Comment on Shen et al, page 1991
Enforced transgenic expression of the T-cell leukemia/lymphoma 1 oncoprotein, TCL1, in lymphocytes in mice results in a range of B-cell neoplasms that require the germinal center to develop. In the key experiment of the present study, Shen and colleagues use mice that are homozygous for a null allele of Pou2af1 to demonstrate that TCL1-driven malignant B-cell transformation does not take place when the TCL1 transgene is crossed onto the genetic background of OCA-B deficiency.
Although TCL1 was originally discovered in patients with T-cell neoplasms, it seems to play an even more prominent role in B-lineage tumors. The mechanism by which TCL1 facilitates the malignant transformation of B lymphocytes is complex and poorly understood. What has been elucidated thus far is that TCL1 augments the activation of the AKT (v-akt murine thymoma viral oncogene homolog) serine/threonine kinase, probably by forming stable heteromeric TCL1-AKT complexes at the inner cell membrane. The resultant enhancement of AKT signaling promotes B-cell proliferation and survival, thus increasing the likelihood of secondary oncogenic mutations and full malignant cell transformation. Stimulation of the protein kinase C–mitogen-activated protein kinase–extracellular signal–regulated kinase (PKC-MAPK-ERK) signaling cascade has also been implicated in TCL1-dependent B-cell transformation, but many details remain unknown.
Realizing that a good mouse model of de-regulated TCL1 expression in lymphocytes may be instrumental in advancing our understanding of TCL1-induced lymphoma development, Shen and colleagues took advantage of an Eμ-B29 promoter to express a TCL1 transgene in B cells and T cells in mice. In previous work,1 they used histopathologic criteria to show that Eμ-B29-TCL1 transgenic mice are prone to B-cell lymphomas of germinal-cell (GC) origin, including follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), and Burkitt-like lymphoma (BLL). This finding was of great interest, since most common B-lymphocyte neoplasms in humans arise via malignant transformationFIG1 of GC B lymphocytes—but accurate mouse models of tumors of this sort are scarce. In this issue of Blood, Shen and colleagues recruit expert collaboration from the laboratories of Drs Fred Alt (Harvard Medical School), Bob Roeder (Rockefeller University), and Sandy Morse (National Institute of Allergy and Infectious Diseases [NIAID]) to continue their studies on tumor development in Eμ-B29-TCL1 transgenics. Shen and colleagues report exciting new insights into the target cell (mature B lymphocyte), microenvironment (GC), and mechanism (c-Myc activation, genomic instability, T-cell help) of TCL1-dependent cell transformation.
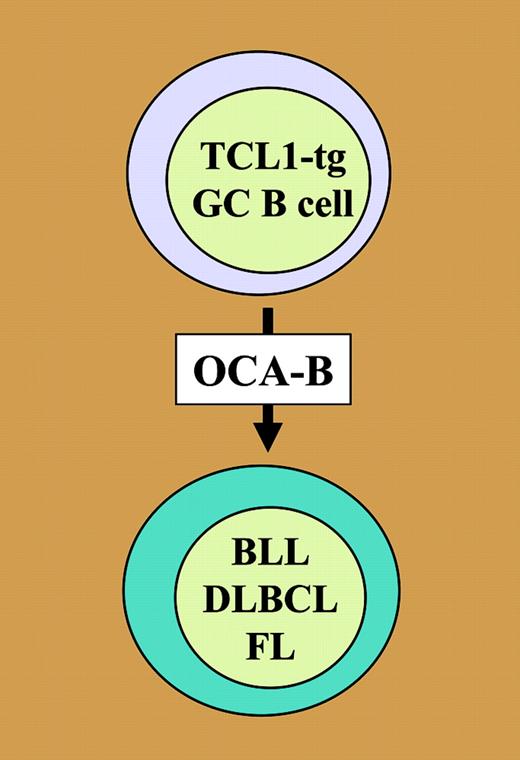
Simplified model on TCL1-driven neoplastic development of GC B lymphocytes. Elevated levels of TCL1 in GC B cells increase proliferation, resistance to Fas-dependent apoptosis, and expression of AID (not shown). Full malignant transformation of these cells is strictly dependent upon OCA-B, which is required for the development of GCs in secondary lymphoid tissues. TCL1-transgenic lymphomas, classified as FL, BLL, or DLBCL, exhibit features of genomic instability and contain elevated Myc (not shown).
Simplified model on TCL1-driven neoplastic development of GC B lymphocytes. Elevated levels of TCL1 in GC B cells increase proliferation, resistance to Fas-dependent apoptosis, and expression of AID (not shown). Full malignant transformation of these cells is strictly dependent upon OCA-B, which is required for the development of GCs in secondary lymphoid tissues. TCL1-transgenic lymphomas, classified as FL, BLL, or DLBCL, exhibit features of genomic instability and contain elevated Myc (not shown).
In the key experiment of the current study, Shen and colleagues use mice that are homozygous for a null allele of Pou2af1 to demonstrate that TCL1-driven malignant B-cell transformation does not take place when the TCL1 transgene is crossed onto the genetic background of OCA-B deficiency. Since Oct coactivator from B cell (OCA-B)–deficient mice are incapable of forming GCs, this result provides definitive genetic evidence that TCL1-induced B lymphomas are dependent on the microenvironment of the GC (see figure). Cytogenetic and molecular analysis of B lymphomas from OCA-B–proficient, TCL1-transgenic mice revealed recurrent chromosomal translocations and an extra copy of the c-Myc containing chromosome 15 (trisomy 15), with corresponding overexpression of the Myc oncoprotein. Further investigation showed that frank lymphoma was preceded by an expansion of TCL1-transgenic B cells that exhibited enhanced resistance to FAS-mediated apoptosis and increased expression of the activation-induced cytidine deaminase (AID). This finding led the authors to propose that elevated AID may be the underlying reason for the observed genomic instability in TCL1-induced lymphomas.
The findings reported by Shen and colleagues have important implications for human B-cell neoplasms, in which small-molecule inhibitors of TCL1, particularly its interaction domain with AKT, might be of therapeutic value. The present Eμ-B29-TCL1 transgenic mice provide a promising preclinical model system for the design and testing of these inhibitors, illustrating once again that genetically engineered mouse models of human cancer may ultimately lead to new clinical applications benefiting cancer patients. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal