Abstract
Host antigen-presenting cells (APCs) are known to be critical for the induction of graft-versus-host disease (GVHD) after allogeneic bone marrow transplantation (BMT), but the relative contribution of specific APC subsets remains unclear. We have studied the role of host B cells in GVHD by using B-cell–deficient μMT mice as BMT recipients in a model of CD4-dependent GVHD to major histocompatibility complex antigens. We demonstrate that acute GVHD is initially augmented in μMT recipients relative to wild-type recipients (mortality: 85% vs 44%, P < .01), and this is the result of an increase in donor T-cell proliferation, expansion, and inflammatory cytokine production early after BMT. Recipient B cells were depleted 28-fold at the time of BMT by total body irradiation (TBI) administered 24 hours earlier, and we demonstrate that TBI rapidly induces sustained interleukin-10 (IL-10) generation from B cells but not dendritic cells (DCs) or other cellular populations within the spleen. Finally, recipient mice in which B cells are unable to produce IL-10 due to homologous gene deletion develop more severe acute GVHD than recipient mice in which B cells are wild type. Thus, the induction of IL-10 in host B cells during conditioning attenuates experimental acute GVHD.
Introduction
Allogeneic stem cell transplantation (SCT) is currently the treatment of choice for a variety of hematologic, neoplastic, and genetic disorders. However, the significant limitation to the efficacy of allogeneic SCT is the occurrence of graft-versus-host disease (GVHD) as a consequence of naive donor T cells recognizing alloantigen on host antigen-presenting cells (APCs).1,2 To date, the specific types of host APCs important in initiating GVHD remain uncertain, although dendritic cells (DCs) appear capable of inducing the full spectrum of GVHD in isolation.3 B cells recognize antigen through surface immunoglobulins and can also present antigen to T cells.4 Importantly, the ability of host B cells to present antigen to T cells is lost following high (3300 cGy) but not low (1000 cGy) radiation doses.5 The role that host B cells play in isolation in GVHD responses, if any, has not been definitively studied, and this is an important issue now that effective monoclonal antibodies are universally available to deplete this cell population.
We have examined the role of B cells in acute GVHD by using B-cell–deficient mutant mice in which the μ immunoglobulin membrane exon has been disrupted (μMT mice). This genetic disruption results in the isolated absence of B-cell development.6 We confirm that B-cell–deficient μMT mice have normal numbers of professional APCs and, when used as bone marrow transplantation (BMT) recipients, develop more severe GVHD than their B-cell–replete wild-type counterparts. Since total body irradiation (TBI) depletes B cells rapidly, the protection from GVHD in the presence of B cells is the result of effects of conditioning on B cells rather than inhibitory effects of B cells on alloreactivity per se. We demonstrate that TBI rapidly induces interleukin-10 (IL-10) generation from host B cells, and IL-10 inhibits subsequent alloreactive T-cell expansion and acute GVHD to the major histocompatibility complex (MHC).
Materials and methods
Mice
Female B6 (H-2b, Ly 5.2+, CD45.2+) or B6 Ptprca (H-2b, Ly 5.1+, CD45.1+) and Balb/c (H-2d) mice were purchased from the Animal Resource Centre (Perth, Western Australia, Australia). μMT (B6, H-2b, CD45.2+) and IL-10–/– (B6, H-2b, CD45.2+) mice were supplied by the Queensland Institute of Medical Research, Herston Medical Research Centre, and Australian National University animal facilities. The age of mice used ranged between 8 and 14 weeks. Mice were housed in microisolator cages and received acidified autoclaved water (pH 2.5) after transplantation. All transplant recipients in experiments that included IL-10–/– animals were fed neomycin-containing drinking water at 1 g/L.
Bone marrow transplantation
Mice received transplants according to a standard protocol, as has been described previously.7,8 At day –1 of transplantation, recipient wild-type and μMT B6 mice received TBI of 1000 cGy (137Cesium source at 108 cGy/min) or 900 cGy in wild-type versus IL-10–/– recipients, split into 2 doses with a 3-hour interval to minimize gastrointestinal (GI) toxicity. Allogeneic (Balb/c) or syngeneic (B6) T-cell–depleted (TCD) donor bone marrow (BM) (5 × 106 per inoculum) with or without T cells (adjusted to 2 or 3 × 106 CD3+ cells per inoculum) purified on nylon wool or by magnetic beads (Qiagen, Hilden, Germany) were administered via intravenous injection in 250 μL of Leibovitz L15 (Gibco Invitrogen cell culture reagents, Invitrogen, Melbourne, Australia). To create bone marrow chimeras, wild-type B6 Ptprca (CD45.1+) mice received 1000 cGy TBI and received transplants 24 hours later of 5 × 106 B6 (CD45.2+) wild-type or μMT TCD BM cells or a combination of 4 × 106μMT TCD BM and 1 × 106 wild-type or IL-10–/– TCD BM, as previously described.9 Chimeras were left for 4 months to reconstitute and subsequently received transplants of allogeneic TCD BM without or with splenic T cells from Balb/c donors. Survival and GVHD clinical score were monitored daily.
Assessment of GVHD
The degree of systemic GVHD was assessed by a scoring system that sums changes in 5 clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity (maximum index = 10).10 Animals with severe GVHD as determined by clinical scores greater than or equal to 6 were killed as required by institutional animal ethics guidelines and the day of death determined as the following day.
FACS and cytokine analysis
Fluorescein isothiocyanate (FITC)–conjugated monoclonal antibodies (mAbs), phycoerythrin (PE)–conjugated mAbs, and biotinylated mAbs were purchased from BD Biosciences Pharmingen (San Diego, CA) and BioLegend (San Diego, CA) and analysis undertaken as previously described.11 B cells, DCs, and residual non-B, non-DC cells were labeled by anti-MHC class II (I-A/I-E), anti-CD19, and anti-CD11c mAb, and MoFlo sorted as MHC class II+/CD19+ (B cells), MHC class II+/CD11c+ (DCs), and MHC class II–/CD19– (non-B, non-DC) populations. Serum levels of interferon-gamma (IFNγ) and tumor necrosis factor-alpha (TNFα) were determined via ELISA (enzyme-linked immunosorbent assay) using antibodies purchased from BD Biosciences Pharmingen. IFNγ, IL-4, IL-5, and TNFα in cell-culture supernatants were determined using the BD Cytometric Bead Array (CBA) system (BD Biosciences Pharmingen). For engraftment studies the percentage of H-2d+ H-2b– cells was divided by the percentage of H-2d+ H-2b– + H-2d– H-2b+ cells. Fluorescein labeling of T cells was performed as described.12 Briefly, purified T cells were resuspended at a density of 3 × 107 cells/mL in phosphate-buffered saline and incubated with 2 μM carboxy-fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 10 minutes at 37°C. Cells were injected into recipients intravenously and analyzed 5 hours and 120 hours later using FACScan (BD Biosciences Pharmingen), and the proliferation indexes were calculated using ModFit LT cell-cycle analysis software (Verity Software House, Topsham, ME) as fold expansion of input donor cells. Apoptotic lymphocytes were determined as annexin V+/7-aminoactinomycin D (7-AAD)– cells by labeling splenocytes with PE-conjugated annexin V and 7-AAD (BD Biosciences Pharmingen), as previously described.13 Intracellular staining of IL-10 was performed on unseparated splenocytes from control or irradiated B6 mice 24 hours after TBI with 1000 cGy. IL-10 was detected with anti–mouse IL-10 mAb (BioLegend) using staining protocol and recommended reagents from BioLegend. Proportion of IL-10–positive B cells was determined in the MHC class II (I-A/I-E)+/CD19+ population.
Real-time IL-10 PCR
For real-time polymerase chain reaction (PCR) analysis of IL-10, equivalent numbers of Mo-Flo sorted cells were resuspended in Trizol (Gibco-BRL, Carlsbad, CA), snap frozen on dry ice, and RNA extracted according to the manufacturer's protocol. cDNA was immediately reverse transcribed using AMVRT (Promega, WI) according to the manufacturer's protocol and cDNA stored at –20°C. Real-time PCR was undertaken using Platinum SYBR Green qPCR SuperMix UDG (Invitrogen, Melbourne, Australia), carried out on a Rotor-Gene3000 (Corbett Research, Sydney, Australia) and data analyzed using Rotor-Gene V-5.0 (Corbett Research). Primers used for IL10 reactions were 5′-GAGAGCGCTCATCTCGATTT-3′ and 5′-GGGTCTCCCAAGGAAAGGTA-3′. Primers used for β-2 microglobulin reactions (B2M) were 5′-TTTCTGGTGCTTGTCTCACTGACCG-3′ and 5′-GCAGTTCAGTATGTTCGGCTTCCCA-3′. IL-10 cDNA copy numbers then were normalized for variations in the efficiency of RNA extraction and cDNA transcription against the B2M housekeeping gene, as previously described.14
Cell cultures
Cell culture was performed in 10% fetal calf serum (FCS) Iscove modified Dulbecco medium (IMDM) supplemented with 1% penicillin streptomycin, 1% l-glutamine, 1% sodium pyruvate, 1% nonessential amino acid, 1% HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 0.02 mM β-mercaptoethanol. All tissue culture reagents were purchased from JRH Biosciences (Melbourne, Australia). Cultures were plated in round-bottom, 96-well plates or flat-bottom, 24-well plates (Falcon, Becton Dickinson, Franklin Lakes, NJ) and incubated at 37°C with 5% CO2. In primary mixed lymphocyte cultures (MLCs) red-cell–lysed splenocytes from μMT and wild-type B6 mice were titrated, plated in 96-well plates, and irradiated at 1500 cGy; 1 × 105 purified Balb/c T cells were added to each well. Supernatants were harvested at 4 days and cultures then pulsed with 3H-thymidine at 1 μCi (0.037 MBq) per well. In secondary MLCs, performed 2 weeks after BMT, donor-derived H-2d+CD4+ T cells were purified (> 99% CD4+) by cell sorting from allogeneic μMT and wild-type recipients and stimulated with H-2b+CD11c+ DCs. Supernatants were harvested at 2 days and cultures then pulsed as described for primary MLCs. 3H-thymidine uptake was determined 12 hours later using Wallac 1205 Beta Plate Reader (GMI, Ramsey, MN). In vitro cytotoxic response to alloantigen was determined in a standard 51Cr release assay as previously published.15 Briefly, 13 days after SCT, splenocytes from transplant recipients were pooled within the treatment group, and equal numbers of sort-purified CD8+ effector cells (> 99% pure) were plated with 51Cr-labeled EL4 (H-2b, host type) and A20 (H-2d, donor type) target cells. Peritoneal macrophages were obtained by peritoneal lavage, plated at 0.2 × 106/well (round-bottom, 96-well plate), and stimulated with lipopolysaccharide (LPS, 1 μg/mL, Sigma, St Louis, MO). Supernatants for assessment of TNFα levels were harvested 5 hours later.
Histopathology
Formalin-preserved skin, liver, and distal small bowel was embedded in paraffin, and 5-μm thick sections were stained with hematoxylin and eosin for histologic examination. Slides were coded and examined in a blinded fashion by A.D.C., using a semiquantitative scoring system for abnormalities known to be associated with GVHD, as previously described.8,11,16,17 Scores were added to provide a total score of 24 for the skin, 28 for the small bowel, and 40 for the liver.
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank analysis. The Mann-Whitney U test was used for the statistical analysis of cytokine data and clinical scores. P values less than or equal to .05 were considered statistically significant.
Results
B-cell–deficient μMT mice have normal professional APC numbers and function
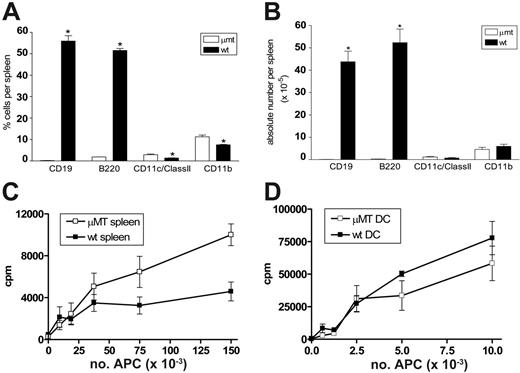
We first determined the APC composition and stimulatory function in μMT mice relative to wild-type control mice. As shown in Figure 1A-B, CD19 and B220-positive B cells are absent from the spleen of μMT mice. While the proportions of DCs (CD11c+/class II+) and monocytes (CD11b+) are increased 2-fold in μMT spleen, the absolute numbers are equivalent. In addition, the numbers of plasmacytoid DCs (CD11cdim/B220+) are also equivalent (data not shown). We next examined the allostimulatory capacity of whole spleen and sort-purified professional APCs (DCs) from μMT mice. As shown in Figure 1C, purified Balb/c (H-2d) T cells proliferated in an enhanced fashion to allogeneic APCs (H-2b) within irradiated spleen from μMT mice relative to those from wild-type mice. However, irradiated DCs from μMT and wild-type mice stimulated allogeneic T cells equally (Figure 1D). There was no significant difference in IFNγ or IL-4 production in these cultures (data not shown), suggesting that the presence or absence of B cells did not alter T-cell differentiation in vitro. Importantly, the presence of normal numbers and function of professional APCs in μMT mice suggested that these animals would be an informative means of studying the role of recipient B cells in GVHD.
B-cell–deficient μMT mice have normal professional APC numbers and function. Splenocytes were phenotyped from naive μMT and wild-type mice. (A) Proportions of APCs within spleen. (B) Absolute numbers of APCs within spleen. Results represent mean ± SE (n = 3, *P < .05). (C) Purified Balb/c T cells were cultured with allogeneic μMT and wild-type B6 irradiated splenocytes. Proliferation was measured via 3H-thymidine incorporation. Results represent 1 of 3 identical experiments. (D) Purified Balb/c T cells were cultured with allogeneic μMT and wild-type B6 CD11c+ DCs, and proliferation was determined by 3H-thymidine incorporation. Results represent mean ± SE of triplicate wells and 1 of 2 replicate experiments.
B-cell–deficient μMT mice have normal professional APC numbers and function. Splenocytes were phenotyped from naive μMT and wild-type mice. (A) Proportions of APCs within spleen. (B) Absolute numbers of APCs within spleen. Results represent mean ± SE (n = 3, *P < .05). (C) Purified Balb/c T cells were cultured with allogeneic μMT and wild-type B6 irradiated splenocytes. Proliferation was measured via 3H-thymidine incorporation. Results represent 1 of 3 identical experiments. (D) Purified Balb/c T cells were cultured with allogeneic μMT and wild-type B6 CD11c+ DCs, and proliferation was determined by 3H-thymidine incorporation. Results represent mean ± SE of triplicate wells and 1 of 2 replicate experiments.
Host B cells attenuate the severity of acute GVHD to MHC following allogeneic BMT
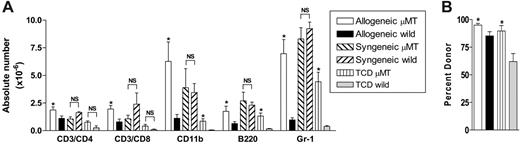
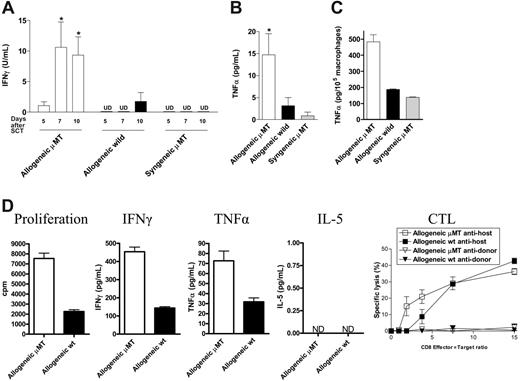
To study the specific effect of host B cells on acute GVHD, we transferred allogeneic Balb/c (H-2d) or syngeneic wild-type B6 (H-2b) TCD BM and splenic T cells into lethally irradiated (1000 cGy) μMT or wild-type mice (H-2b). As previously published,18 GVHD lethality in this model is entirely CD4 dependent. We chose this model because acute GVHD develops rapidly after transplantation, and B-cell–deficient mice have impaired development of Peyer patches,19 which are not required for the development of GVHD to MHC after myeloablative conditioning.20 Surprisingly, the absence of host B cells in allogeneic recipients resulted in a significant increase in early acute GVHD severity, with an increase in mortality and GVHD clinical scores early after BMT (Figure 2A-B). In contrast, only a small minority of μMT recipient mice receiving syngeneic grafts died after BMT, and long-term survivors did not develop features of GVHD. Furthermore, survival and clinical scores in wild-type syngeneic recipients was equivalent to these μMT recipients of syngeneic grafts (data not shown). Histopathologic examination of GVHD target organs 21 days after BMT confirmed increased GVHD in allogeneic B-cell–deficient recipients (Figure 2C). Interestingly, clinical GVHD after day 30 was not different in surviving allogeneic BMT recipients. Thus, the presence of recipient B cells appeared to be responsible only for the attenuation of early acute GVHD, in this allogeneic BMT model. To determine whether the increase in acute GVHD observed was due to effects of host B cells on donor engraftment and T-cell expansion, we next examined the splenic phenotype of animals that received transplants, 10 days after BMT. The absence of B cells in μMT recipients resulted in significantly increased expansion of allogeneic T cells, monocytes, B cells, and granulocytes (Figure 3A) and improved donor engraftment (Figure 3B). However, this effect was not seen in syngeneic recipients, suggesting that this is an allogeneic effect rather than increased donor cellular expansion due to enhanced homeostatic expansion in μMT recipients. Since acute GVHD is a consequence of both donor T-cell function and inflammatory cytokine generation, we next determined IFNγ and TNFα levels in the sera of animals after transplantation. As shown in Figure 4A-B, serum levels of IFNγ on days 5, 7, and 10, and TNFα on day 10 after BMT are significantly increased in μMT mice receiving allogeneic grafts. Furthermore, macrophages from μMT recipients receiving allogeneic grafts produced significantly more TNFα than those from wild-type recipients in response to LPS (Figure 4C), consistent with enhanced priming by IFNγ.21
Host B cells attenuate the severity of acute GVHD following allogeneic BMT. (A) Survival by Kaplan-Meier analysis. Bone marrow and purified splenic T cells from Balb/c (allogeneic) donors were transplanted into lethally irradiated μMT (n = 23) or wild-type (n = 25) recipient B6 mice. B6 (syngeneic) BM and T cells were transplanted into μMT (n = 8) mice as non-GVHD controls. **P < .01 μMT versus wild-type allogeneic recipients. Data combined from 4 experiments. (B) Clinical scores as described in “Assessment of GVHD” were determined as a measure of GVHD severity in surviving animals. ***P < .001 μMT versus wild-type allogeneic recipients. (C) Histopathology of GVHD target organs. Bone marrow and purified splenic T cells from Balb/c (allogeneic) donors was transplanted into irradiated μMT (n = 7) or wild-type (n = 6) recipient B6 mice. μMT recipients (n = 4) receiving syngeneic B6 BM were non-GVHD controls. Semiquantitative histopathology as described in “Histopathology.” Data expressed as mean ± SE. #P < .01 and *P = .05 μMT allo versus wild-type allo.
Host B cells attenuate the severity of acute GVHD following allogeneic BMT. (A) Survival by Kaplan-Meier analysis. Bone marrow and purified splenic T cells from Balb/c (allogeneic) donors were transplanted into lethally irradiated μMT (n = 23) or wild-type (n = 25) recipient B6 mice. B6 (syngeneic) BM and T cells were transplanted into μMT (n = 8) mice as non-GVHD controls. **P < .01 μMT versus wild-type allogeneic recipients. Data combined from 4 experiments. (B) Clinical scores as described in “Assessment of GVHD” were determined as a measure of GVHD severity in surviving animals. ***P < .001 μMT versus wild-type allogeneic recipients. (C) Histopathology of GVHD target organs. Bone marrow and purified splenic T cells from Balb/c (allogeneic) donors was transplanted into irradiated μMT (n = 7) or wild-type (n = 6) recipient B6 mice. μMT recipients (n = 4) receiving syngeneic B6 BM were non-GVHD controls. Semiquantitative histopathology as described in “Histopathology.” Data expressed as mean ± SE. #P < .01 and *P = .05 μMT allo versus wild-type allo.
In addition, 2 weeks after BMT, donor-derived splenic CD4+ T cells from μMT allogeneic recipients showed a higher proliferative response when stimulated in MLC with allogeneic DCs and produced significantly more IFNγ and TNFα (Figure 4D) compared to T cells from wild-type recipients. In contrast, the cytotoxic responses of CD8+ donor T cells were of the same magnitude in allogeneic μMT compared to wild-type recipients.
Host B cells inhibit donor cellular expansion and engraftment. Bone marrow and T cells from allogeneic Balb/c donors were transplanted into lethally irradiated μMT (n = 8) and wild-type (n = 8) recipient mice. Non-GVHD wild-type (n = 4) and μMT (n = 4) controls received BM and T cells from syngeneic or TCD allogeneic donors. Splenocytes were phenotyped on day 10 after transplantation. (A) Absolute numbers of each cell lineage. Results represent mean ± SE of individual animals. (B) Relative proportions of donor engraftment as described in “FACS and cytokine analysis.” Results represent mean ± SE of individual animals. *P < .05 versus respective wild-type groups. NS indicates not significant.
Host B cells inhibit donor cellular expansion and engraftment. Bone marrow and T cells from allogeneic Balb/c donors were transplanted into lethally irradiated μMT (n = 8) and wild-type (n = 8) recipient mice. Non-GVHD wild-type (n = 4) and μMT (n = 4) controls received BM and T cells from syngeneic or TCD allogeneic donors. Splenocytes were phenotyped on day 10 after transplantation. (A) Absolute numbers of each cell lineage. Results represent mean ± SE of individual animals. (B) Relative proportions of donor engraftment as described in “FACS and cytokine analysis.” Results represent mean ± SE of individual animals. *P < .05 versus respective wild-type groups. NS indicates not significant.
Host B cells inhibit T-cell proliferation following allogeneic transplantation
To determine the cellular mechanisms responsible for enhanced engraftment and expansion of donor T cells in the absence of host B cells, we next examined allogeneic donor T-cell proliferation and apoptosis during acute GVHD in μMT and wild-type recipients. Allogeneic Balb/c (H-2d) or syngeneic B6 (H-2b) purified CFSE-labeled T cells were transferred into lethally irradiated μMT or wild-type recipients (H-2b). At 4 hours and 120 hours after transplantation we examined surface expression of CD69, T-cell division by CFSE dilution, and apoptosis using annexin V and 7-AAD. Donor T-cell activation early after BMT in response to alloantigen, as measured by CD69 expression, was not augmented in the absence of host B cells (Figure 5A). However, by 120 hours after T-cell transfer, proliferation of allogeneic donor T cells was increased in μMT recipients (Figure 5B,C). Apoptosis (determined by the expression of annexin V in the absence of 7-AAD incorporation) was similar or increased in the absence of host B cells (Figure 5D,E), consistent with the enhanced state of activation of these cells as determined by proliferation and cytokine analysis. Thus, the increase in donor T-cell expansion in the absence of host B cells is due to an increased rate of proliferation following activation by alloantigen and not a consequence of reduced apoptosis.
Host B cells attenuate the production of inflammatory cytokines after allogeneic BMT. μMT allogeneic recipients (□, n = 8), wild-type allogeneic recipients (▪, n = 8), and μMT syngeneic recipients (▦, n = 4) mice received transplants as in Figure 3. (A) IFNγ levels were measured in sera on days 5, 7, and 10 after BMT, and (B) TNFα at day 10 as described in “FACS and cytokine analysis.” Results represent mean ± SE of individual animals. (C) Peritoneal macrophages were harvested from animals on day 10 after BMT and stimulated with LPS (1 μg/mL) as described in “Cell cultures.” TNFα was determined in culture supernatant by ELISA. Results are normalized to production per 105 macrophages based on CD11b staining. *P < .05 versus wild-type allogeneic recipients. (D) Donor H-2d+CD4+ and CD8+ cells were sorted 2 weeks after BMT from allogeneic μMT (□) or wild-type (▪) recipients. CD4+ cells were stimulated in MLC with allogenic H-2b+ CD11c+ DCs. Levels of IFNγ, TNFα, and IL-5 were determined in cell-culture supernatants as described in “FACS and cytokine analysis.” Cytotoxic T lymphocyte (CTL) activity of donor CD8+ T cells was tested in a standard 51Cr release assay as described in “Cell cultures.” Results are expressed as mean ± SE of triplicate wells. ND indicates not detected.
Host B cells attenuate the production of inflammatory cytokines after allogeneic BMT. μMT allogeneic recipients (□, n = 8), wild-type allogeneic recipients (▪, n = 8), and μMT syngeneic recipients (▦, n = 4) mice received transplants as in Figure 3. (A) IFNγ levels were measured in sera on days 5, 7, and 10 after BMT, and (B) TNFα at day 10 as described in “FACS and cytokine analysis.” Results represent mean ± SE of individual animals. (C) Peritoneal macrophages were harvested from animals on day 10 after BMT and stimulated with LPS (1 μg/mL) as described in “Cell cultures.” TNFα was determined in culture supernatant by ELISA. Results are normalized to production per 105 macrophages based on CD11b staining. *P < .05 versus wild-type allogeneic recipients. (D) Donor H-2d+CD4+ and CD8+ cells were sorted 2 weeks after BMT from allogeneic μMT (□) or wild-type (▪) recipients. CD4+ cells were stimulated in MLC with allogenic H-2b+ CD11c+ DCs. Levels of IFNγ, TNFα, and IL-5 were determined in cell-culture supernatants as described in “FACS and cytokine analysis.” Cytotoxic T lymphocyte (CTL) activity of donor CD8+ T cells was tested in a standard 51Cr release assay as described in “Cell cultures.” Results are expressed as mean ± SE of triplicate wells. ND indicates not detected.
Total body irradiation rapidly depletes host APCs and induces production of IL-10 from host B cells
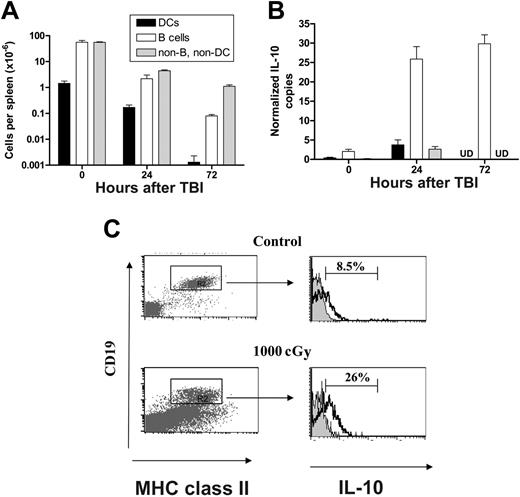
Since BMT was undertaken in this system the day after TBI, we examined the numbers of APC remaining at the time of transplantation. Splenic B cells and DCs were reduced 28-fold and 8-fold, respectively, 24 hours after TBI, while remaining non-B, non-DCs were depleted 12-fold (Figure 6A). Thus, there was already a significant depletion of host B cells by the time of BMT, suggesting that the previously described effect of B cells on GVHD may reflect the result of radiation on B cells rather than an effect of B cells on GVHD per se. Radiation is known to be immunosuppressive, and although this is related primarily to its cytotoxic effect on the immune system, it is also known to induce the transcription of IL-10 in lymphoid tissue.22 We therefore characterized IL-10 transcription in splenic cellular populations at various time points after TBI. As shown in Figure 6B, TBI induced a sustained 10-fold increase in IL-10 mRNA levels, which was exclusively within the B-cell fraction of the spleen rather than within any other APC compartments. The effect was extremely rapid, and increased levels of IL-10 transcript were readily detectable in B cells within 4 hours of TBI (data not shown). In addition, there was a 3-fold increase in the proportion of IL-10 producing splenic B cells 24 hours after exposure to TBI relative to nonirradiated control mice (Figure 6C), confirming an effect on protein production. Interestingly, preparative regimens with fludarabine (2 mg/dose, day –3 to –1) and cyclophosphamide (2 mg/dose, day –2 to –1) did not induce changes in IL-10 mRNA levels within splenic B cells, DCs, and residual non-B, non-DC compartments compared to untreated control animals (data not shown).
Host B cells inhibit donor T-cell proliferation. Purified CFSE-labeled Balb/c T cells were transplanted into lethally irradiated μMT (n = 4) or wild-type (n = 4) recipient mice. (A) Four hours after BMT, donor (H-2d+) T cells were recovered from the spleens of wild-type (solid line) or μMT recipients (dotted line) and the expression of the activation marker CD69 determined relative to isotype control (solid histogram). (B) At 4 and 120 hours after BMT, proliferation of donor T cells (H-2d+) was determined by reduction of CFSE intensity. (C) ModFit analysis of proliferation index in μMT (□, n = 5) and wild-type (▪, n = 5) allogeneic recipients. Results represent mean ± SE of individual animals *P < .05 versus wild-type. (D) Donor cells (H-2d+) were stained for annexin V and 7-AAD and proportions of annexin V+/7-AAD– apoptotic cells determined as described in “FACS and cytokine analysis.” (E) Absolute numbers of apoptotic cells per spleen in μMT (□, n = 5) and wild-type (▪, n = 5) allogeneic recipients. Results represent mean ± SE of individual animals.
Host B cells inhibit donor T-cell proliferation. Purified CFSE-labeled Balb/c T cells were transplanted into lethally irradiated μMT (n = 4) or wild-type (n = 4) recipient mice. (A) Four hours after BMT, donor (H-2d+) T cells were recovered from the spleens of wild-type (solid line) or μMT recipients (dotted line) and the expression of the activation marker CD69 determined relative to isotype control (solid histogram). (B) At 4 and 120 hours after BMT, proliferation of donor T cells (H-2d+) was determined by reduction of CFSE intensity. (C) ModFit analysis of proliferation index in μMT (□, n = 5) and wild-type (▪, n = 5) allogeneic recipients. Results represent mean ± SE of individual animals *P < .05 versus wild-type. (D) Donor cells (H-2d+) were stained for annexin V and 7-AAD and proportions of annexin V+/7-AAD– apoptotic cells determined as described in “FACS and cytokine analysis.” (E) Absolute numbers of apoptotic cells per spleen in μMT (□, n = 5) and wild-type (▪, n = 5) allogeneic recipients. Results represent mean ± SE of individual animals.
TBI rapidly depletes host APCs and induces IL-10 transcription in host B cells. (A) Absolute numbers of splenic DCs, B cells, and non-B, non-DC cells prior to and at 24 hours and 72 hours after TBI. Results are expressed as mean ± SE of 3 to 5 individual animals. (B) IL-10 copy number (normalized to B2M) in DCs, B cells, and non-B, non-DC populations prior to and at 24 and 72 hours after TBI. Results are expressed as mean ± SE of replicate samples from 3 experiments in which B cells (CD19+/class II+), DCs (CD11c+/class II+), or non-B, non-DC (CD19–/class II–) were FACS sorted from the spleen of 2 to 5 animals at various times after TBI. UD indicates undetected. (C) Intracellular staining of IL-10. Splenocytes from control (top panels) or irradiated wild-type B6 mice, 24 hours after TBI with 1000 cGy (bottom panels) were stained as described in “FACS and cytokine analysis.” Proportion of IL-10–positive B cells was determined in MHC class II (I-A/I-E)+/CD19+ population. Representative plots from 1 of 2 identical experiments shown.
TBI rapidly depletes host APCs and induces IL-10 transcription in host B cells. (A) Absolute numbers of splenic DCs, B cells, and non-B, non-DC cells prior to and at 24 hours and 72 hours after TBI. Results are expressed as mean ± SE of 3 to 5 individual animals. (B) IL-10 copy number (normalized to B2M) in DCs, B cells, and non-B, non-DC populations prior to and at 24 and 72 hours after TBI. Results are expressed as mean ± SE of replicate samples from 3 experiments in which B cells (CD19+/class II+), DCs (CD11c+/class II+), or non-B, non-DC (CD19–/class II–) were FACS sorted from the spleen of 2 to 5 animals at various times after TBI. UD indicates undetected. (C) Intracellular staining of IL-10. Splenocytes from control (top panels) or irradiated wild-type B6 mice, 24 hours after TBI with 1000 cGy (bottom panels) were stained as described in “FACS and cytokine analysis.” Proportion of IL-10–positive B cells was determined in MHC class II (I-A/I-E)+/CD19+ population. Representative plots from 1 of 2 identical experiments shown.
Recipient-derived IL-10 attenuates allogeneic donor T-cell proliferation, expansion, and subsequent GVHD
To confirm that host-derived IL-10 was indeed capable of modulating donor T-cell responses, we next examined the effect of recipient-derived IL-10 on donor T-cell function by transplanting allogeneic CFSE-labeled donor T cells into irradiated wild-type or IL-10–deficient (IL-10–/–) B6 animals and determining their subsequent proliferation and expansion. As seen in Figure 7A-B, viable donor (7-AAD–/H-2d+) T cells underwent greater proliferation and expansion in allogeneic recipients that were deficient in IL-10 than in respective wild-type recipients. Furthermore, the absence of recipient-derived IL-10 was associated with increased acute GVHD mortality and morbidity following BMT (Figure 7C-D), confirming that the generation of this cytokine from BZMT recipients was indeed capable of modulating acute GVHD.
Reconstitution of B cells prior to BMT improves survival of genetically B-cell–deficient hosts
Our results suggest that the absence of host B cells and IL-10 at the time of TBI-based conditioning exacerbates severe acute GVHD to MHC. The transfer of B cells back into μMT mice is not feasible due to very poor and only transient reconstitution, at least in part, because they are actively rejected.23 To confirm that increased mortality from acute GVHD observed in genetically B-cell–deficient μMT mice is related to the absence of IL-10 generation from B cells, we created mixed bone marrow chimeras in which μMT hemopoiesis coexists with either wild-type or IL-10–/– B cells as described in “Materials and methods.” As previously described, in the mixed (wild-type and IL-10–/–) chimeras only a small fraction (20%) of non–B cells are IL-10–/–.9 As shown in Table 1, the use of these chimeras as BMT recipients confirmed that the reconstitution of μMT hemopoiesis with B cells prior to BMT significantly improved survival (65% vs 29%, P < .05). However, protection from GVHD induced by wild-type host B cells was lost when IL-10–deficient B cells were reconstituted (65% vs 33%, P < .05).
Effect of B-cell-derived IL-10 on GVHD
Graft . | TCD Allo . | Allo . | Allo . | Allo . |
|---|---|---|---|---|
| Recipient chimera | μMT → wt | μMT → wt | μMT + wt → wt | μMT + IL-10-/- → wt |
| μMT/wt or IL-10-/- BM ratio | 1:0 | 1:0 | 4:1 | 4:1 |
| Recipient B cell no. | 0.2 ± 0.1 | 0.2 ± 0.1 | 32 ± 8 | 21 ± 3 |
| B-cell IL-10 | NA | NA | 929 ± 83 | <30 |
| Survival, % | 90 | 29 | 65* | 33 |
Graft . | TCD Allo . | Allo . | Allo . | Allo . |
|---|---|---|---|---|
| Recipient chimera | μMT → wt | μMT → wt | μMT + wt → wt | μMT + IL-10-/- → wt |
| μMT/wt or IL-10-/- BM ratio | 1:0 | 1:0 | 4:1 | 4:1 |
| Recipient B cell no. | 0.2 ± 0.1 | 0.2 ± 0.1 | 32 ± 8 | 21 ± 3 |
| B-cell IL-10 | NA | NA | 929 ± 83 | <30 |
| Survival, % | 90 | 29 | 65* | 33 |
Wild-type (wt) CD45.1+ B6 mice were transplanted with CD45.2+ μMT or a combination of wild-type and μMT or IL-10-/- and μMT T-cell-depleted (TCD) BM in the ratios described and left for 4 months to reconstitute. At this time B-cell numbers were quantitated within the spleen (expressed as mean ± SE per spleen [×106]), and FACS-sorted B cells were stimulated in culture for 48 hours with LPS (10 μg/mL) and IL-10 determined in culture supernatant (pg/mL). Chimeras were subsequently transplanted with allogeneic TCD BM without (TCD Allo, n = 10) or with splenic T cells (Allo, n = 18-20 per group in 2 experiments) from Balb/c donors and survival determined at day 45 by Kaplan-Meier estimates.
NA indicates not applicable.
P < .05 versus μMT → wild type and μMT + IL-10-/- → wild type.
Recipient-derived IL-10 attenuates the severity of acute GVHD following allogeneic BMT. (A) Purified CFSE-labeled Balb/c T cells were transplanted into lethally irradiated wild-type and IL-10–/– recipient mice. Five days later, proliferation of splenic donor T cells (H-2d+) was determined by CFSE intensity, and representative examples are shown. (B) ModFit analysis of proliferation index (via CFSE intensity) and donor T-cell expansion in IL-10–/– (□, n = 8) and wild-type (▪, n = 8) allogeneic recipients. Results are mean ± SE of individual animals from 2 replicate experiments. *P < .05 and **P < .01 versus wild-type. (C) Survival by Kaplan-Meier analysis. Bone marrow and purified splenic T cells from Balb/c (allogeneic) donors were transplanted into lethally (900 cGy) irradiated IL-10–/– (n = 12) or wild-type (n = 12) recipient B6 mice. B6 (syngeneic) bone marrow was transplanted into IL-10–/– B6 mice (n = 9) as non-GVHD controls. **P < .01, IL-10–/– versus wild-type allogeneic recipients. Data combined from 2 experiments. (D) Clinical scores as described in “Assessment of GVHD.” **P < .01 and *P < .05, IL-10–/– versus wild-type allogeneic recipients.
Recipient-derived IL-10 attenuates the severity of acute GVHD following allogeneic BMT. (A) Purified CFSE-labeled Balb/c T cells were transplanted into lethally irradiated wild-type and IL-10–/– recipient mice. Five days later, proliferation of splenic donor T cells (H-2d+) was determined by CFSE intensity, and representative examples are shown. (B) ModFit analysis of proliferation index (via CFSE intensity) and donor T-cell expansion in IL-10–/– (□, n = 8) and wild-type (▪, n = 8) allogeneic recipients. Results are mean ± SE of individual animals from 2 replicate experiments. *P < .05 and **P < .01 versus wild-type. (C) Survival by Kaplan-Meier analysis. Bone marrow and purified splenic T cells from Balb/c (allogeneic) donors were transplanted into lethally (900 cGy) irradiated IL-10–/– (n = 12) or wild-type (n = 12) recipient B6 mice. B6 (syngeneic) bone marrow was transplanted into IL-10–/– B6 mice (n = 9) as non-GVHD controls. **P < .01, IL-10–/– versus wild-type allogeneic recipients. Data combined from 2 experiments. (D) Clinical scores as described in “Assessment of GVHD.” **P < .01 and *P < .05, IL-10–/– versus wild-type allogeneic recipients.
Discussion
We have demonstrated that host B cells transiently attenuate CD4+-dependent acute GVHD directed against major histocompatibility antigens. This protection from acute GVHD occurred in association with quantitative reductions in the expansion, proliferation, and IFNγ generation by donor cells following BMT. The diminished GVHD was associated with IL-10 production from host B cells in response to TBI, and recipient-derived IL-10 attenuated the severity of acute GVHD. Thus, the anti-inflammatory response of host B cells to conditioning is one mechanism regulating the severity of acute GVHD to MHC after allogeneic BMT.
The role of APCs in GVHD pathophysiology is becoming increasingly clear. Using a CD8+-dependent model of GVHD and recipient chimeric mice in which host APCs were class I deficient, Shlomchik and colleagues1 confirmed the absolute requirement for host APCs in the induction of GVHD. Although donor APCs are not required for the initiation of GVHD, cross-priming of alloreactive CD8+ T cells augments GVHD in the same model.24 The importance of specific host APC subsets in the induction of acute GVHD has not been definitively studied, but 2 recent studies suggest that DCs are likely to be critical cell populations. In the study of Duffner et al,3 host-type DCs were the only APCs required for the induction of GVHD, and host B cells were unable to induce GVHD in isolation. Importantly, however, this study did not assess the effect of conditioning on APCs and the subsequent ability of DCs and B cells to modulate GVHD. In contrast, TBI has been shown to activate DCs and macrophages such that their ability to induce alloreactive T-cell responses and inflammatory cytokines is greatly increased.16,25 Our study confirms that within 3 days of lethal TBI, there is a 3-log depletion of host splenic APCs. However, the ratio of B cells to DCs increases after TBI, and those B cells that remain produce IL-10 in response to conditioning. Thus, with increasing time after TBI, the lymphoid environment in which donor T cells become activated and expand becomes increasingly characterized by IL-10 derived from residual B cells. This B-cell–dependent generation of anti-inflammatory cytokines therefore appears as a natural counterbalance to the early production of proinflammatory cytokines induced by radiation in cells of the monocyte-macrophage lineage.16
B cells are known to play an important role in the limitation of type 1 T-cell autoreactivity by producing IL-10 in response to CD40 ligation9 and are required to produce IL-10 to attenuate Th1 responses driven by IL-12 derived from DCs.26 Furthermore, recently it has become clear that B cells can assume regulatory function by producing IL-10 and TGFβ in otherwise proinflammatory states (reviewed in Mizoguchi and Bhan27 ). Only a few studies to date have investigated the role of recipient B cells in GVHD. One report confirmed that B cells were important in priming responses to minor histocompatibility antigens (HAs), but GVHD studies involved depletion of both donor and host B cells with ongoing antibody administration, and definite effects on GVHD were not demonstrable.28 In addition, the effects of B-cell depletion with exogenous anti-μ antibody itself (eg, on cytokine induction) were not studied. A second more recent study in a model of chronic GVHD directed to minor HA did not demonstrate changes in GVHD when B-cell–deficient recipients were used with a different background and genetic mutation to that in the recipients used in this study.29,30 Interestingly, that study did not reveal effects of recipient γδ T cells on GVHD that were evident in a second report in the same model of GVHD to MHC as was used in our studies.31 Acute GVHD is a Th1-dominant disease and in MHC disparate models, donor B cells are largely absent.32 In contrast, pathogenic autoreactive B cells are expanded during chronic GVHD,33,34 and the depletion of B cells by administration of monoclonal antibodies against CD20 is increasingly reported as successful therapy for resistant chronic GVHD.35,36 Thus, in contrast to acute GVHD, donor but not recipient B cells appear to be important in the pathophysiology of chronic GVHD. Since B-cell–depleting antibodies are being added to conditioning regimens in the setting of allogeneic BMT for the treatment of indolent B-cell lymphomas, the role of both normal and malignant recipient B cells (and their response to depletion) in directing GVHD outcome becomes increasingly important.
The role of IL-10 in modulating GVHD has been extensively studied and, when exogenously administered to BMT recipients, has protective and detrimental effects on GVHD at low and high doses, respectively.37 During GVHD, the generation of IL-10 from regulatory T cells appears to be the predominant immunomodulatory source of this cytokine.38,39 Recently, it has become clear that the immunomodulatory effect of IL-10 is critically dependent on local production within the lymphoid microenvironment. Thus, the modulation of GVHD by IL-10 from regulatory T cells requires these cells to appropriately enter the lymph node and is thus dependent on the expression of CD62L.40,41 Our data suggest that although B cells are largely depleted by TBI, residual IL-10–producing B cells in fact eventually dominate the lymphoid APC environment and also exert regulatory effects on subsequent alloreactive immune responses. Importantly, recipient gene polymorphisms associated with high IL-10 production are known to be associated with protection from GVHD,42 and it has been hypothesized that host APCs are an important source of this cytokine.43 While our experiments using mixed (wild-type and IL-10–/–) chimeras as BMT recipients (Table 1) do not exclude the possibility that the minority of non–B-cell IL-10–/– hemopoiesis (20%) may contribute to the increase in GVHD seen in these recipients, this seems unlikely as sustained increases in IL-10 mRNA levels were not detected in other recipient spleen cells. In contrast, IL-10 from nonhemopoietic recipient tissue may well be an important modulator of GVHD, and our studies have not addressed this issue. At the present time, the data suggest that recipient B cells and perhaps regulatory T cells29 are major producers of recipient-derived IL-10, which acts to attenuate the severity of GVHD putatively induced by professional host APCs. It is important to stress, however, that transient protective effects of B cells are dependent on TBI, and it will be of interest to now study the effect of B-cell–depleting antibodies on the induction of IL-10 in vivo.
The data presented here suggest that the response of host B cells to conditioning plays an important role in modulating GVHD to MHC after allogeneic BMT. This principle is in line with the known ability of apoptotic lymphocytes to exert IL-10–dependent immunosuppression.44 Since these studies invoke CD4-dependent acute GVHD to MHC, it is important to consider that effects may differ when CD8-dependent immune responses are directed to minor HA or result in chronic GVHD, since inflammatory cytokines are less dominant in mediating pathology in this setting.45 Thus, in appropriate circumstances, the manipulation of host B cells and their responses to conditioning may have important effects on GVHD that can be exploited to improve the outcome of allogeneic BMT.
Prepublished online as Blood First Edition Paper, June 20, 2006; DOI 10.1182/blood-2006-04-016063.
Supported in part by grants from the National Health and Medical Research Council (NHMRC) and Queensland Cancer Fund. G.R.H. is a Wellcome Trust Senior Overseas Research Fellow.
V.R. guided experimental procedures, designed and wrote the manuscript; T.B. guided experimental procedures, and designed and wrote the manuscript; K.P.M. helped with experimental design; R.K. undertook experimental transplant procedures; A.L.D. undertook all real-time PCR; E.S.M. helped with experimental design and undertook CTL assays; A.C.B. guided experimental procedures with IL-10 knockout animals; H.M.B. undertook experimental transplant procedures; A.D.C. performed all histology analyses; and G.R.H. guided experimental design and helped with manuscript preparation.
V.R. and T.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal