Comment on Martínez et al, page 3012
In this issue of Blood, Martínez and colleagues report that Hedgehog (Hh) membrane–anchored morphogens that are associated with microvesicles (MVs) shed from activated T lymphocytes induce megakaryocytic differentiation of K562 cells, as well as stimulating megakaryocytic development from primary human CD34+ progenitors. Thus, the authors provide further evidence that MV-related mechanisms operate in normal hematopoiesis.
Generally, cells communicate by secreted growth factors, cytokines, chemokines, small molecular mediators, and cell-to-cell adhesion contacts. However, attention is now being focused on circular membrane fragments called microvesicles (MVs), which for many years have been largely overlooked. This MV-mediated cell-cell communication system probably emerged very early during evolution and was a kind of template for the development of cell-cell interaction mechanisms involving soluble bioactive mediators and finetuned ligand-receptor interactions.1-3
MVs are shed from the surface membranes of normal and malignant cells, as well as secreted from the endosomal compartment as circular membrane fragments. MVs contain numerous proteins and lipids similar to those present in the membranes of the cells from which MVs originate. Furthermore, since they engulf some cytoplasm during membrane blebbing, they may also contain proteins and mRNA derived from it. Moreover, MVs may “hijack” infectious particles (eg, HIV or prions) from the cytoplasm or possibly even whole intact organelles (eg, mitochondria).
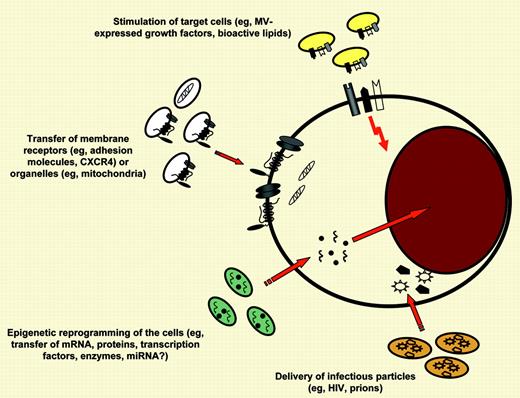
Shedding of membrane-derived MVs is a physiological phenomenon that accompanies cell activation and growth, and the number of MVs shed from cells increases upon (1) cell activation, (2) hypoxia or irradiation, (3) oxidative injury, (4) shearing stress, and (5) exposure to proteins from an activated complement cascade.2 Growing experimental evidence indicates that the enrichment of MVs in various bioactive molecules plays an important pleiotropic role in many biologic processes, including cancerogenesis, coagulation, immune responses, and modulation of susceptibility/infectability of cells to retroviruses or prions (see figure).2-5 FIG1
Different mechanisms by which MVs may interact with target cells. MVs may (1) stimulate target cells directly by surface-expressed ligands acting as a kind of “signaling complex”; (2) transfer surface receptors from one cell to another; (3) deliver proteins, mRNA, bioactive lipids, and even whole organelles (eg, mitochondria) into target cells; and, finally, (4) serve as a vehicle (“Trojan horse” mechanism) to transfer infectious particles between cells (eg, HIV or prions). In this issue of Blood, Martínez and colleagues describe that MVs derived from T lymphocytes express Hh morphogens that may induce megakaryopoietic differentiation in hematopoietic progenitors.
Different mechanisms by which MVs may interact with target cells. MVs may (1) stimulate target cells directly by surface-expressed ligands acting as a kind of “signaling complex”; (2) transfer surface receptors from one cell to another; (3) deliver proteins, mRNA, bioactive lipids, and even whole organelles (eg, mitochondria) into target cells; and, finally, (4) serve as a vehicle (“Trojan horse” mechanism) to transfer infectious particles between cells (eg, HIV or prions). In this issue of Blood, Martínez and colleagues describe that MVs derived from T lymphocytes express Hh morphogens that may induce megakaryopoietic differentiation in hematopoietic progenitors.
MVs are released by various cell types and differ in composition depending on their cell of origin and status. Their level is elevated in the peripheral blood (PB) of patients suffering from infection, cancer, or cardiovascular disorders. The number of MVs circulating in PB increases during injury, inflammation, thrombosis, and cell activation.
In this issue of Blood, Martínez and colleagues describe that MVs derived from T lymphocytes (1) circulate in peripheral blood, (2) express Hh morphogens on the surface, and (3) stimulate megakaryopoiesis. Thus, a novel MV-related mechanism for transferring/spreading morphogens has been described, similar to that observed during early embryogenesis.3 Furthermore, the Hh signaling pathway had been proposed to regulate Meg differentiation. Interestingly, the number of Hh+ MVs increases in diabetic patients, which triggers thrombopoiesis and may contribute to thrombocytic complications. Further work, however, requires the identification of other biologic compounds that are expressed by T-cell–derived MVs and their additional potential targets (eg, perhaps the endothelium).
Since all cells present in the hematopoietic microenvironment secrete MVs, the MV-related network modulates hematopoietic development. Thus, MVs should no longer be considered cell debris or biologically irrelevant cell dust. Augmenting evidence demonstrates that they are important mediators of intercellular communication and underappreciated components of the hematopoietic niche. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal