Abstract
While investigating 2592 patients enrolled in multicenter myeloma trials, we found light chain–only (LCO) patients had worse median survival times (1.9 years) than patients with IgA and IgG paraproteins (2.3 and 2.5 years, respectively) (P < .001). However, IgA and IgG patients with levels of LC excretion similar to those of LCO patients also had poor survival times because of renal failure, resulting in worse survival during induction therapy and at relapse with no difference in progression-free survival between LCO and IgG patients. LC excretion was higher for λ than for κ types, but there was no difference in survival between the 2 LC types when stratified for level of LC excretion, indicating that care of renal function is vital to improving the survival of any patient with LC excretion. LCO patients were younger (P = .001), had worse performance status (P = .001), and had more lytic lesions (P < .001), perhaps reflecting late and missed diagnoses in younger and older LCO patients, respectively. No differences were observed between IgA and IgG patients in presentation characteristics, response, or survival from disease progression. The worse survival of IgA patients was attributed to shorter progression-free survival (median, 1.2 vs 1.6 years; P < .001), which is important for maintenance therapy.
Introduction
Multiple myeloma (MM) results from the proliferation of a neoplastic clone of plasma cells in bone marrow and accounts for 10% of hematologic malignancies.1 The disease is heterogeneous in severity and manifestations, which variably include skeletal disease (70%), renal impairment (20%), anemia (40%), and impaired humoral immunity (80%).1-4 This study assesses whether that heterogeneity of disease is linked to the type of monoclonal immunoglobulin proteins (paraproteins) the malignant plasma cells secrete.
Immunoglobulin consists of 2 identical heavy chain regions, variable and constant, which are encoded on chromosome 14, and 2 identical light chains also consisting of variable and constant regions. The light chains are either κ encoded on chromosome 2 or λ encoded on chromosome 22. Rearrangement of immunoglobulin variable region gene segments, during B lymphopoiesis, dictates the specificity for antigen binding.5 Variable regions of immunoglobulin are specific to the B cell that makes it and to any neoplastic clone that may arise from its progeny. Immunoglobulin secreted by myeloma plasma cells exhibits mutations in the germline variable region gene segments, indicating that their precursor cell, at some stage of ontogeny, has undergone the physiological site-directed hypermutation process typically seen in germinal center centroblasts.6,7
Virgin B cells use μ and δ gene segments to make the constant regions of their heavy chains and thus express surface IgM and IgD. After antigen stimulation, they may use other γ, α, or ϵ heavy chain constant region gene segments, a process that requires cognate interaction with an antigen-specific T cell.7,8 This immunoglobulin class switch is more likely to be to IgG in immune responses to antigen entering through skin and to IgA in immune response to antigen entering through mucosal surfaces. In MM, the immunoglobulin class is most commonly IgG and then IgA. Evidence indicates that IgA myeloma is different from IgG myeloma in terms of disease characteristics.3,9-11 In an international study of staging in myeloma, 2375 IgA patients had a median overall survival of 40 months compared with 49 months for 5894 IgG patients; the reason for this difference was not explored.12
This international study also noted a median overall survival time of 35 months for 1035 light chain–only (LCO) patients but did not explore the significance of light chain (LC) excretion in patients with IgG or IgA paraproteins.12 Normal plasma cells produce more free light chain (flc) than heavy chain. The flc is secreted into the blood, filtered at the renal glomeruli, and reabsorbed by the renal tubule cells. In two thirds or more of MM patients, the excess of flc production exceeds renal tubular reabsorption capacity, and flc is easily detected in the urine.1,3 In many patients, flc is nephrotoxic, and the consequent renal impairment is associated with worse survival.1,13-17 In some patients the dysregulation in heavy, and light chain production is so extreme that only free light chains are secreted in detectable quantities, commonly called Bence-Jones or light chain–only (LCO) myeloma. It is uncertain whether, in addition to the nephrotoxicity of flc, the extreme dysregulation in heavy and light chain production is associated with different or worse disease for LCO myeloma patients.
Patients receiving conventional dose chemotherapy in the multicenter Medical Research Council (MRC) United Kingdom myelomatosis trials from 1980 to 2002 were the subjects of this study, in which paraprotein type and levels were monitored by central laboratory analysis. We assessed for differences in clinical presentation and in overall and progression-free survival among the 3 main groups of patients (IgG, IgA, and LCO myeloma) and by light chain type. We have compared survival between patients with IgG or IgA myeloma and patients with LCO myeloma stratified by level of urinary light chain excretion.
Patients, materials, and methods
Patients with IgG, IgA, and LCO myeloma enrolled in the IV, V, VI, and VIII MRC UK myelomatosis trials between 1980 and 2002 (2592 total patients) were eligible for this study. Approval was obtained by local ethics committees of the participating hospitals' institutional review boards. Informed consent was provided according to the Declaration of Helsinki.
Our diagnostic criteria were consistent with the recently agreed criteria for symptomatic myeloma as defined and published by the International Myeloma Working Group.18 The characteristics of these trials are listed in Table 1. Patients were assigned to standard dose melphalan-based chemotherapy (822 patients) or melphalan-based conventional dose combination chemotherapy (1712 patients) as exemplified by doxorubicin, carmustine, cyclophosphamide, and melphalan (ABCM).19-21 Fifty-eight patients with thrombocytopenia received cyclophosphamide therapy. Twenty-six patients in the VI trial received melphalan 140 mg without stem cell rescue. Analysis did not include the Myeloma VII Trial because half these patients received high-dose chemotherapy with autologous hematopoietic stem cell rescue.22 Little difference was observed in survival, and no significant difference was observed in the distribution of paraprotein types between the trials or between the treatment arms within individual trials. Serum creatinine, β-2 microglobulin (Sβ2m), paraprotein type and levels, and urine creatinine and light chain levels were measured by central laboratory investigation in Birmingham. Participating centers provided clinical details, information on skeletal disease–related events, full blood count (FBC), serum albumin and urea, and plasma cell infiltration of bone marrow. Follow-up serum and urine samples were assessed at 3-month intervals, and clinical details were recorded. Disease response and progression were defined according to trial protocol criteria. A final report to ascertain the cause of death and a summary of the clinical course was recorded in the event of a death.
Characteristics of patients enrolled in IV, V, VI, and VIII MRC Multiple Myeloma trials
Trial, dates (patient ages), and induction treatment . | No. patients . | Median OS, y . | Median DP, y . | IgG, % . | IgA, % . | LCO, % . |
|---|---|---|---|---|---|---|
| IV, 1980-1982 (under 80 y) | ||||||
| MP | 254 | 2.3 | 1.1 | 59 | 29 | 12 |
| MPV | 251 | 2.2 | 1.5 | 57 | 24 | 19 |
| V, 1982-1986 (under 75 y) | ||||||
| C-weekly-plts < 80 | 58 | 1.8 | 1.7 | 53 | 28 | 19 |
| M7 | 291 | 2.0 | 1.4 | 56 | 29 | 15 |
| ABCM | 293 | 2.7 | 1.6 | 56 | 27 | 17 |
| VI (under 75 y) | ||||||
| 1986-1991 | ||||||
| HDM (M140) | 12 | 2.5 | 2.5 | 59 | 33 | 8 |
| HDMP | 14 | 5.0 | 1.9 | 71 | 29 | 0 |
| ABCM | 322 | 2.7 | 1.5 | 58 | 28 | 14 |
| ABCM-P | 328 | 2.5 | 1.4 | 58 | 29 | 13 |
| 1991-1993 | ||||||
| NR ABCM | 271 | 2.6 | 1.4 | 58 | 28 | 14 |
| VIII, 1993-2002 (at least 65 y, or under 65 y*) | ||||||
| ABCM to plateau | 135 | 3.1 | 1.4 | 69 | 25 | 6 |
| ABCM ×3 then C-weekly | 140 | 3.1 | 1.5 | 56 | 36 | 8 |
| NR | 223 | 1.2 | 1.3 | 63 | 24 | 13 |
| Total | 2592 | NA | NA | 58 | 28 | 14 |
Trial, dates (patient ages), and induction treatment . | No. patients . | Median OS, y . | Median DP, y . | IgG, % . | IgA, % . | LCO, % . |
|---|---|---|---|---|---|---|
| IV, 1980-1982 (under 80 y) | ||||||
| MP | 254 | 2.3 | 1.1 | 59 | 29 | 12 |
| MPV | 251 | 2.2 | 1.5 | 57 | 24 | 19 |
| V, 1982-1986 (under 75 y) | ||||||
| C-weekly-plts < 80 | 58 | 1.8 | 1.7 | 53 | 28 | 19 |
| M7 | 291 | 2.0 | 1.4 | 56 | 29 | 15 |
| ABCM | 293 | 2.7 | 1.6 | 56 | 27 | 17 |
| VI (under 75 y) | ||||||
| 1986-1991 | ||||||
| HDM (M140) | 12 | 2.5 | 2.5 | 59 | 33 | 8 |
| HDMP | 14 | 5.0 | 1.9 | 71 | 29 | 0 |
| ABCM | 322 | 2.7 | 1.5 | 58 | 28 | 14 |
| ABCM-P | 328 | 2.5 | 1.4 | 58 | 29 | 13 |
| 1991-1993 | ||||||
| NR ABCM | 271 | 2.6 | 1.4 | 58 | 28 | 14 |
| VIII, 1993-2002 (at least 65 y, or under 65 y*) | ||||||
| ABCM to plateau | 135 | 3.1 | 1.4 | 69 | 25 | 6 |
| ABCM ×3 then C-weekly | 140 | 3.1 | 1.5 | 56 | 36 | 8 |
| NR | 223 | 1.2 | 1.3 | 63 | 24 | 13 |
| Total | 2592 | NA | NA | 58 | 28 | 14 |
IgG, 1513 patients; IgA, 718 patients; LCO, 361 patients.
MP indicates melphalan, prednisolone; MPV, melphalan, prednisolone, vincristine; C-weekly plts, cyclophosphamide weekly for low platelets; M7, melphalan; ABCM, doxorubicin, carmustine, cyclophosphamide, melphalan; NR, nonrandomized; C-VAMP, cyclophosphamide, vincristine, doxorubicin, methylprednisolone; OS, overall survival; DP, duration of plateau; IgG, immunoglobulin G; IgA, immunoglobulin A; LCO, light chain only; and NA, not applicable.
If high-dose therapy (HDT) contraindicated.
Statistical analysis
Differences in patient characteristics and causes of death by paraprotein class group were investigated using Pearson χ2 test. The primary end point for analysis was survival defined as date of entry to date of death or date last seen. Survival curves were constructed using the Kaplan-Meier method,23 and the log-rank test24 was used to assess differences between paraprotein class groups. Relapse-free interval or duration of first plateau was calculated from the date of plateau to the date of disease progression, or it was censored at the date of death for patients who died without disease progression or at the date a patient was last seen alive without disease progression.
Results
Patient characteristics
IgA and IgG patients were alike in all presentation characteristics with the exception of corrected calcium, with hypercalcemia in 45% of IgA patients compared with 28% of IgG patients (Table 2). However, there was no difference in the rates of bone fractures, bone pain, or lytic lesions between these 2 groups of patients, and the increased hypercalcemia levels in IgA patients might have been artifactual. Presentation characteristics of patients with LCO myeloma were unlike those of IgA or IgG patients. Sixty percent of LCO patients were younger than 65 years of age, whereas the rate was 48% in IgA and IgG patients (P = .001). Sixty-six percent of IgA and IgG patients had serum albumin concentrations lower than 35 g/L compared with 28% of LCO patients (P < .001), but this might partly have reflected a compensation for high total serum protein levels attributable to paraprotein. Despite their younger age, LCO patients had poorer performance status; 61% had restricted activity or worse compared with 51% of IgA patients and 50% of IgG patients (P = .001). LCO patients had a higher incidence of renal impairment with elevated serum creatinine (42% had serum creatinine greater than 199 mM compared with 16% of IgA and 13% of IgG patients; P = .001). LCO patients also had a higher incidence of elevated Sβ2m (54% had levels greater than 8 mg/L compared with 34% and 30% of IgA and IgG patients, respectively). An increased incidence of multiple lytic bone lesions (P < .001) was observed in LCO patients compared with IgG and IgA patients but was not apparent in bone pain or fractures (Table 2).
Patient characteristics by paraprotein class
. | Paraprotein class, no. (%) . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Factor and grouping . | IgG . | IgA . | LCO . | χ2 . | P . | ||
| General characteristics | |||||||
| Sex | 4.52 | .15 | |||||
| Male | 880 (58) | 413 (58) | 188 (52) | ||||
| Female | 632 (42) | 305 (42) | 173 (48) | ||||
| Age, y | 16.88 | .001 | |||||
| Under 65 | 744 (49) | 334 (47) | 215 (60) | ||||
| 65 or more | 767 (51) | 383 (53) | 146 (40) | ||||
| Performance status | 26.68 | .001 | |||||
| Asymptomatic | 193 (14) | 69 (10) | 19 (6) | ||||
| Minimal symptoms | 516 (37) | 257 (38) | 111 (33) | ||||
| Restricted activity | 544 (39) | 273 (41) | 166 (49) | ||||
| Bedridden | 151 (11) | 69 (10) | 41 (12) | ||||
| Serum albumin, g/L | 149.03 | < .001 | |||||
| Under 30 | 375 (33) | 153 (28) | 19 (7) | ||||
| 30 to 35 | 371 (33) | 206 (38) | 55 (21) | ||||
| Above 35 | 383 (34) | 185 (34) | 188 (72) | ||||
| Tumor burden/activity | |||||||
| Bone marrow plasma cells, % | 14.76 | .005 | |||||
| Under 20 | 290 (25) | 116 (22) | 75 (26) | ||||
| 20 to 50 | 532 (46) | 231 (43) | 107 (38) | ||||
| Above 50 | 326 (28) | 190 (35) | 103 (36) | ||||
| C-reactive protein, mg/L | 3.86 | .14 | |||||
| 10 or less | 455 (67) | 214 (64) | 118 (60) | ||||
| Above 10 | 220 (33) | 121 (36) | 78 (40) | ||||
| Serum β-2 microglobulin, mg/L | 76.38 | < .001 | |||||
| 4 or less | 431 (29) | 171 (24) | 63 (18) | ||||
| 4 to 8 | 608 (41) | 291 (42) | 98 (28) | ||||
| Above8 | 438 (30) | 237 (34) | 188 (54) | ||||
| Hemopoietic function | |||||||
| Anemia, Hb g/dL | 12.64 | .01 | |||||
| Less than 7.5 | 138 (10) | 84 (13) | 50 (15) | ||||
| 7.5 to 10 | 467 (33) | 242 (36) | 100 (30) | ||||
| Above 10 | 797 (57) | 344 (51) | 184 (55) | ||||
| Thrombocytopenia, platelets × 109/L | 17.67 | .001 | |||||
| Less than 80 | 24 (2) | 18 (3) | 11 (4) | ||||
| 80 to 150 | 141 (11) | 103 (16) | 44 (14) | ||||
| Above 150 | 1169 (88) | 521 (81) | 256 (82) | ||||
| Neutrophils, × 109/L | 25.73 | < .001 | |||||
| Less than 1.8 | 81 (6) | 45 (7) | 11 (3) | ||||
| 1.8 to 3 | 384 (29) | 204 (32) | 61 (19) | ||||
| Above 3 | 851 (65) | 390 (61) | 245 (77) | ||||
| Skeletal disease | |||||||
| Corrected calcium, μM | 51.55 | < .001 | |||||
| 2.63 or less | 812 (72) | 296 (55) | 177 (69) | ||||
| Above 2.63 | 311 (28) | 245 (45) | 80 (31) | ||||
| Bone pain | 1.99 | .74 | |||||
| Present | 512 (34) | 259 (36) | 131 (36) | ||||
| Absent | 174 (12) | 75 (10) | 36 (10) | ||||
| No information | 827 (55) | 384 (53) | 194 (54) | ||||
| Fractures | 6.54 | .16 | |||||
| Present | 468 (31) | 214 (30) | 125 (35) | ||||
| Absent | 587 (39) | 261 (36) | 120 (33) | ||||
| No information | 458 (30) | 243 (34) | 116 (32) | ||||
| Lytic lesions | 45.23 | < .001 | |||||
| None | 382 (25) | 166 (23) | 36 (10) | ||||
| Isolated | 100 (7) | 43 (6) | 22 (6) | ||||
| Multiple | 867 (57) | 421 (59) | 266 (74) | ||||
| No information | 164 (11) | 88 (12) | 37 (10) | ||||
| Serum phosphate, μM | 7.90 | .10 | |||||
| Less than 0.99 | 66 (18) | 30 (17) | 13 (15) | ||||
| 0.99 to 1.5 | 233 (64) | 117 (67) | 50 (56) | ||||
| Above 1.5 | 63 (17) | 28 (16) | 26 (29) | ||||
| Renal disease | |||||||
| Serum creatinine, μM | 170.52 | < .001 | |||||
| Less than 130 | 936 (64) | 436 (62) | 128 (36) | ||||
| 130 to 199 | 330 (23) | 151 (22) | 81 (23) | ||||
| Above 199 | 193 (13) | 113 (16) | 149 (42) | ||||
| Blood urea, μM | 98.17 | < .001 | |||||
| Less than 6.5 | 760 (51) | 363 (51) | 122 (34) | ||||
| 6.5 to 10 | 454 (30) | 195 (28) | 85 (24) | ||||
| Above 10 | 280 (19) | 150 (21) | 154 (43) | ||||
. | Paraprotein class, no. (%) . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|
| Factor and grouping . | IgG . | IgA . | LCO . | χ2 . | P . | ||
| General characteristics | |||||||
| Sex | 4.52 | .15 | |||||
| Male | 880 (58) | 413 (58) | 188 (52) | ||||
| Female | 632 (42) | 305 (42) | 173 (48) | ||||
| Age, y | 16.88 | .001 | |||||
| Under 65 | 744 (49) | 334 (47) | 215 (60) | ||||
| 65 or more | 767 (51) | 383 (53) | 146 (40) | ||||
| Performance status | 26.68 | .001 | |||||
| Asymptomatic | 193 (14) | 69 (10) | 19 (6) | ||||
| Minimal symptoms | 516 (37) | 257 (38) | 111 (33) | ||||
| Restricted activity | 544 (39) | 273 (41) | 166 (49) | ||||
| Bedridden | 151 (11) | 69 (10) | 41 (12) | ||||
| Serum albumin, g/L | 149.03 | < .001 | |||||
| Under 30 | 375 (33) | 153 (28) | 19 (7) | ||||
| 30 to 35 | 371 (33) | 206 (38) | 55 (21) | ||||
| Above 35 | 383 (34) | 185 (34) | 188 (72) | ||||
| Tumor burden/activity | |||||||
| Bone marrow plasma cells, % | 14.76 | .005 | |||||
| Under 20 | 290 (25) | 116 (22) | 75 (26) | ||||
| 20 to 50 | 532 (46) | 231 (43) | 107 (38) | ||||
| Above 50 | 326 (28) | 190 (35) | 103 (36) | ||||
| C-reactive protein, mg/L | 3.86 | .14 | |||||
| 10 or less | 455 (67) | 214 (64) | 118 (60) | ||||
| Above 10 | 220 (33) | 121 (36) | 78 (40) | ||||
| Serum β-2 microglobulin, mg/L | 76.38 | < .001 | |||||
| 4 or less | 431 (29) | 171 (24) | 63 (18) | ||||
| 4 to 8 | 608 (41) | 291 (42) | 98 (28) | ||||
| Above8 | 438 (30) | 237 (34) | 188 (54) | ||||
| Hemopoietic function | |||||||
| Anemia, Hb g/dL | 12.64 | .01 | |||||
| Less than 7.5 | 138 (10) | 84 (13) | 50 (15) | ||||
| 7.5 to 10 | 467 (33) | 242 (36) | 100 (30) | ||||
| Above 10 | 797 (57) | 344 (51) | 184 (55) | ||||
| Thrombocytopenia, platelets × 109/L | 17.67 | .001 | |||||
| Less than 80 | 24 (2) | 18 (3) | 11 (4) | ||||
| 80 to 150 | 141 (11) | 103 (16) | 44 (14) | ||||
| Above 150 | 1169 (88) | 521 (81) | 256 (82) | ||||
| Neutrophils, × 109/L | 25.73 | < .001 | |||||
| Less than 1.8 | 81 (6) | 45 (7) | 11 (3) | ||||
| 1.8 to 3 | 384 (29) | 204 (32) | 61 (19) | ||||
| Above 3 | 851 (65) | 390 (61) | 245 (77) | ||||
| Skeletal disease | |||||||
| Corrected calcium, μM | 51.55 | < .001 | |||||
| 2.63 or less | 812 (72) | 296 (55) | 177 (69) | ||||
| Above 2.63 | 311 (28) | 245 (45) | 80 (31) | ||||
| Bone pain | 1.99 | .74 | |||||
| Present | 512 (34) | 259 (36) | 131 (36) | ||||
| Absent | 174 (12) | 75 (10) | 36 (10) | ||||
| No information | 827 (55) | 384 (53) | 194 (54) | ||||
| Fractures | 6.54 | .16 | |||||
| Present | 468 (31) | 214 (30) | 125 (35) | ||||
| Absent | 587 (39) | 261 (36) | 120 (33) | ||||
| No information | 458 (30) | 243 (34) | 116 (32) | ||||
| Lytic lesions | 45.23 | < .001 | |||||
| None | 382 (25) | 166 (23) | 36 (10) | ||||
| Isolated | 100 (7) | 43 (6) | 22 (6) | ||||
| Multiple | 867 (57) | 421 (59) | 266 (74) | ||||
| No information | 164 (11) | 88 (12) | 37 (10) | ||||
| Serum phosphate, μM | 7.90 | .10 | |||||
| Less than 0.99 | 66 (18) | 30 (17) | 13 (15) | ||||
| 0.99 to 1.5 | 233 (64) | 117 (67) | 50 (56) | ||||
| Above 1.5 | 63 (17) | 28 (16) | 26 (29) | ||||
| Renal disease | |||||||
| Serum creatinine, μM | 170.52 | < .001 | |||||
| Less than 130 | 936 (64) | 436 (62) | 128 (36) | ||||
| 130 to 199 | 330 (23) | 151 (22) | 81 (23) | ||||
| Above 199 | 193 (13) | 113 (16) | 149 (42) | ||||
| Blood urea, μM | 98.17 | < .001 | |||||
| Less than 6.5 | 760 (51) | 363 (51) | 122 (34) | ||||
| 6.5 to 10 | 454 (30) | 195 (28) | 85 (24) | ||||
| Above 10 | 280 (19) | 150 (21) | 154 (43) | ||||
Overall survival by paraprotein class
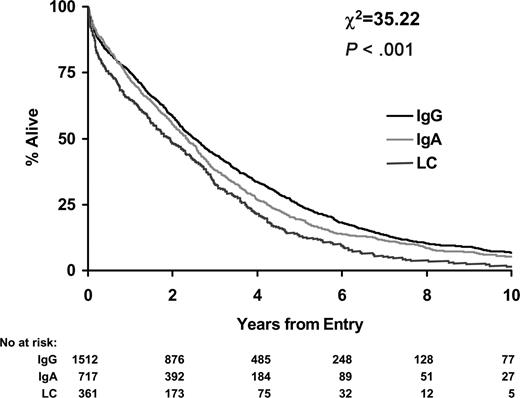
IgG patients had the longest overall survival (median, 2.5 years; 95% CI, 2.3-2.7 years) followed by IgA patients (median, 2.3 years; 95% CI, 2.1-2.6 years; P = .01). LCO patients had the shortest overall survival (median, 1.9 years; 95% CI, 1.5-2.3 years; P < .001; Figure 1).
Early death and failure to reach plateau
LCO myeloma was associated with early death, with 19% of LCO patients dying within 100 days compared with 11% of patients with IgA and 12% with IgG myeloma; this increase in early deaths was primarily attributable to renal failure (P = .01; Table 3). LCO patients were less likely to achieve plateau than IgA or IgG patients (48% compared with 56%; P = .008).
Causes of early death
Cause of death . | IgG, % . | IgA, % . | LCO, % . | χ2 . |
|---|---|---|---|---|
| Progressive tumor | 36 | 39 | 31 | NS |
| Infection, tumor under control | 28 | 18 | 13 | NS |
| Mainly renal | 6 | 8 | 19 | 15.94* |
| Died of other cause | 30 | 36 | 37 | NS |
Cause of death . | IgG, % . | IgA, % . | LCO, % . | χ2 . |
|---|---|---|---|---|
| Progressive tumor | 36 | 39 | 31 | NS |
| Infection, tumor under control | 28 | 18 | 13 | NS |
| Mainly renal | 6 | 8 | 19 | 15.94* |
| Died of other cause | 30 | 36 | 37 | NS |
Early death was defined as death within 100 days of entry.
IgG, n = 185 of 1513 (12%) patients; IgA, n = 80 of 718 (11%) patients; LCO, n = 70 of 361 (19%) patients.
NS indicates not significant.
P = .01.
Duration of first plateau phase by paraprotein class
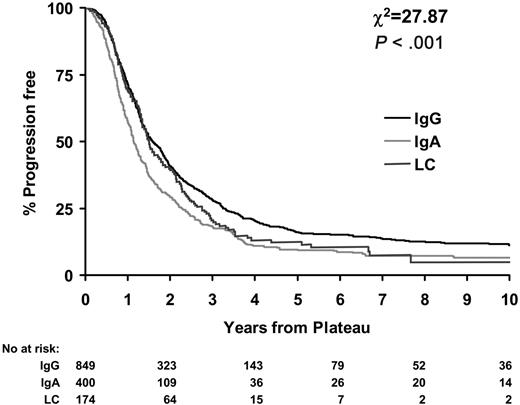
Little difference in duration of plateau was observed between LCO and IgG patients (P = .08). Median durations of plateau were 1.6 years (95% CI, 1.5-1.8 years) for IgG patients and 1.5 years (95% CI, 1.3-1.8 years) for LCO myeloma patients. However, IgA patients had significantly shorter durations of plateau than IgG and LCO patients (median, 1.2; 95% CI, 1.1-1.3; P < .001; Figure 2.)
Survival from first disease progression
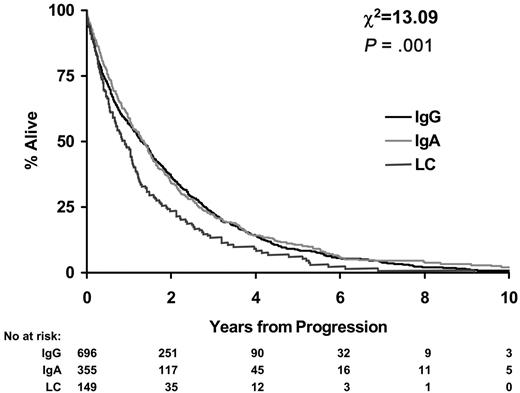
Survival from disease progression was worse for LCO patients. Median survival from disease progression for IgA and IgG patients was 1.3 years (95% CI, 1.2-1.5 years) compared with 0.9 years for LCO myeloma patients (95% CI, 0.7-1.1 years; P < .001; Figure 3).
Effect of free light chain (flc) excretion on survival
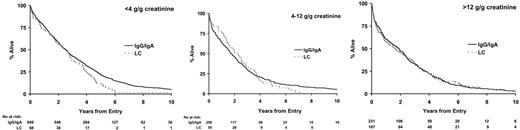
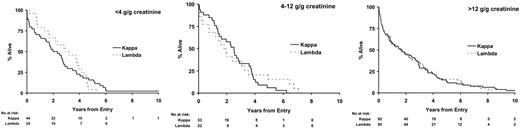
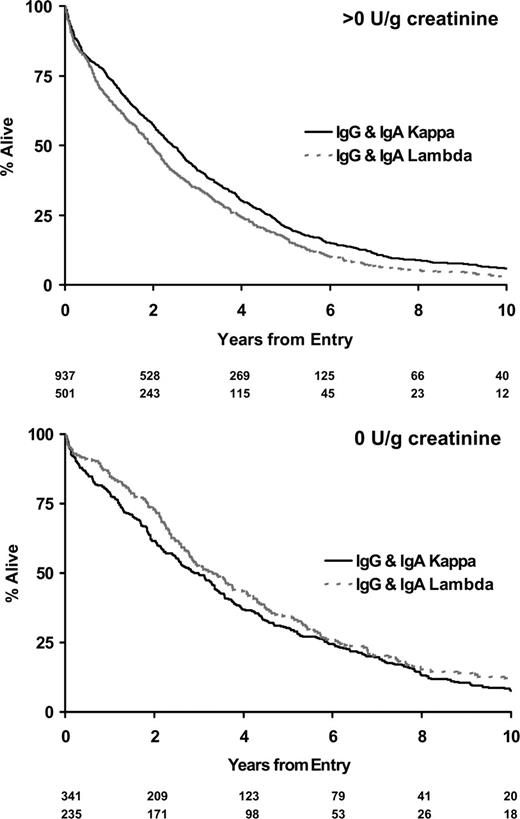
Twenty-eight percent of IgA and IgG myeloma patients did not have significant urinary flc excretion, and within this group only 2% had renal impairment (Table 4). Eleven percent of IgG and 13% of IgA patients had high levels of urine flc excretion (greater than 12 g/g creatinine), and 48% of these patients had renal impairment; this compares to a 54% rate of renal impairment in LCO patients with greater than 12 g/g creatinine flc excretion. There was no difference between IgG, IgA, and LCO myeloma in the probability of renal impairment for a given level of flc excretion (Table 4). No differences in overall survival between paraprotein classes stratified by level of urinary light chain excretion were detected (Figure 4). No differences in survival were identified between the κ and λ light chain types for patients with LCO myeloma when stratified by levels of urinary light chain excretion (Figure 5). Evidence suggested superior survival in patients with IgA/IgG κ light chain type compared with survival in patients with IgA/IgG λ light chain. This was only apparent in patients with detectable urinary light chain excretion; patients with IgA/IgG κ light chain had a median survival of 2.4 years (95% CI, 2.2-2.7 years) compared with 2.0 years (95% CI, 1.7-2.2 years) in patients with IgA/IgG λ light chain (P = .007; Figure 6). This probably reflected higher levels (greater than 4 g/g creatinine) of flc excretion in λ (30% of λ patients) than in κ (21% of κ patients) light chain (P < .001). Increasing levels of urinary flc are only associated with worse survival if they are associated with renal impairment at presentation. In patients with serum creatinine lower than 100 μM, light chain levels did not influence the percentage of patients achieving plateau (P = .86) or survival in the first 3 years (P = .48).
Urinary flc excretion and renal failure by paraprotein class
Urinary flc excretion, g/g creatinine . | Patients with renal failure, no.(%) . | . | . | ||
|---|---|---|---|---|---|
| . | IgG . | IgA . | LCO . | ||
| 0 g/g | 28 (2) | 29 (3) | 0 (0) | ||
| Less than 4 g/g | 48 (8) | 46 (11) | 22 (18) | ||
| 4-12 g/g | 13 (29) | 12 (28) | 18 (38) | ||
| More than 12 g/g | 11 (48) | 13 (48) | 60 (54) | ||
Urinary flc excretion, g/g creatinine . | Patients with renal failure, no.(%) . | . | . | ||
|---|---|---|---|---|---|
| . | IgG . | IgA . | LCO . | ||
| 0 g/g | 28 (2) | 29 (3) | 0 (0) | ||
| Less than 4 g/g | 48 (8) | 46 (11) | 22 (18) | ||
| 4-12 g/g | 13 (29) | 12 (28) | 18 (38) | ||
| More than 12 g/g | 11 (48) | 13 (48) | 60 (54) | ||
IgG, 1367 patients; IgA, 649 patients; LCO, 310 patients.
Discussion
The paraprotein class and degree of dysregulation between heavy and light chain production reflect differences in original immune response and biology of the malignant cells. Whether the 3 main paraprotein types of multiple myeloma—IgG, IgA, and light chain only (LCO)—are associated with significantly different disease at presentation and subsequent survival is relevant to prognosis and clinical management. An international study of staging in myeloma noted median survival for IgG, IgA, and LCO patients to be 49 months, 40 months, and 35 months, respectively.12 However, this study did not explore why IgA patients fared worse than IgG patients, nor did it explore the significance of LC excretion in IgG and IgA patients or whether the extreme dysregulation in heavy and light chain production was associated with different or worse disease for patients with LCO myeloma. This study assesses presentation characteristics and survival in 2592 patients receiving conventional dose chemotherapy in the IV, V, VI, and VIII MRC myeloma trials over 22 years. Most (822) patients received standard dose melphalan-based chemotherapy or melphalan-based-conventional dose combination chemotherapy (ABCM; 1712 patients). Paraprotein types and levels were assessed by central laboratory analysis (Table 1).
Survival by paraprotein class stratified by urinary light chain excretion.
We found no differences in the presentation characteristics of IgG and IgA myeloma patients, with the exception of a higher incidence of hypercalcemia in IgA patients (45% vs 28%; P = .001; Table 2). This higher incidence of hypercalcemia was not associated with any increased incidence of bone pain, lytic lesions, or fractures, and we are investigating whether it might reflect an interference of IgA paraproteins with the measurement of corrected serum calcium levels. We found no difference between IgG and IgA patients with respect to early deaths (12% and 11%), percentages of patients achieving plateau phase (56% for both groups), or survival from disease progression (Figure 3). The difference in overall survival was wholly attributable to a shorter duration of plateau phase for IgA compared with IgG patients (1.2 vs 1.6 years; P < .001; Figure 2). This clinically important difference should be taken into account when considering maintenance therapy and in the interpretation of results of trials of maintenance therapy. The biologic cause for this difference in the duration of the plateau phase might reflect susceptibility to the translocation of genes into IgG versus IgA switch regions. IgA patients have been shown to have a higher incidence of t(4;14) and associated early disease progression,25 but we did not have cytogenetic data in our study.
Survival by type for patients with light chain myeloma stratified by urinary light chain excretion.
Survival by type for patients with light chain myeloma stratified by urinary light chain excretion.
Evidence that light chain disease is a variant of myeloma dates from the 1941 report of Gutman et al,26 who described a patient with myeloma, hypoproteinemia, and Bence-Jones proteinuria. Light chain myeloma accounts for 10% to 20% of all cases of myeloma, and it is well established that LCO myeloma is associated with a higher incidence of renal impairment and worse survival.1-4,13-17 Whether this worse survival is attributable solely to light chain nephrotoxicity or also reflects a biologically more aggressive disease is unclear, and the importance of LC excretion to survival of IgG and IgA patients also requires clarification.
LCO myeloma patients were younger than IgG and IgA patients. Only 40% of LCO myeloma patients were older than 65 years compared with 52% of IgG and IgA patients (P = .002; Table 2). This might have reflected biologic differences, failure to diagnose LCO myeloma in older patients, or failure to enter them into trials. Despite their younger age, LCO patients had worse performance status (P = .001) and greater incidence of multiple lytic lesions at diagnosis that might have reflected the biology of the disease or a delay in diagnosis because of the lack of a serum paraprotein, emphasizing the need to assess urine for flc and indicating that failure to send urine to the laboratory can delay the diagnosis of myeloma; assay of serum for flc may mitigate this problem. Paraproteins cause high total serum protein levels and a compensatory reduction in albumin concentration, so it was not surprising that twice as many LCO patients (72%) as IgG and IgA patients (34%) had normal serum albumin levels, a factor that might have confounded the use of albumin as a prognostic marker. LCO patients had a 3-fold higher incidence of renal impairment and elevated levels of Sβ2m at presentation than IgG/IgA patients (P = .001).
Death within 100 days occurred in 19% of LCO patients compared with 12% of IgG and IgA patients (Table 3), primarily because of renal failure attributable to higher incidence and amounts of urinary flc in LCO patients (Table 4). Seventy-eight percent of LCO patients had greater than 4 g/g creatinine flc excretion compared with only 24% of IgG and IgA myeloma patients. IgG and IgA patients with the same levels of urinary flc excretion as LCO myeloma patients had the same incidence of renal failure and poor survival (Table 4; Figure 4). There has been conjecture as to whether light chain type affects survival. In LCO patients, there was no difference in survival between κ and λ types when stratified by level of LC excretion (Figure 5). Similarly, the inferior outcome of IgG/IgA λ myeloma compared with IgG/IgA κ is a function of the higher level of flc excretion in the former group (Figure 6). The significantly different survival between LCO and IgG/IgA myeloma reflects the incidence of renal failure that is a function of the incidence and level of flc excretion associated with those paraprotein and light chain types. In patients without renal impairment, levels of light chain excretion do not influence early survival, reinforcing that it is the renal impairment caused by LCs that affects survival, not the LCs themselves.
Survival by light chain type for IgG and IgA myeloma stratified by the presence or absence of urinary light chain excretion.
Survival by light chain type for IgG and IgA myeloma stratified by the presence or absence of urinary light chain excretion.
Progression-free survival reflects the intrinsic biology of the disease and is also an indicator of any treatment benefit. We noted no difference in progression-free survival between IgG and LCO myeloma patients. However, survival from progression was worse for LCO myeloma patients. Response to treatment and relapse in LCO myeloma was conventionally assessed by urine light chain measurements. Studies suggest that kidneys metabolize 20 to 30 g/d flc.27,28 Free light chains are not present in urine until their production exceeds the renal threshold; thus, serum levels are greatly increased before flc is detectable in the urine. Recent studies suggest urine flc measurements are relatively insensitive for assessing residual disease or early relapse in LCO myeloma.29 The increase in mortality at relapse may, therefore, reflect a delay in diagnosing relapse and the higher incidence of associated renal impairment. In these patients, careful monitoring of disease in the plateau phase and preemptive therapy early in relapse may reduce further renal damage and thus improve survival. Newly available serum flc analysis may prove to be a more sensitive way of monitoring disease response and relapse in LCO myeloma.29,30
This large study demonstrates a strong association between level of flc excretion, renal impairment, and poor survival irrespective of paraprotein type or light chain class (κ or λ). Survival is related to preservation of renal function. Supportive measures to minimize further renal damage, including avoidance of aminoglycosides and NSAIDs and maintenance of hydration, are important to avoid death during induction therapy and at progression.31,32 Choice of chemotherapy and recent expansion of therapeutic options to agents including thalidomide, lenalidomide, and bortezomib should take into account their interaction with renal impairment and now provide a greater potential for controlling myeloma in these patients.32-35 Comparable survival between LC secretors and nonsecretors might be achieved.
Prepublished online as Blood First Edition Paper, May 25, 2006; DOI 10.1182/blood-2006-03-008953.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal