Abstract
We report a novel fibrinogen variant (fibrinogen Seoul II), which has a heterozygous point mutation from CAA to CCA leading to AαGln328Pro. The mutation site is among several glutamine residues that serve as α-chain cross-linking acceptor sites. Fibrinogen Seoul II was found in a 51-year-old male patient and his family in Seoul, Korea. The patient was diagnosed with myocardial infarction at age 43. Eight years later he was admitted to the emergency room due to recurrence of the disease, where he expired under treatment with tissue plasminogen activator (t-PA). Fibrin polymerization curves, made using purified fibrinogen from the patient's relatives, showed a decreased final turbidity, suggesting Seoul II fibrin clots are composed of thinner fibers. This supposition was verified using scanning electron microscopy. Alpha-polymer formation by the mutant fibrinogen upon thrombin treatment in the presence of factor XIII and calcium was distinctly impaired. This result confirms that the residue Aα328 plays a pivotal role in α-chain cross-linking.
Introduction
Fibrinogen, one of the critical plasma proteins, is a 340-kDa glycoprotein1 synthesized in the liver2,3 and has essential roles in both blood coagulation and platelet aggregation.4 Fibrinogen is a dimer consisting of 2 identical pairs of Aα, Bβ, and γ chains intertwined to form a trinodular molecule with 2 terminal D regions and a central E region.5,6 The D region includes the carboxyl termini of the Bβ and γ chains, while that of the Aα chain goes beyond the D region to form the αC domain, which is composed of residues Aα220-6107 and normally interacts with the central E region.
In the process of blood coagulation, thrombin cleaves fibrinopeptides A and B from Aα and Bβ chains to form a fibrin monomer, exposing the GPR and GHRP sequences, respectively. Subsequently, protofibrils in a half-staggered array are formed, and the release of αC domains from the central E region is facilitated, allowing lateral aggregations through intermolecular associations between αC domains.8,9 The final phase of fibrin clot formation involves the covalent modification of fibrin molecules by factor XIIIa. The factor XIIIa–mediated cross-linking process, where α-α cross-linking follows γ-γ cross-linking, contributes to stabilization of the fibrin clot and resistance to thrombolytic agents.10,11 Until now, a variety of mutations in each of the fibrinogen chain genes have been reported in more than 350 families all over the world, with Aα-chain mutation as the most common form.12 Those mutations usually have been associated with dysfibrinogenemia or hypofibrinogenemia, both of which feature decreased levels of plasma fibrinogen activity. Each Aα-chain has at least 2 glutamine acceptor sites located at amino acid residues 328 and 366 and 5 potential lysine donor sites between residues 518 and 584.13 Aα glutamine 328 is among several glutamine residues that serve as α-chain cross-linking acceptor sites,10 for which only one fibrinogen variant has been described: compound heterozygotes characterized by a stop mutation at AαGln328 resulting in truncation of the Aα-chain (Fibrinogen Keokuk14 ).
Here we present a novel fibrinogen variant (fibrinogen Seoul II), which has a heterozygous point mutation from CAA to CCA, resulting in a glutamine-to-proline substitution at residue 328 in the carboxyl-terminal region of the fibrinogen Aα-chain. We investigated how this mutation impacted fibrin polymerization and cross-linking through various in vitro tests on purified fibrinogens from the index subject and his family members.
Patients, materials, and methods
Patients studied
Fibrinogen Seoul II was found in a 51-year-old male patient with myocardial infarction and his 8-year-old son and 79-year-old mother. The patient's illness was diagnosed as myocardial infarction at age 43. Eight years later he was admitted to the emergency room, where he expired during treatment with tissue plasminogen activator (t-PA). The patient's son and mother also were enrolled in this study to evaluate possible functional or structural defects of fibrinogen in his family. Only the subject received t-PA treatment. All participants or their representatives gave informed consent.
Sample collection and preparation
Blood was collected into 0.109 M trisodium citrate tubes by venipuncture. The patient's plasma was collected under the conditions of t-PA treatment, and his relatives were in normal condition. Platelet-poor plasma was prepared by centrifugation for 15 minutes at 2300g and stored at –70°C. Blood for DNA analysis was collected into 15% K3 EDTA (ethylenediaminetetraacetic acid) tubes. Genomic DNA was isolated from white blood cells as previously described15 and also stored at –70°C.
Coagulation tests
Prepared plasma was used to measure plasma fibrinogen activity based on the Clauss method16 using an STA coagulation analyzer and reagent kit (Diagnostica Stago, Asniers, France). Plasma fibrinogen antigen level was measured by the immunologic method using a nephelometer and antiserum (Dade-Behring, Deerfield, IL) if needed. Thrombin time was measured using the STA coagulation analyzer and reagent kit.
DNA sequence analysis
Primers were designed for all exons, exon-intron boundaries, and promoter regions of the fibrinogen Aα (FGA, GenBank M64982), Bβ (FGB, GenBank M64983), and γ (FGG, GenBank M10014) chain genes. These genes were amplified from genomic DNA using standard polymerase chain reaction (PCR) protocols.17 The point mutation causing fibrinogen Seoul II, located in exon 5 of the fibrinogen Aα-chain, was amplified using the primers 5′GACTGCAACCTGGAAACCTG3′ (forward) and 5′TGGTTGTGCTACCAGAGGTG3′ (reverse). PCR products were purified using the QIAquick PCR purification kit (QIAGEN, Valencia, CA) and sequenced using a Dye Terminator Cycle Sequencing Ready Reaction Kit with AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA) and an ABI 3700 sequencer (Applied Biosystems) following the protocols supplied by the manufacturer.
Fibrinogen purification
Plasma samples from subjects with and without the molecular defect were fractionated using gel filtration chromatography on Hiload 16/60 superdex 200 columns (Amersham Biosciences, Piscataway, NJ) equilibrated with 10 mM sodium phosphate, pH 7.4, 0.5 M NaCl buffer. The first peak among 3 main peaks, which included fibrinogen, was pooled and precipitated with 22.5% ammonium sulfate solution for 30 minutes at 4°C in ice followed by centrifugation at 14 000g for 20 minutes. The pellet was washed twice with 25% ammonium sulfate solution, resuspended in 20 mM Hepes (N-2 hydroxyethylpiperazine-N′-2ethanesulfonic acid) buffer, pH 7.4, 150 mM NaCl, and dialyzed against the same buffer overnight at 4°C. The protein concentration was determined by the BCA (bicinchoninic acid) method using a BCA protein assay kit (Pierce Biotechnology, Rockford, IL). Fibrinogen purity was assessed by separation on 4% to 20% gradient sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel.
Thrombin-catalyzed fibrin polymerization
Purified fibrinogen in 20 mM Hepes buffer, pH 7.4, 150 mM NaCl, was used to perform fibrin polymerization tests. Fibrinogen concentration was adjusted to 0.15 mg/mL with 50 mM TBS (tris[hydroxymethyl]aminomethane–buffered saline), 150 mM NaCl, pH 7.4 buffer. Fibrin polymerization for normal and heterozygous Seoul II fibrinogen from the patient's son was initiated by adding 1 μL of 20 National Institutes of Health (NIH) unit/mL thrombin (final concentration, 0.1 NIH unit/mL, HYPHEN BioMed, Andresy, France) to the purified fibrinogens. Change in optical density at 350 nm was monitored at 30-second intervals for 60 minutes at room temperature in a SpectraMax Plus 384 (Molecular Devices, Union City, CA). All experiments were performed 3 times.
Fibrinogen clottability
Clottability of the purified fibrinogens was determined essentially as previously described.18 Purified fibrinogen in 20 mM Hepes buffer, pH 7.4, 150 mM NaCl, was used for clottability testing. The fibrinogen concentration was adjusted to 0.45 mg/mL with 50 mM TBS, 150 mM NaCl, pH 7.4, and 1 mM CaCl2. Human α-thrombin (final concentration, 0.1 U/mL) was added for clottability of normal and heterozygous Seoul II fibrinogen. Samples were incubated for 3 hours at 37°C, followed by an overnight incubation at 4°C. The reaction was terminated by centrifugation at 2300g for 20 minutes. The fibrin(ogen) that was not incorporated into the fibrin gel was determined from the A280 of the supernatant, and the ability to clot was calculated as (A280 at zero time – A280 of the supernatant)/(A280 at zero time) × 100%.
SDS-PAGE analysis of factor XIIIa–catalyzed cross-linking of fibrin
Formation of γ-dimer and α-polymer upon thrombin treatment in the presence of FXIII and 2.5 mM calcium chloride was analyzed using SDS-PAGE. Purified fibrinogen concentration was adjusted to 0.8 mg/mL in 20 mM Hepes buffer, pH 7.4, 150 mM NaCl, and incubated in distilled water containing 1 NIH unit/mL thrombin (HYPHEN BioMed) in the presence of 1 U/mL factor XIII (Merck Biosciences, Darmstadt, Germany) and 2.5 mM calcium. SDS was added after incubation times of 0, 1, 2, 5, 10, and 30 minutes to stop the clotting reaction. Boiling in a reducing SDS-PAGE solution immediately dissolved each clot. The extent of α-chain cross-linking for heterozygous Seoul II and normal fibrin clots was assessed by SDS-PAGE. Densitometry of α-monomer bands was carried out using Gelscope 1.5 software (Biobud Co, Ltd, Seoul, Korea).
t-PA–mediated plasmin digestion on fibrin (fibrinolysis profile)
To analyze the profile of t-PA–mediated plasmin degradation, purified fibrinogen (0.2 mg/mL) was mixed with tPA (Sigma-Aldrich, St Louis, MO) (27.4 ng/mL, about 0.25 U/mL), plasminogen (10 μg/mL) (HYPHEN BioMed), and FXIII (from 2% fresh human plasma) in 50 mM TBS containing 5 mM CaCl2. 0.2 U/mL of thrombin was added, and the turbidity of polymerizing or digesting fibrin at 350 nm was monitored every 20 seconds for 65 minutes using a SpectraMax Plus-384 plate reader (Molecular Devices) at 37°C. All experiments were performed 3 times.
Scanning electron microscopy and measurement of fiber diameter and branch point number
To investigate the structure of Seoul II fibrin clots, thrombin (1 NIH unit/mL, HYPHEN BioMed), CaCl2 (final concentration, 5 mM), and factor XIII (final concentration, 10 μg/mL) were added to purified fibrinogen (final concentration, 1 mg/mL) from the subject's relatives. After a 90-minute incubation in a moist environment, the resulting clots were processed for scanning electron microscopy by fixation, dehydration, critical point drying, and coating as described previously.19 Clots were observed and photographed digitally in at least 2 different areas per clot, using a scanning electron microscope (S-800, Hitachi, Tokyo, Japan). Fiber thickness and branch point number per square micrometer were measured using MediScreen 1.0 software (Biobud). Statistical analyses were performed by means of SPSS 11.0 for Windows (SPSS, Chicago, IL). One-way analysis of variance (ANOVA) followed by Scheffé and Tukey tests was used to compare fiber thickness.
Results
Study of the index case
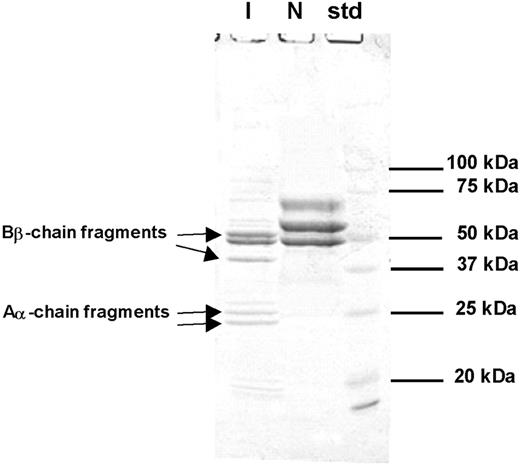
Coagulation tests in the emergency room for the index subject showed plasma fibrinogen activity of less than 1.32 μM (45 mg/dL), but fibrinogen antigen levels of 6.68 μM (227 mg/dL; within normal range). This discrepant result prompted the investigation of the possibility of hereditary dysfibrinogenemia, as the subject had no other risk factors for dysfibrinogenemia except for the t-PA treatment in the emergency room. The patient showed markedly prolonged prothrombin time (PT, 29.5 seconds; reference range, 11-15 seconds), activated partial thromboplastin time (aPTT, 180 seconds; reference range, 29-45 seconds), and thrombin time (> 240 seconds). DNA sequencing of all exons and their boundaries of the fibrinogen Aα, Bβ, and γ chains revealed the presence of Seoul II fibrinogen (Figure 1). SDS-PAGE on purified fibrinogen from the subject showed the presence of various sizes of fragments considered to have originated from fibrinogen (Figure 2). In addition, thrombin-catalyzed polymerization of fibrin from the subject was markedly impaired, compared with that of normal fibrinogen (data not shown). N-terminal sequencing was performed on the various SDS-PAGE fragments, demonstrating that α-chain fragments of 24 kDa and 26 kDa and β-chain fragments of 38 kDa and 50 kDa were formed by plasmin activated from plasminogen by t-PA (Figure 2).
DNA sequencing. DNA sequence analysis of exon 5 of the Aα-chain gene in Seoul II mutation and normal control. Seoul II is shown to have a heterozygous point mutation, with the conversion of CAA to CCA resulting in Gln to Pro substitution at Aα residue 328.
DNA sequencing. DNA sequence analysis of exon 5 of the Aα-chain gene in Seoul II mutation and normal control. Seoul II is shown to have a heterozygous point mutation, with the conversion of CAA to CCA resulting in Gln to Pro substitution at Aα residue 328.
Coagulation tests
Coagulation studies of the subject's family members showed that PT and aPTT were within normal ranges. In both the subject's son and mother, plasma fibrinogen activities were 8.23 μM (280 mg/dL) and 10.06 μM (342 mg/dL), respectively, and thrombin times were 17.9 seconds and 16.5 seconds, respectively. These results were all within normal range.
DNA sequencing result
DNA sequencing of fibrinogen genes from the subject and his family members revealed that all 3 had a point mutation changing the triplet CAA coding for AαGln328 to CCA, resulting in substitution of glutamine (Gln) to proline (Pro). As shown in Figure 1, both adenine and guanine were found at position 4315 in the DNA sequencing of exon 5 derived from the Aα-chain genes of the subject and his relatives, indicating a heterozygous mutation. No other mutations were found in the full sequences of all exons and exon-intron boundaries of the Aα, Bβ, and γ chains from this family.
SDS-PAGE of reduced fibrinogen from index subject (I) and normal control (N). Aα- or Bβ-chain fragments were identified through N-terminal sequencing. Intact Aα- or Bβ-chain bands are seen in the normal control, but not the index subject. N-terminal sequence of each band is as follows: 24 kDa, ADSGEGDFLA; 25 kDa, ADSGEGDFLA; 38 kDa, DNENVVNEYS; 50 kDa, ARPAKAAATQ.
SDS-PAGE of reduced fibrinogen from index subject (I) and normal control (N). Aα- or Bβ-chain fragments were identified through N-terminal sequencing. Intact Aα- or Bβ-chain bands are seen in the normal control, but not the index subject. N-terminal sequence of each band is as follows: 24 kDa, ADSGEGDFLA; 25 kDa, ADSGEGDFLA; 38 kDa, DNENVVNEYS; 50 kDa, ARPAKAAATQ.
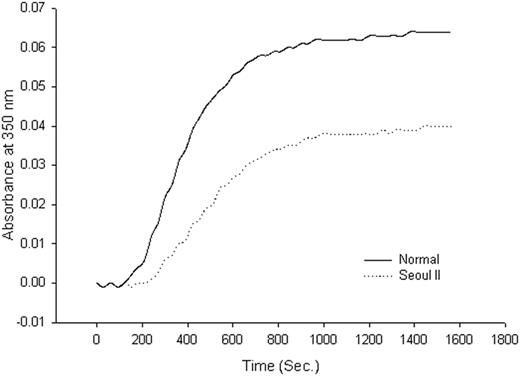
Thrombin-catalyzed fibrin polymerization curve. Thrombin-catalyzed fibrin polymerization curve of heterozygous Seoul II fibrinogen (dotted) and normal control (solid). The final turbidity of heterozygous Seoul II fibrin clots was moderately decreased compared to the normal control.
Thrombin-catalyzed fibrin polymerization curve. Thrombin-catalyzed fibrin polymerization curve of heterozygous Seoul II fibrinogen (dotted) and normal control (solid). The final turbidity of heterozygous Seoul II fibrin clots was moderately decreased compared to the normal control.
Thrombin-catalyzed fibrin polymerization and fibrinogen clottability
Although family member fibrinogen concentration was normal, fibrin polymerization of heterozygous Seoul II fibrinogen from the subject's son, as monitored by turbidity at 350 nm at room temperature, showed a decreased final turbidity compared to that of the normal fibrinogen (Figure 3). The kinetic curve for the turbidity of fibrin polymerization revealed that heterozygous Seoul II fibrin polymerization had a lag phase of 240 seconds, a Vmax of 0.53 × 10–4 U/s, and a final turbidity of 0.040 U compared with the control values of 150 seconds, 1.04 × 10–4 U/s, and 0.064 U, respectively. Fibrin(ogen) clottability was 98% and 97% in normal and heterozygous Seoul II fibrin(ogen), respectively.
SDS-PAGE analysis of factor XIIIa–induced fibrin cross-linking
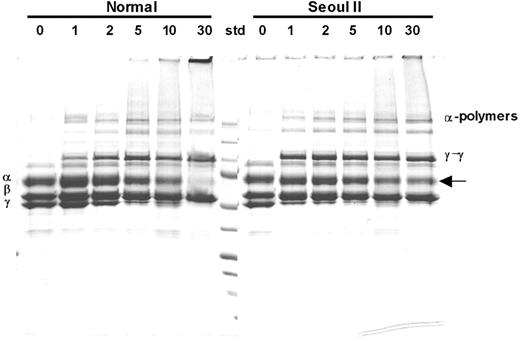
Time-interval SDS-PAGE analysis of fibrin cross-linking induced by factor XIIIa demonstrated that more α-monomers remained in the heterozygous Seoul II fibrin clot after 30 minutes of reaction time than in normal fibrin clots (Figure 4), suggesting incomplete cross-link formation. Densitometry studies showed that approximately 41% of heterozygous Seoul II α-monomer was cross-linked by FXIII, as compared to 80% of normal α-monomer after 30 minutes' reaction time. On the contrary, γ-chain cross-linking of heterozygous Seoul II fibrin was accelerated in 1 to 5 minutes when treated with factor XIIIa, compared to that of normal fibrin.
SDS-PAGE analysis of factor XIIIa–catalyzed cross-linking of fibrin. SDS-PAGE analysis of γ-dimer and α-polymer formation upon thrombin treatment in the presence of factor XIII and calcium in heterozygous Seoul II fibrinogen (right) and normal control. Incubation with thrombin for 0, 1, 2, 5, 10, and 30 minutes formed clots, respectively. More Aα-chain bands remain for the heterozygous Seoul II fibrin clot (arrow) after 30 minutes of reaction time compared to the normal control, suggesting that Aα-chain cross-linking is impaired by Seoul II mutation. Standard (Std) size equals 250, 150, 100, 75, 50, 37, 25, 15, and 10 kDa from top to bottom, respectively.
SDS-PAGE analysis of factor XIIIa–catalyzed cross-linking of fibrin. SDS-PAGE analysis of γ-dimer and α-polymer formation upon thrombin treatment in the presence of factor XIII and calcium in heterozygous Seoul II fibrinogen (right) and normal control. Incubation with thrombin for 0, 1, 2, 5, 10, and 30 minutes formed clots, respectively. More Aα-chain bands remain for the heterozygous Seoul II fibrin clot (arrow) after 30 minutes of reaction time compared to the normal control, suggesting that Aα-chain cross-linking is impaired by Seoul II mutation. Standard (Std) size equals 250, 150, 100, 75, 50, 37, 25, 15, and 10 kDa from top to bottom, respectively.
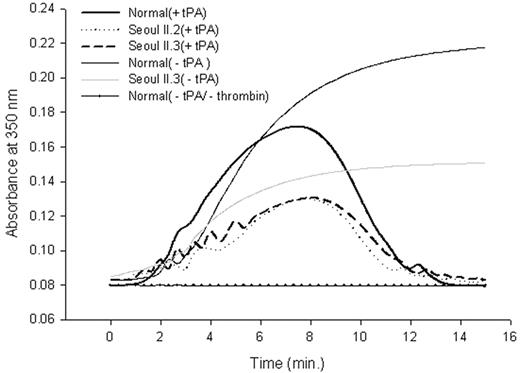
Fibrinolysis profile of purified fibrinogen. Peak absorbance came 40 seconds later in heterozygous Seoul II than in normal clots. The subject's mother (Seoul II.2) and son (Seoul II.3), who also have Seoul II fibrinogen, showed similar absorbance changes.
Fibrinolysis profile of purified fibrinogen. Peak absorbance came 40 seconds later in heterozygous Seoul II than in normal clots. The subject's mother (Seoul II.2) and son (Seoul II.3), who also have Seoul II fibrinogen, showed similar absorbance changes.
t-PA–mediated plasmin digestion of fibrin (fibrinolysis profile)
Differences between fibrin polymerization to fibrinolysis catalyzed by plasmin activated from plasminogen by t-PA for normal and heterozygous Seoul II fibrinogen are shown in Figure 5. Baseline absorbance was established using only normal fibrinogen without thrombin activity. Peak absorbance, where fibrinolysis begins to exceed polymerization, came 40 seconds later in heterozygous Seoul II fibrin than in normal fibrin. As with the thrombin-induced fibrin polymerization curve (Figure 3), the difference in peak turbidity between normal and heterozygous Seoul II fibrin clot was evident.
Scanning electron microscopy of fibrin clots
Fiber thickness differed significantly between normal and heterozygous Seoul II fibrin clots, based on diameter measurements using scanning electron microscopy (Table 1). Heterozygous Seoul II fibrin clots showed thinner fibers than normal fibrin clots (Figure 6). This result corresponds with the observation that final turbidity of thrombin-catalyzed fibrin polymerization was lower in heterozygous Seoul II fibrin than in normal fibrin. The subject's mother and son had similar fiber thickness by scanning electron microscopy. There was no significant difference in branch point number per square micrometer between normal and heterozygous Seoul II fibrin clots (Table 2).
Measurement of fibrin fiber diameter
. | No. of fibers . | Mean, nm . | SD . |
|---|---|---|---|
| Normal | 307 | 133 | 20 |
| Son (heterozygous Seoul II) | 307 | 105* | 21 |
| Mother (heterozygous Seoul II) | 307 | 109* | 21 |
. | No. of fibers . | Mean, nm . | SD . |
|---|---|---|---|
| Normal | 307 | 133 | 20 |
| Son (heterozygous Seoul II) | 307 | 105* | 21 |
| Mother (heterozygous Seoul II) | 307 | 109* | 21 |
Significant differences (P < .01) between normal fibrin and heterozygous Seoul II fibrins (ANOVA with Scheffé and Tukey post hoc analysis).
Measurement of branch point number
. | No. of μm2 counted . | Mean branch point number per μm2 . | SD . |
|---|---|---|---|
| Normal | 350 | 1.39 | 0.74 |
| Son (heterozygous Seoul II) | 344 | 1.45* | 0.73 |
| Mother (heterozygous Seoul II) | 346 | 1.36* | 0.70 |
. | No. of μm2 counted . | Mean branch point number per μm2 . | SD . |
|---|---|---|---|
| Normal | 350 | 1.39 | 0.74 |
| Son (heterozygous Seoul II) | 344 | 1.45* | 0.73 |
| Mother (heterozygous Seoul II) | 346 | 1.36* | 0.70 |
No significant differences (P > .05) between normal fibrin and heterozygous Seoul II fibrins (ANOVA).
Scanning electron microscopy of fibrin clots. Scanning electron microscopy images of heterozygous Seoul II fibrin clots (right) and normal fibrin clots (left) are shown. Heterozygous Seoul II fibrin clot showed thinner fibers than normal control (bar equals 3 μm).
Scanning electron microscopy of fibrin clots. Scanning electron microscopy images of heterozygous Seoul II fibrin clots (right) and normal fibrin clots (left) are shown. Heterozygous Seoul II fibrin clot showed thinner fibers than normal control (bar equals 3 μm).
Discussion
Dysfibrinogenemia was suggested in the index subject, according to emergency room measurements of initial plasma fibrinogen activity and antigen. In addition, DNA sequence analysis showed hereditary dysfibrinogenemia. However, we conclude that the abnormal coagulation profile of the patient, including plasma fibrinogen activity level, resulted from his treatment with t-PA. A study of 3 generations demonstrated that the subject's mother and the only son also shared the same mutation. They were shown to have normal plasma fibrinogen activities. Fibrin polymerization curve revealed low turbidity (data not shown) in the index subject, which was considered exaggerated when taking a heterozygous mutation into account. More information was elucidated by reducing SDS-PAGE of purified fibrinogen showing various sizes of bands derived from fibrinogen. N-terminal sequencing proved that these are α- or β-chain fragments (Figure 2). This finding was supported by the fact that the patient received a large amount of t-PA in the emergency room. To further understand the characteristics of fibrinogen Seoul II, purified fibrinogens from the subject's 8-year-old son and 79-year-old mother also were closely examined.
AαGlu328, located in the αC-domain, is composed of residues Aα220-6107 and is one of the places where isopeptide γ-glutamyl-ϵ-lysyl bond forms when fibrin assembly is followed by cross-linking induced by factor XIIIa.10,13,20 Two αC-domains interact with the central portion of the molecule, from which they dissociate and become available for intermolecular interaction when fibrin assembly takes place.8,9 These intermolecular interactions between αC-domains are important for the enhancement of lateral aggregation during fibrin polymerization.7 Therefore, αC-domains are thought to play a major role in fibrin polymerization and stabilization through covalent α-cross-linking. Approximately 22 αC-domain mutations have been described. Sixteen mutations cause dysfibrinogenemia characterized by prematurely truncated Aα-chains due to nonsense or frame-shift mutations, including Otago, Switzerland B6,21 Turkey B5,21 France A7,22 France A12,22 Keokuk, United States B3,22 Milano III, Nieuwegein, Marburg, Caracas I, Lincoln, Perth, San Giovanni Rotondo, Paris VII, and Indianapolis IV. One mutation (Champagne Au Mont d'Or) is an insertion of 39 amino acids. The other 5 are missense mutations; Caracas II is an N-glycosylated Asn substitution, Indianapolis and Indianapolis II are associated with renal amyloidosis, and Caracas V and Dusart/Paris V are related to fibrinogen-albumin complex formation. As a missense mutation of the αC-domain, fibrinogen Seoul II is unique because it is related to the α-chain cross-linking site.
Until now, the recently reported fibrinogen variant Keokuk14 has been the only described cross-linking site mutation. This compound heterozygous mutation of AαGln328stop and AαIVS4 + 1G → T results in truncated fibrinogen formation, leading to impaired polymerization and α-cross-linking. In contrast, the Seoul II mutation causes an amino acid substitution (AαGln328Pro) but does not form a stop codon.
In characterizing heterozygous Seoul II fibrinogen, 2 findings were of particular interest. First, polymerization curves for the clotting of purified heterozygous Seoul II fibrinogen showed approximately two thirds of final clot turbidity compared with that of normal fibrinogen. The mechanism by which the mutation affects the final polymerization turbidity is uncertain. One possibility is that the substitution of proline for glutamine at residue Aα328 may alter the tertiary structure of the αC domain, leading to a turn in the polypeptide chain and impairing intermolecular interactions (lateral aggregation). In general, the replacement of an amino acid with proline has been known to give rise to conformational change of proteins through the distortion of the regular helical structure and loss of a backbone hydrogen bond.23-27 Scanning electron microscopic observations of thinner fibers in the heterozygous Seoul II fibrin clots (Figure 6) seem to support this idea. Although clots prepared for scanning electron microscopy were treated with FXIIIa, this reaction did not seem to substantially affect clot morphology, except for making fibrin fibers a little thinner and longer.10,28
Second, when heterozygous Seoul II fibrin clots were formed by incubation with thrombin and FXIIIa (Figure 4), incomplete α-chain cross-link formation was shown, as demonstrated by reducing SDS-PAGE analysis. Approximately 41% of heterozygous Seoul II α-monomer was cross-linked by FXIIIa, as compared to 80% of normal α-monomer after 30 minutes' reaction time. This finding indicates that the residue Aα328 plays an important role in α-chain cross-linking and that the AαGln328Pro mutation prevents FXIIIa from functioning as an active transglutaminase between glutamine and lysine to stabilize fibrin clots. So far, several glutamine residues, such as AαGln 221,29 237,29 328,20,29 and 36620 , are known to be involved in the α-chain cross-linking reaction. Recently, a C-terminal portion of the αC-domain, including residues 392-610,30 also was revealed to contain an FXIIIa incorporation site. As we can see it in some amino acid substitutions not associated with the α cross-linking site, such as Caracas II31 (AαSer434AsnN-glycosylated), Tokyo V32 (γAla327thr), and Thr312Ala polymorphism,33 the amount of α-cross-linking is proportionate to fiber thickness. Therefore, in addition to the mutation itself, we think that the fact that thicker fibers tend to have more α-chain cross-links28 also can account for the difference in the amount of α-chain cross-linking between normal and heterozygous Seoul II fibrin clots. Heterozygous Seoul II fibrin α-chains were still present as monomers after 90 minutes of incubation by the reducing SDS-PAGE (data not shown). Considering the mutational site and the SDS-PAGE analysis, α-cross-linking of Seoul II fibrinogen is thought to be impaired.
Interestingly, the γ-chains of the heterozygous Seoul II fibrin formed γ-γ dimers more rapidly than normal fibrin in 1 to 5 minutes when treated with factor XIIIa. We think this finding suggests that protofibril elongation is predominant over lateral aggregation in the early process of fibrin assembly for the heterozygous Seoul II fibrin, possibly due to impaired lateral aggregation. Further studies are necessary to clarify this finding.
In fibrinolysis profiles, where turbidity was continuously monitored from the beginning of fibrin formation to fibrinolysis, peak absorbance in heterozygous Seoul II fibrin clots occurred 40 seconds later than in normal clots (Figure 5), suggesting delayed fibrinolysis in Seoul II fibrin clots. This finding suggests that the Seoul II fibrin mutation might have precipitated thrombotic processes, although it should be noted that the subject already had risk factors for thrombosis, including hypertension and a history of heavy smoking. The mutation might also have induced a conformational change that alters the t-PA and plasminogen-binding site in residues 392-61034 of αC-domain, making it difficult to access. Studies about stiffness, compaction, and permeation will be required to make the effect of the Seoul II mutation on fibrinolysis clear.
As for the Seoul II mutation, molecular modeling would be helpful to confirm conformational changes in the αC domain. However, numerous attempts to crystallize preparations for the αC domain have not succeeded because the region is highly mobile and there is little evidence for features such as α helices or β sheets.35 The study of a homozygous Seoul II mutation would be helpful in understanding the impact of the mutation on disease but, unfortunately, all family members were not available to study the transmission of the mutation. In conclusion, this report describes a new fibrinogen variant characterized by an amino acid substitution (AαGln328Pro) in a site involved in α-cross-linking. Seoul II fibrin shows a decrease in final turbidity on polymerization and impaired fibrin α-chain cross-linking. This characterization of fibrinogen Seoul II describes its influence on fibrin assembly and cross-linking.
Prepublished online as Blood First Edition Paper, May 30, 2006; DOI 10.1182/blood-2005-11-007591.
R.P. and H.-J.D. contributed equally to this work.
Supported by research funding (grant no. 20050188 [R.P.]) from Soon Chun Hyang University, Korea.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Chang-Seok Ki (Department of Laboratory Medicine, Samsung Medical Center, Seoul, Korea) for helping with primer design. We also thank the family for their participation in this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal