) Jurkat cells were cultured in FCS-free medium, remained as controls (VCM), or were treated with 0.5 μg/mL staurosporine with (ACM-DMS) or without (ACM) the previous addition of DMS for 3 hours. After changing medium, incubation continued for another 2 hours and conditioned media were analyzed for the S1P content with liquid chromatography-tandem mass spectrometry. (B) Exemplary chromatograms of S1P representing 22.0, 42.1, and 12.2 ng/mL. (C) Quantification of S1P release in the supernatant of Jurkat cells. Data are presented as the mean ± SD from 6 independent experiments. Significant differences between samples are marked with an asterisk (P < .05).
Measurement of S1P secretion by apoptotic cells. (A) Fragment spectra of S1P and the internal standard C17-S1P by using product ion scans in the positive ionization mode. Product ion scans were obtained by infusion of 100 to 1000 ng/mL of the respective analyte in methanol with a flow of 10 μL/min. (B-C) Jurkat cells were cultured in FCS-free medium, remained as controls (VCM), or were treated with 0.5 μg/mL staurosporine with (ACM-DMS) or without (ACM) the previous addition of DMS for 3 hours. After changing medium, incubation continued for another 2 hours and conditioned media were analyzed for the S1P content with liquid chromatography-tandem mass spectrometry. (B) Exemplary chromatograms of S1P representing 22.0, 42.1, and 12.2 ng/mL. (C) Quantification of S1P release in the supernatant of Jurkat cells. Data are presented as the mean ± SD from 6 independent experiments. Significant differences between samples are marked with an asterisk (P < .05).
Sphk2 expression in apoptotic cells contributes to the release of S1P. (A) Apoptosis in human primary macrophages was induced for 8 hours with 10 ng/mL TNF-α and 10 μg/mL CHX. Cells remained untreated or were treated with ACM or ACM from Jurkat cells nucleofected with siRNA to target Sphk1 or Sphk2, 16 hours prior to induction of cell death. Caspase-3 activity was quantified as described in “Materials and methods.” Data are presented as the mean ± SD from 4 independent experiments. Significant differences between samples are marked with an asterisk (P < .05). (B) Jurkat cells were nucleofected with control siRNA or siRNA against Sphk1 or Sphk2. Western analysis shows Sphk1 and Sphk2 expression. One representative experiment of 3 is displayed.
Sphk2 expression in apoptotic cells contributes to the release of S1P. (A) Apoptosis in human primary macrophages was induced for 8 hours with 10 ng/mL TNF-α and 10 μg/mL CHX. Cells remained untreated or were treated with ACM or ACM from Jurkat cells nucleofected with siRNA to target Sphk1 or Sphk2, 16 hours prior to induction of cell death. Caspase-3 activity was quantified as described in “Materials and methods.” Data are presented as the mean ± SD from 4 independent experiments. Significant differences between samples are marked with an asterisk (P < .05). (B) Jurkat cells were nucleofected with control siRNA or siRNA against Sphk1 or Sphk2. Western analysis shows Sphk1 and Sphk2 expression. One representative experiment of 3 is displayed.
Discussion
Macrophages display opposing activities in human (patho)physiology by adopting different phenotypes, thereby shaping inflammation.5 Our study supports the notion that apoptotic cells not only modulate proversus anti-inflammatory mediator release from macrophages but also affect their susceptibility toward apoptosis. Cell protection was initiated by liberating sphingosine-1-phosphate from apoptotic cells.
S1P is known to provoke survival in different cell types by binding to high-affinity G protein-coupled receptors.14,29,30 Five different receptors for S1P are known to date, of which subtypes 1 to 4 are shown to be expressed on human macrophages.27,28 Down-regulation of S1P1 on primary macrophage partially diminished protection by ACM. This may indicate that protection of S1P in macrophages is transmitted by more than one subtype expressed on their surface. Further studies using knock-down approaches will clarify the relative contribution of S1P-receptor subtypes toward macrophage protection by S1P. S1P receptors activate PI3K and/or ERK1/2.29 Inhibitor studies pointed to these pathways in conveying protection by ACM, which resulted in phosphorylation of Bad at Ser136. It is an established function of the PI3K/Akt pathway to attenuate the apoptotic machinery,31 among other cells in macrophages.32 ERK1/2 activation in macrophages usually is a signal downstream to growth factors or cytokines such as M-CSF, IL-3, or TGF-β.33,34 TGF-β seems of interest, because it is secreted by apoptotic cells.13 Yet the possibility that ERK1/2 is activated by TGF-β to contribute to Bcl-2 up-regulation must be excluded under our experimental conditions, concerning the lipophilic nature of the factor under investigation. It was shown previously that extracellular S1P inhibits bone marrow-derived macrophage cell death induced by M-CSF withdrawal.26 This was related to PKB activation, increased Bcl-XL expression, and decreased acid sphingomyelinase activity, thus reducing ceramide production. Although we noticed increased expression of Bcl-XL, its induction was not affected by blocking PKB. These data provide evidence that besides PKB another pathway seems to be involved in Bcl-XL induction. However, expression of Bcl-2 and Bcl-XL as well as Bad phosphorylation were noticed with ACM and authentic S1P. S1P receptor activation triggers intracellular Ca2+ signals.14,29,30 Ca2+ signals are involved in ACM-mediated macrophage survival, as shown by the use of the intracellular Ca2+ scavenger Bapta-AM. Although Ca2+ signals usually promote apoptosis by mitochondrial Ca2+ overload and subsequent release of proapoptotic factors,35 there are indications that a modest Ca2+ increase elicits antiapoptotic responses via Ca2+/calmodulin-dependent kinase kinase-induced PKB activation, which results in Bad phosphorylation.36 This agrees quite well with our findings showing Bad phosphorylation depending not only on PI3K but also on Ca2+ signaling. Whether PI3K and Ca2+ pathways work synergistically or independently remains to be clarified. Since either reducing the expression of Bcl-2 or diminishing Ser136-Bad phosphorylation was sufficient to recover caspase-3 activity, we assume that the Bcl-2 family rheostat is more important than expression modulation or phosphorylation of individual proteins such as Bcl-2 or Bad.
Previously, Golpon et al11 reported VEGF release from epithelial cells following phagocytosis of apoptotic cells with the notion that VEGF acted as a survival factor in blocking UV- or TNF-α/CHX-induced apoptosis. Another study noticed survival of macrophages when cocultured with apoptotic lymphocytes for 72 hours.10 As the phagocytosis inhibitor colchicine abrogated protection, this was taken as evidence for phagocytosis being required. However, colchicine also suppresses Ca2+ release from intracellular stores,37 which in our system was needed for protection without the prerequisite of phagocytosis.
Release of S1P from apoptotic Jurkat cells was necessary for macrophage protection, as demonstrated by transwell experiments, the use of ACM in combination with the sphingosine kinase inhibitor DMS, and liquid chromatography-tandem mass spectrometry. Knock-down studies using siRNA revealed a vital role of Sphk2 in apoptosis-dependent S1P production. Sphk2 activity during apoptosis has been previously reported to be linked to promoting apoptosis.38,39 However, knock-down of Sphk2 did not diminish the induction of apoptosis in Jurkat cells. Our data therefore expand the role of Sphk2 in regulating apoptosis, from participating in apoptosis induction inside cells to acting as an inhibitor, once releasing its product. As the production and release of S1P during apoptosis have not been reported so far, future studies need to define details of Sphk2 activation as well as release pathways.
Other potential mediators released from apoptotic cells can formally be excluded to contribute to ACM protection. TGF-β does not fulfill criteria of a lipid messenger. LPC40 attenuated macrophage apoptosis only when supplied at high concentrations of roughly 100 μM, a concentration that is unlikely to be of physiologic relevance. Arachidonic acid and/or derived metabolites released during apoptosis41 could also be excluded, because they are not extractable with chloroform.12 The new role of S1P as an antiapoptotic mediator released from apoptotic cells adds to existing concepts on the anti-inflammatory role of S1P. Our study shows that apoptotic cells communicate with their neighborhood by releasing S1P, which prevents apoptosis in macrophages. This might be relevant to fully understand the phenotype switch in macrophages that is induced by apoptotic cells.
Prepublished online as Blood First Edition Paper, May 11, 2006; DOI 10.1182/blood-2006-04-014852.
Supported by grants from the Deutsche Forschungsgemeinschaft (BR 999, FOG 784) and European Community.
A.W. performed the research and analyzed data; A.W., A.M.J., A.v.K., and B.B. participated in designing the research; H.S. and G.G. performed and designed mass spectrometry measurements; A.W. and B.B. wrote the paper; and all authors checked the final version of the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal