Abstract
Neutrophils play a crucial early role during the innate response, but little is known about their possible contribution when an adaptive immune response is installed. A robust neutrophilia and a T helper 1 (Th1) immune response are present after immunization with Complete Freund Adjuvant (CFA). We show that when FITC-labeled OVA was injected into the footpad of OVA/CFA immunized mice, the main OVA-FITC+ cells recruited in draining popliteal lymph nodes (LNs) were neutrophils, with most of them arriving at the LN by means of lymphatic vessels. The development of this OVA-FITC+ neutrophil influx requires an immune response against OVA. The OVA-FITC+ neutrophils present in LNs displayed mainly intracellular TNF-α, and their depletion resulted in an increase in the specific IL-5 levels. These data provide new evidence about the role played by neutrophils in vivo in adaptive immunity.
Introduction
It is generally known that neutrophils (NEs) play a key role in innate immune defense. They are present in the bloodstream and help in recognizing and neutralizing microorganisms. By being able to adhere to the blood vessel walls at sites of tissue damage, they transmigrate through endothelial cells to the infection site, where they are stimulated to phagocytose and kill microorganisms. This accumulation of leukocytes forms the first step in immune surveillance and plays a key role in innate immunity to infection.1 However, some reports have described the involvement of NEs in directing T-cell polarization to the type of pathogens occurring during an infection process.2-5 To date, it is still unclear whether this is a common phenomenon in other situations, such as in models with inflammation but without infection.
In previous studies, we have observed differences in the behavior of the immune system, in terms of intensity and quality of humoral and cellular responses, depending on the adjuvant used.6-8 In the case of Complete Freund Adjuvant (CFA), a T helper 1 (Th1) immune response was induced, and studies have reported a high hemopoiesis in the spleen, along with an expansion of myeloid CD11b+ cells and an important neutrophilia when CFA was used as the adjuvant.6,7,9 However, this scenario was not present when synthetic oligodeoxynucleotides containing CpG motifs and Bordetella pertussis were used as adjuvants.7 Therefore, taking into account that there was an increase in the number of neutrophils in the blood, we decided to explore the possible contribution of NEs in this model of immune inflammation when an immune response is taking place. With this objective, we injected the antigen into the footpad of previously immunized animals and analyzed the cells involved in antigen capture in the skin and lymphoid organs. Interestingly, we found that not only did NEs uptake antigen in skin, but they also comprised the main population of cells bearing the antigen in lymphoid organs. The influx of NEs was present only if there was a specific immune response occurring, in which case they drained popliteal lymph nodes (LNs) through the lymphatic vessels, and remained there for at least 48 hours, before undergoing apoptosis. Moreover, NEs in lymph nodes mainly expressed TNF-α and contributed to the quality of the established secondary immune response. Our results emphasize the involvement of NEs as cells not only involved in innate immune responses but also in adaptive ones.
Materials and methods
Mice
Female 8-to 12-week-old BALB/c mice were used. Mice were originally obtained from the Comisión Nacional de Energía Atómica (CNEA), Argentina. The Institutional Experimentation Animal Committee (authorization no. 15-01-44195) approved animal handling and experimental procedures.
Immunization
Mice were subcutaneously immunized on the base of the tail and in the neck region on days 0, 15, and 30 with 0.5 mL albumin, chicken egg (OVA), Keyhole Limpet Hemocyanin (KLH), or phosphate-buffered saline (PBS) emulsified in CFA (60 μg OVA or KLH/per animal/per dose) (OVA/CFA, KLH/CFA, or PBS/CFA). OVA and CFA were both from Sigma-Aldrich Argentina SA (Buenos Aires, Argentina). KLH was obtained from Pierce Biotechnology (Rockford, IL). Mice were injected in the hind footpad with 0.1 mg OVA labeled with FITC (OVA-FITC; mol wt 45 000; Molecular Probes, Eugene, OR), KLH was conjugated with FITC following a previously described protocol,10 PBS, or immune complexes.
Immune complex formation
To produce OVA–anti-OVA antibody complex, 50 μL OVA-FITC (0.75 mg/mL) was preincubated for 30 minutes at 37°C with 100 μL plasma from OVA/CFA mice (diluted 1:2 in sterile PBS). To eliminate free antigen, we used Microcon centrifugal filters (Millipore, Billerica, MA).
Cell preparation
Samples of blood were collected by periorbital bleeding. Lymph nodes and spleens were collected in RPMI 1640 medium supplemented with 2% heat-inactivated FCS (Natocor, Córdoba, Argentina). Erythrocytes from spleen and peripheral blood were removed by red blood cell (RBC)–lysing buffer (Sigma-Aldrich). A single-cell suspension of lymph nodes and spleen was prepared by gently pressing the organs through a stainless-steel mesh. In all cases, cell viability was greater than 95%, as determined by Trypan Blue dye exclusion. In some experiments, LNs were treated for 45 minutes at 37°C with 400 U/mL collagenase D and 50 μg/mL DNase I (Roche Diagnostics GmbH, Mannheim, Germany) in RPMI 1640 medium. After inhibition of collagenase activity with 6 mM EDTA in PBS, cell suspension of lymph nodes was obtained as indicated above.
Cell cultures
Cell suspensions were cultured in RPMI 1640 medium supplemented with 10% FCS, 2 mM L-glutamine, 50 μM 2-ME (Sigma-Aldrich), and gentamicin (40 μg/mL; Schering-Plough S.A., Lomas del Mirador, Argentina) at 37°C in a 5% CO2 humidified incubator. Cell suspensions were cultured with medium alone or with OVA (100 μg/mL).
Antibody assays
Specific antibodies against OVA were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well flat-bottom plates were coated and incubated overnight at 4°C with OVA (1 μg/well) in 0.1 M sodium carbonate-bicarbonate buffer (pH 9.6). Plates were then blocked with PBS containing 0.5% gelatin for 1 hour at 37°C. After washing, the plates were incubated for 1 hour at 37°C with plasma samples diluted in PBS with 0.05% Tween containing 0.5% gelatin. For IgG detection, plates were incubated with HRP-conjugate anti–mouse IgG (Sigma-Aldrich). Plates were examined on a microplate (model 450; Bio-Rad, Hercules, CA) at 490 nm after incubation with H2O2 and o-phenylendiamine.
Cytokine-specific ELISA
Levels of IFN-γ and IL-5 were measured in culture supernatants by capture ELISA following instructions from the manufacturer (BD Pharmingen, San Diego, CA). The following antibodies were used for coating and detection, respectively: R4-6A2 and XMG1.2 clones for anti–IFN-γ, TRFK5 and TRFK4 clones for anti–IL-5. Values of IFN-γ and IL-5 were calculated by reference to a standard curve constructed by assaying serial dilutions of the respective murine standard cytokines.
Flow cytometry
The cells were first incubated with anti-CD32/CD16 (clone 2.4G2) for 30 minutes. Then, the cells were stained on the surface for 30 minutes with the corresponding phycoerythrin- or biotin-conjugated antibodies. When biotin-conjugated antibody was used, a third step involving a 30-minute incubation was performed with streptavidin-Cy-chrome. Surface staining was performed in Hanks balanced salt solution with 0.1% albumin bovine, 0.1% sodium azide, and 0.005 mM Na3EDTA at 4°C. Cells were analyzed on a Cytoron Absolute cytometer (Ortho Diagnostic Systems, Raritan, NJ), and the data were processed using WinMDI 2.8 software (http//facs.scripps.edu. Copyright by Joseph Trotter).
To detect intracellular cytokines, cells (1.5 × 106/mL) were incubated in RPMI medium 10% FCS containing 4.8 μM monensin (Sigma-Aldrich) for 3 to 5 hours at 37°C in a moist atmosphere of 5% CO2 in air. After this time period, cells were washed and stained with anti–Gr-1 antibody as described above. Cells were then fixed with 4% paraformaldehyde for 20 minutes at 4°C and permeabilized with Perm/Wash solution (BD Pharmingen) for 30 minutes at room temperature. Intracellular cytokines were detected by the addition of a specific antibody diluted in Perm/Wash solution for 60 minutes at 4°C. After final washes with Perm/Wash solution, cells were analyzed by flow cytometry.
Antibodies against murine CD3 (145-2C11), CD19 (1D3), CD11b (M1/70), Gr-1 (Ly-6G and Ly6-C) (RB6-8C5), I-Ad/I-Ed (2G9), CD40 (HM40-3), CD80 (16-10A-1), CD86 (GL1), CD11c (HL3), B220 (RA3-6B2), Ly-6G (1A8), TNF-α (MP6-XT22), IL-4 (11B11), IL-12 (C15.6), IFN-γ (XMG1.2), matching isotype controls and streptavidin-Cy-chrome were purchased from BD Pharmingen (San Diego, CA). Antibody anti-F4/80 (A3-1) was purchased from Caltag (Burlingame, CA). Isotype controls were obtained from BD Pharmingen or eBioscience (San Diego, CA).
Apoptosis assay
For apoptotic-cell detection propidium iodide staining was performed to analyze the hypodiploid DNA peak as described previously by Nicoletti et al.11 Briefly, 1 × 106 cells were fixed overnight in 500 μL 70% ethanol at 4°C. Cell pellets were gently resuspended in 500 μL hypotonic fluorochrome solution (50 μg/mL propidium iodide in 0.1% sodium citrate plus 0.3% nonidet P-40) and kept at 4°C for 18 hours. After that, cells were analyzed as indicated under “Flow cytometry.”
Morphologic analysis of cells by cytospin
Cell suspensions diluted in 10% FCS RPMI 1640 medium were centrifuged on microscope slides in a Cytospin apparatus (Shandon, Cheshire, United Kingdom) and stained with DAPI (0.1 μg/mL; Molecular Probes) or May-Grünwald-Giemsa (Merck, Darmstadt, Germany).
Fluorescent microscopy
LNs and skin (footpad) were inserted into OCT-embedding compound (Miles, Elkhart, IN) and snap-frozen in liquid air. Cryostat sections (8 μm thick) of tissues were sliced, air-dried, and fixed in citrate-acetoneformaldehyde at room temperature for 1 minute. Next, samples were rehydrated in PBS and mounted on glycerine. These sections and cytospins slides were observed using a Nikon Eclipse TE 2000-U (Nikon, Melville, NY) system equipped with a Nikon Plan Fluor 20×/0.45 NA objective lens, a Nikon Plan Fluor EL WD 40×/0.60 NA objective lens, a Nikon Plan Apo VC 60×/1.4 NA oil objective lens, a Nikon Plan Apo VC 100×/1.4 NA oil objective lens, a Nikon DS-5M digital camera head, and Nikon ACT-2U software. In some cases, slides were examined with a Carl Zeiss LSM 5 Pascal laser scanning confocal microscope (Carl Zeiss, Jena, Germany) equipped with an argon/helium/neon laser, a C-apochromat 40×/1.2 NA objective lens, and Zeiss LS software. Images were then processed with Adobe Photoshop (Adobe, San Jose, CA).
Neutrophil depletion
To deplete NEs, an anti–mouse Gr-1 antibody (RB6-8C5) was used. Mice received 0.5 mg/mouse anti–Gr-1 antibody or control rat IgG intraperitoneally on days 37, 38, 39, 40, and 41 after first immunization. This treatment resulted in greater than 98% depletion of NEs (Gr-1high CD11bhigh). On day 40, mice were injected with OVA-FITC into the footpad, killed 48 hours later, and had their LNs removed; the effect of NE depletion on the immune response to OVA was evaluated.
Statistical analysis
The data were analyzed using a one-way ANOVA followed by Tukey posttest for multiple comparisons. We used the Student t test on 2 groups. All data were considered statistically significant if P values were less than .05.
Results
Analysis of antigen-capturing cells in lymph organs from immune mice
CFA is an adjuvant that induces an inflammatory state with a corresponding increase in neutrophils in the blood when used with an antigen or with PBS.7 To analyze the possible contribution of neutrophils during the course of the immune response, we injected animals with the antigen for which they had previously been immunized and examined the cells involved in antigen-capture in lymphoid organs, using the antigen labeled with FITC dye (OVA-FITC). To this purpose, unimmunized, OVA/CFA-immunized or PBS/CFA-immunized mice were challenged with OVA-FITC in the footpad. In peripheral blood and spleen we found OVA-FITC+ cells in OVA/CFA-immunized mice (Figure 1A). In unimmunized or PBS/CFA-immunized mice injected with OVA-FITC, the percentage of OVA-FITC+ cells was very low (data not shown). To analyze the antigen-capturing cells in the lymphoid organ that drained the site of injection, we removed the LNs. A cell population was found with a forward and side-scatter higher than that of the lymphocytes appearing in LNs from OVA/CFA-immunized mice (Figure 1B). This population did not appear in either unimmunized or in PBS/CFA mice injected with OVA-FITC. Also, these cells were not encountered in OVA/CFA-immunized mice when they were injected with PBS instead of OVA-FITC. Interestingly, the proportion of these cells increased according to the number of OVA/CFA immunizations. Similar results were obtained when experiments were done with uncoupled OVA (data not shown). Thus, in blood as well as in lymphoid organs, after an antigen exposure, there were OVA-FITC+ cells with a higher side scatter than lymphocytes.
Most OVA-FITC+ cells in popliteal lymph nodes are neutrophils
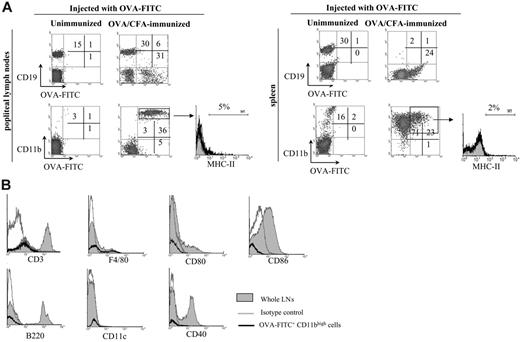
We then characterized the OVA-FITC+ cells in LNs. We found that most OVA-FITC+ cells were CD11bhigh (Figure 2A), with very few OVA-FITC+ cells being CD11c+ (1% ± 0.5%) or CD3+ (3% ± 1%) and with F4/80high cells not being found at all (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). Also, few OVA-FITC+ cells expressed CD19 (Figure 2A), and, considering that these cells fell within the normal lymphocyte forward scatter/side scatter gate, these cells were probably antigen-specific B cells. The population of OVA-FITC+ CD11bhigh CD19– cells had high side-scatter, and they were located in the gate indicated in Figure 1B, suggesting that these cells were not lymphocytes. The discovery of CD11b+ cells in LNs after antigen injection was not surprising, taking into account that the integrin CD11b is expressed in macrophages and in some dendritic-cell subsets, and it is widely recognized that these cells are able to take up antigen in the periphery and to transport it to draining lymph nodes.12,13 However, most of these OVA-FITC+ CD11bhigh cells were negative for MHC class II (Figure 2A). Further phenotypic analysis showed that these OVA-FITC+ CD11bhigh cells were F4/80low (20%) and CD11c–, excluding the presence of macrophage and dendritic cells in this OVA-FITC+ CD11bhigh cell population (Figure 2B). Moreover, these cells did not express costimulatory molecules such as CD86, CD80, and CD40.
Analysis of antigen-capturing cells in lymphoid organs from immune mice. Unimmunized, OVA/CFA-immunized, and PBS/CFA-immunized mice were injected with OVA-FITC or PBS into the footpad, and 6 hours later blood, spleen, and LNs were processed for flow cytometry. (A) The histograms show the percentage of OVA-FITC+ cells in blood and spleen from OVA/CFA-immunized mice injected with OVA-FITC (black line) or PBS (gray area). (B) Top panels show forward and side light scatter profiles of LN cells. The bottom panel shows the percentage of OVA-FITC+ LN cells in OVA-FITC–injected (black line) or in PBS-injected (gray area) mice from the total cell population. A graph showing side light scatter profile of OVA-FITC+ cells is also included. Data shown in parentheses depict MFI. One typical experiment of 4 performed is shown (n = 3-4 mice per group).
Analysis of antigen-capturing cells in lymphoid organs from immune mice. Unimmunized, OVA/CFA-immunized, and PBS/CFA-immunized mice were injected with OVA-FITC or PBS into the footpad, and 6 hours later blood, spleen, and LNs were processed for flow cytometry. (A) The histograms show the percentage of OVA-FITC+ cells in blood and spleen from OVA/CFA-immunized mice injected with OVA-FITC (black line) or PBS (gray area). (B) Top panels show forward and side light scatter profiles of LN cells. The bottom panel shows the percentage of OVA-FITC+ LN cells in OVA-FITC–injected (black line) or in PBS-injected (gray area) mice from the total cell population. A graph showing side light scatter profile of OVA-FITC+ cells is also included. Data shown in parentheses depict MFI. One typical experiment of 4 performed is shown (n = 3-4 mice per group).
We then analyzed the expression of Gr-1 (Ly-6G and Ly-6C) and found that most OVA-FITC+ cells strongly expressed Gr-1 (Figure 3A) suggesting that they were NEs. In addition, Figure 3B shows that all OVA-FITC+ Gr-1high cells are Ly-6Ghigh. Furthermore, in cytospin preparations of LNs we observed abundant cells that displayed the characteristic polylobed nuclei of NEs, whereas in PBS-injected animals the majority of cells showed the typical nuclei of mononuclear cells (Figure 3C). OVA-FITC was detected inside cells with polylobed nuclei as is shown by DAPI staining (Figure 3Di-ii). Confocal microscopy revealed that OVA-FITC was located in the cytoplasm of NEs which had had the surface stained by anti–Gr-1 antibody (Figure 3Diii-v), confirming the results observed by fluorescence-activated cell sorting (FACS), and excluding the possibility of cell doublets in FACS analysis. On the whole, these observations indicated that in OVA/CFA-immunized mice, most OVA-FITC+ cells present in LNs were NEs.
Surface phenotype of OVA-FITC+ cells. Unimmunized and OVA/CFA-immunized mice (day 40) were injected with OVA-FITC into the footpad, and 6 hours later LNs and spleen were obtained and processed by flow cytometry. (A) Density plot graphs show CD19 and CD11b expression versus green fluorescence in OVA-FITC–injected mice. The histograms show staining for MHC-II of the gated population; the percentage of cells with a fluorescent intensity over the marker (black line), corresponding to the upper limit of control background staining (gray area) is indicated. (B) Histograms show in black lines the expression of the indicated markers in OVA-FITC+ CD11bhigh LN cells, and the gray shaded area denotes the expression of the same marker in whole LN cells. The gray lines represent cells stained with an isotype-matched control antibody. One typical experiment of 4 performed is shown (n = 3-4 mice per group).
Surface phenotype of OVA-FITC+ cells. Unimmunized and OVA/CFA-immunized mice (day 40) were injected with OVA-FITC into the footpad, and 6 hours later LNs and spleen were obtained and processed by flow cytometry. (A) Density plot graphs show CD19 and CD11b expression versus green fluorescence in OVA-FITC–injected mice. The histograms show staining for MHC-II of the gated population; the percentage of cells with a fluorescent intensity over the marker (black line), corresponding to the upper limit of control background staining (gray area) is indicated. (B) Histograms show in black lines the expression of the indicated markers in OVA-FITC+ CD11bhigh LN cells, and the gray shaded area denotes the expression of the same marker in whole LN cells. The gray lines represent cells stained with an isotype-matched control antibody. One typical experiment of 4 performed is shown (n = 3-4 mice per group).
Influx of OVA-FITC+ neutrophils in popliteal lymph nodes increases with the number of immunizations and requires an antigen-specific response
During the course of the immunization we observed an increase in the percentage as well as in the mean fluorescence intensity (MIF) of OVA-FITC+ NEs after the first, second, or third immunizations (Figure 4A). A 2.8-fold increase in the influx of NEs occurred between days 15 and 30, and a 3.3-fold increase was presented between days 30 and 40. This increase in the influx of NEs into LNs was not associated with a corresponding increase in the levels of NEs in peripheral blood during the immunization course (Figure 4B). However, in LNs from unimmunized or PBS/CFA-immunized mice, where no antibodies against OVA were present, very few OVA-FITC+ cells were detected (Figure 1B). We then studied whether OVA-specific antibodies can mediate the influx of NEs. First, we studied the kinetics and strength of OVA-specific IgG responses in immunized OVA/CFA mice (Figure 4C). On day 30, OVA-antibody levels were significantly higher than those seen on day 15, and similar levels were found between days 30 and 40. Second, unimmunized and PBS/CFA immunized mice were injected in the footpad with soluble OVA-FITC or with OVA-FITC immune complexes, and 6 hours later the presence of OVA-FITC+ cells was analyzed in LNs (Figure 4D). We encountered a higher number of OVA-FITC+ cells in mice injected with immune complexes than in those injected with soluble antigen, in both groups of animals. Furthermore, in PBS/CFA-immunized mice, the number of OVA-FITC+ cells after immune complex injections was higher than that in unimmunized mice, probably because of the higher number of NEs in blood (52% ± 2% in PBS/CFA versus 20% ± 5% in unimmunized mice) or to the higher inflammatory state in PBS/CFA-immunized mice.
OVA-FITC+ cells in lymphoid organs are mainly neutrophils. OVA/CFA-immunized mice were injected in the footpad with OVA-FITC, and 6 hours later lymphoid organs were obtained. (A) Density plot graphs show Gr-1 expression versus green fluorescence. (B) Gr-1high OVA-FITC+ cells show high levels of Ly-6G. (C) May-Grünwald-Giemsa–stained cytospin preparations of LNs from OVA-FITC, or PBS-injected, OVA/CFA-immunized mice. Arrows indicate NEs. (D) LNs from OVA/CFA-immunized mice injected with OVA-FITC: (i-ii) (original magnification, ×600) typical polylobed nucleus of OVA-FITC+ NEs in cytospin preparation stained with DAPI alone (in blue) (i) or DAPI plus anti–Gr-1–labeled with Alexa Fluor 546 (red) (ii); (iii-iv) (original magnification, ×400) confocal microscopy: OVA-FITC+ cells (green) and Gr-1+ cells (red) as well as a merged image are shown. Panels C and Di-ii were obtained with conventional microscopy. One typical experiment of 4 performed is shown (n = 3-4 mice per group).
OVA-FITC+ cells in lymphoid organs are mainly neutrophils. OVA/CFA-immunized mice were injected in the footpad with OVA-FITC, and 6 hours later lymphoid organs were obtained. (A) Density plot graphs show Gr-1 expression versus green fluorescence. (B) Gr-1high OVA-FITC+ cells show high levels of Ly-6G. (C) May-Grünwald-Giemsa–stained cytospin preparations of LNs from OVA-FITC, or PBS-injected, OVA/CFA-immunized mice. Arrows indicate NEs. (D) LNs from OVA/CFA-immunized mice injected with OVA-FITC: (i-ii) (original magnification, ×600) typical polylobed nucleus of OVA-FITC+ NEs in cytospin preparation stained with DAPI alone (in blue) (i) or DAPI plus anti–Gr-1–labeled with Alexa Fluor 546 (red) (ii); (iii-iv) (original magnification, ×400) confocal microscopy: OVA-FITC+ cells (green) and Gr-1+ cells (red) as well as a merged image are shown. Panels C and Di-ii were obtained with conventional microscopy. One typical experiment of 4 performed is shown (n = 3-4 mice per group).
Influx of OVA-FITC+ neutrophils in LNs increases with the number of immunizations and requires an antigen-specific response. (A-C) Mice were immunized with OVA/CFA on days 0, 15, and 30. (A) After the first (day 15), second (day 30), or third (day 40) immunization, mice were injected with OVA-FITC (▴) or PBS (▪), and 6 hours later LNs were obtained and the percentages of OVA-FITC+ NEs were measured by flow cytometry. Each point represents the mean ± SD. (B) The percentage of NEs in smears of peripheral blood stained with May-Grünwald-Giemsa was evaluated on days 15, 30, and 40. Each point represents the mean ± SD. The value of NEs in peripheral blood from unimmunized mice was 20% ± 5%. (C) Plasma from individual immune mice was assayed for anti–OVA IgG by ELISA on days 15, 30, and 40. Each point represents the mean of specific antibody titers (log10) ± SD. IgG antibody titers were calculated as the reciprocal of the last plasma dilution that yielded an A490 above that of the double-mean value of preimmune plasma. (D) OVA-antibody complexes or soluble OVA-FITC were injected in unimmunized or PBS/CFA-immunized animals. Six hours after injection, LN cells were counted, and fluorescent cells were analyzed by flow cytometry. Results are expressed as the mean number of OVA-FITC+ NEs/LNs ± SD. (E) OVA/CFA-immunized or KLH/CFA-immunized mice (day 40) were injected in the footpad with OVA or KLH. As the control, a group of mice were immunized with PBS/CFA and injected (day 40) with OVA. After 6 hours, LNs were obtained, and the cells were stained with anti–Gr-1 and analyzed by flow cytometry. The percentage of NEs (Gr-1high) is indicated (black line); the gray area corresponds to cells stained with isotype-matched control antibody. One typical experiment of 4 performed is shown (n = 3-4 mice per group).
Influx of OVA-FITC+ neutrophils in LNs increases with the number of immunizations and requires an antigen-specific response. (A-C) Mice were immunized with OVA/CFA on days 0, 15, and 30. (A) After the first (day 15), second (day 30), or third (day 40) immunization, mice were injected with OVA-FITC (▴) or PBS (▪), and 6 hours later LNs were obtained and the percentages of OVA-FITC+ NEs were measured by flow cytometry. Each point represents the mean ± SD. (B) The percentage of NEs in smears of peripheral blood stained with May-Grünwald-Giemsa was evaluated on days 15, 30, and 40. Each point represents the mean ± SD. The value of NEs in peripheral blood from unimmunized mice was 20% ± 5%. (C) Plasma from individual immune mice was assayed for anti–OVA IgG by ELISA on days 15, 30, and 40. Each point represents the mean of specific antibody titers (log10) ± SD. IgG antibody titers were calculated as the reciprocal of the last plasma dilution that yielded an A490 above that of the double-mean value of preimmune plasma. (D) OVA-antibody complexes or soluble OVA-FITC were injected in unimmunized or PBS/CFA-immunized animals. Six hours after injection, LN cells were counted, and fluorescent cells were analyzed by flow cytometry. Results are expressed as the mean number of OVA-FITC+ NEs/LNs ± SD. (E) OVA/CFA-immunized or KLH/CFA-immunized mice (day 40) were injected in the footpad with OVA or KLH. As the control, a group of mice were immunized with PBS/CFA and injected (day 40) with OVA. After 6 hours, LNs were obtained, and the cells were stained with anti–Gr-1 and analyzed by flow cytometry. The percentage of NEs (Gr-1high) is indicated (black line); the gray area corresponds to cells stained with isotype-matched control antibody. One typical experiment of 4 performed is shown (n = 3-4 mice per group).
To test whether this NE influx needs an antigen-specific immune response, mice were immunized with OVA/CFA or KLH/CFA and subsequently injected with OVA or KLH. An NE population was detected in mice immunized and injected with the same antigen, whereas this population was very low in LNs of mice injected with the unrelated antigen (Figure 4E). Also, in PBS/CFA immune mice injected with OVA, very few Gr-1+ cells were observed. Taken together, these results showed that the development of this NE influx requires the presence of an antigen-specific response.
Localization and kinetics of neutrophil influx at the site of inoculation and in popliteal lymph nodes
In LNs we observed that independent of the number of immunizations carried out, 100% of Gr-1high cells were OVA-FITC+ 6 hours after antigen injection. We then asked ourselves whether NEs took up the antigen in the periphery and then migrated to the LNs, or whether the antigen arrived alone at the LNs where it was then taken up by NEs. To answer this question, we analyzed by FACS the kinetics of NE influx in LNs over different short time periods. We found that 2 hours after antigen injection, all Gr-1high cells in LNs were OVA-FITC+ (Figure 5A). The presence of OVA-FITC+ NEs in LNs suggests that NEs are in fact capturing antigen in the skin and then migrating to LNs. If this were not the case, we would find Gr-1high/OVA-FITC+ cells as well as Gr-1high/OVA-FITC– cells.
To analyze the possibility that NEs take up OVA-FITC in skin, we performed kinetic analysis in hind footpad sections (site of inoculation). OVA-FITC+ cells were found in the dermis as little as 2 hours after the OVA-FITC injection, and their number increased 6 hours after injection, as NEs infiltrated the skin (Table 1). The number of NEs in skin in PBS/CFA-immunized mice was comparable to that of OVA/CFA-immunized mice, but few of them were OVA-FITC+ and migrated to LNs, strongly indicating that uptake of antigen in skin is a prerequisite for the migration of NEs to LNs. We then analyzed the distribution of NEs in LN sections. Figure 5B shows that 2 hours after injection, OVA-FITC+ cells mainly infiltrated the subcapsular sinus, whereas 6 hours after injection they filled the cortex. The fact that after a short time we observed a predominant proportion of NEs under the capsule, suggests that NEs had arrived at LNs via the lymphatics. To confirm this route, mice immunized with OVA/CFA were injected with OVA-FITC into the right footpad, and 6 hours later, LNs draining the right and left footpad were removed. Figure 5C shows that only 1% of NEs was observed in the contralateral LNs. Thus, migration was restricted to the ipsilateral LNs, supporting the idea that NEs had reached the LNs via afferent lymphatics.
Kinetics of neutrophil influx in skin
. | OVA/CFA . | . | PBS/CFA . | . | ||
|---|---|---|---|---|---|---|
| Time, h . | OVA-FITC+ cells . | Neutrophil infiltrates . | OVA-FITC+ cells . | Neutrophil infiltrates . | ||
| 2 | 1 ± 1 | 6 ± 1 | 1 ± 1 | 5 ± 1 | ||
| 6 | 35 ± 2 | 40 ± 2 | 1 ± 1 | 33 ± 2 | ||
| 24 | 2 ± 1 | 58 ± 2 | ND | 35 ± 2 | ||
| 240 | ND | 8 ± 2 | ND | 6 ± 1 | ||
. | OVA/CFA . | . | PBS/CFA . | . | ||
|---|---|---|---|---|---|---|
| Time, h . | OVA-FITC+ cells . | Neutrophil infiltrates . | OVA-FITC+ cells . | Neutrophil infiltrates . | ||
| 2 | 1 ± 1 | 6 ± 1 | 1 ± 1 | 5 ± 1 | ||
| 6 | 35 ± 2 | 40 ± 2 | 1 ± 1 | 33 ± 2 | ||
| 24 | 2 ± 1 | 58 ± 2 | ND | 35 ± 2 | ||
| 240 | ND | 8 ± 2 | ND | 6 ± 1 | ||
Kinetics of OVA-FITC+ cells or NE influx in skin section evaluated by microscopic analysis (×400). The data are expressed as number of cells per field. At least 20 and 40 fields of each smear were evaluated.
Localization and kinetics of neutrophil influx in the site of inoculation and in LNs. Mice immunized with OVA/CFA or PBS/CFA were injected with OVA-FITC in the footpads on day 40. (A) Kinetics of NEs (Gr-1, black line) in LNs at different short time periods (10 minutes, 20 minutes, 30 minutes, or 2 hours), evaluated by FACS. The percentage of NEs (Gr-1high) is also indicated with the gray area corresponding to control background staining. (B) Localization of OVA-FITC+ cells in LNs. The left panels show fluorescent microphotographs of LN sections 2 hours (top panels) or 6 hours (bottom panels) after OVA-FITC injection in the footpad of OVA/CFA-immunized mice. The right panels show the corresponding hematoxylin/eosin-stained tissue sections at ×250 and at higher magnification (×1000). Arrows indicate NEs; SS, subcapsular sinuses; C, cortex. (C) Gr-1high cells (black line) from ipsilateral and contralateral LNs 6 hours after OVA-FITC injection in OVA/CFA-immune mice, processed by FACS. The gray area corresponds to control background staining. (D) Analysis of apoptosis of NEs in LNs or blood obtained 12 or 18 hours after OVA-FITC injection in the footpad of OVA/CFA-immune mice. Cells were stained with FITC-anti–Gr-1 antibody and propidium iodide and then analyzed by flow cytometry. The percentage of cells with hypodiploid DNA in the gate of Gr-1+ cells is indicated in each panel. One typical experiment of 3 performed is shown (n = 3-4 mice per group).
Localization and kinetics of neutrophil influx in the site of inoculation and in LNs. Mice immunized with OVA/CFA or PBS/CFA were injected with OVA-FITC in the footpads on day 40. (A) Kinetics of NEs (Gr-1, black line) in LNs at different short time periods (10 minutes, 20 minutes, 30 minutes, or 2 hours), evaluated by FACS. The percentage of NEs (Gr-1high) is also indicated with the gray area corresponding to control background staining. (B) Localization of OVA-FITC+ cells in LNs. The left panels show fluorescent microphotographs of LN sections 2 hours (top panels) or 6 hours (bottom panels) after OVA-FITC injection in the footpad of OVA/CFA-immunized mice. The right panels show the corresponding hematoxylin/eosin-stained tissue sections at ×250 and at higher magnification (×1000). Arrows indicate NEs; SS, subcapsular sinuses; C, cortex. (C) Gr-1high cells (black line) from ipsilateral and contralateral LNs 6 hours after OVA-FITC injection in OVA/CFA-immune mice, processed by FACS. The gray area corresponds to control background staining. (D) Analysis of apoptosis of NEs in LNs or blood obtained 12 or 18 hours after OVA-FITC injection in the footpad of OVA/CFA-immune mice. Cells were stained with FITC-anti–Gr-1 antibody and propidium iodide and then analyzed by flow cytometry. The percentage of cells with hypodiploid DNA in the gate of Gr-1+ cells is indicated in each panel. One typical experiment of 3 performed is shown (n = 3-4 mice per group).
We also performed a kinetic analysis of NE influx in LN sections over longer time periods (Table 2). Twenty-four hours after antigen injection, there was still an important number of NEs in LNs, although they were no longer OVA-FITC+. This result was confirmed by FACS analysis (after 24 hours there were 17% of NEs but only 0.8% of them were OVA-FITC+). At 48 hours, NEs started to disappear from the LNs, and at 72 hours there were no more NEs.
Kinetics of neutrophil influx in popliteal lymph nodes
. | OVA/CFA . | . | PBS/CFA . | . | ||
|---|---|---|---|---|---|---|
| Time, h* . | OVA-FITC+ cells . | Neutrophil infiltrates . | OVA-FITC+ cells . | Neutrophil infiltrates . | ||
| 2 | 5 ± 1 | 8 ± 2 | 1 ± 1 | 2 ± 1 | ||
| 6 | 30 ± 2 | 39 ± 2 | 1 ± 1 | 2 ± 1 | ||
| 24 | ND | 15 ± 2 | ND | ND | ||
| 48 | ND | 2 ± 1 | ND | ND | ||
. | OVA/CFA . | . | PBS/CFA . | . | ||
|---|---|---|---|---|---|---|
| Time, h* . | OVA-FITC+ cells . | Neutrophil infiltrates . | OVA-FITC+ cells . | Neutrophil infiltrates . | ||
| 2 | 5 ± 1 | 8 ± 2 | 1 ± 1 | 2 ± 1 | ||
| 6 | 30 ± 2 | 39 ± 2 | 1 ± 1 | 2 ± 1 | ||
| 24 | ND | 15 ± 2 | ND | ND | ||
| 48 | ND | 2 ± 1 | ND | ND | ||
Kinetics of OVA-FITC+ cells or NE influx at longer time periods in LN sections evaluated by microscopic analysis (×400). The data are expressed as the number of cells per field. At least 20 and 40 fields of each smear were evaluated.
The results were not detected (ND) for 72 hours and 10 days.
Because 72 hours after OVA injection NEs had disappeared from LNs, we asked ourselves whether the resolution of this acute inflammation involved programmed cell death of invading inflammatory cells.14 Twelve hours after the injection of antigen we observed that only 1% of NEs in LNs had a hypodiploid DNA content, whereas after 18 hours this figure was 22%, indicating that the number of apoptotic NEs increased over time. Very few NEs with hypodiploid DNA were detected in blood from OVA/CFA mice injected with OVA at these times (Figure 5D).
In summary, NE influx in LNs was rapid (2 hours after a challenge) and transient (48 hours). Because we did not observe OVA-FITC+ cells 24 hours after a challenge, we hypothesize that the NEs arrive at the LNs between 2 and 6 hours later, then NEs become OVA-FITC–, remaining in LNs up to 48 hours after an injection. This inflammatory state was resolved 72 hours after injection, and death by apoptosis could have been one of the mechanisms that contributed to its resolution.
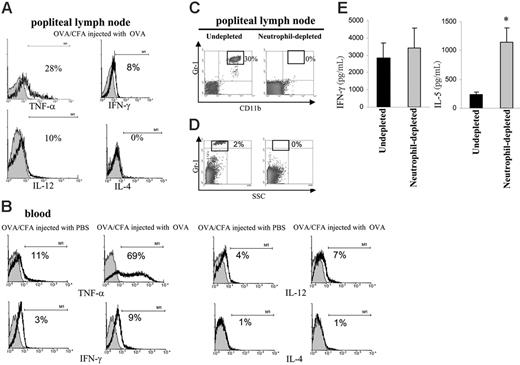
Neutrophils present in popliteal lymph nodes mainly expressed TNF-α, and their depletion resulted in an antigen-specific increase in IL-5 levels
The preceding results show that on injection of OVA in OVA/CFA-immunized mice, a population of NEs arrived at the LNs after an uptake of OVA, probably in the form of immune complexes. We investigated whether NEs could have contributed to the immune response by secreting cytokines. By evaluating the in vivo production of TNF-α, IFN-γ, IL-12, and IL-4 by NEs, we found that NEs present in LNs exhibited a positive cytoplasmic staining for TNF-α, and a slight expression of IFN-γ and IL-12 (Figure 6A). This profile of cytokine expression was similar for NEs present in blood (Figure 6B), whereas NEs from OVA/CFA mice injected with PBS showed lower levels of cytokines, especially TNF-α. We then assessed the effect of NEs on the OVA-specific T-cell response by removing in vivo NEs with anti-Gr1 treatment. Mice depleted of NEs were then injected with OVA-FITC, and 2 days later LN cells were challenged in vitro with OVA. Flow cytometric analysis demonstrated that administration of anti-Gr1 in OVA/CFA-immunized mice was effective in NE depletion, because 6 hours (Figure 6C) or 48 hours (Figure 6D) after antigen injection, there were no Gr-1high cells found in the LNs. LN cells from NE-depleted mice secreted 5 times more IL-5 than LN cells from IgG-treated mice, whereas no difference was found in terms of IFN-γ secretion between both mice groups (Figure 6E). In summary, we have shown that after an antigen challenge, and in the presence of specific antibodies, NEs are recruited into the secondary lymphoid organs, principally secreting TNF-α, and were able to modify the cytokine balance in OVA-specific T-cell responses.
Neutrophils present in LNs mainly expressed TNF-α and their depletion resulted in an antigen-specific increase in IL-5 levels. (A-B) Mice immunized with OVA/CFA were injected with PBS or OVA, and 6 hours later LN cells and peripheral blood were obtained and analyzed for intracellular cytokines. Cells were stained with anti–TNF-α, anti–IFN-γ, anti–IL-12, anti–IL-4 (black line), or an isotype-matched control antibody (gray area) (histograms gated on Gr-1high cells). One typical experiment of 3 performed is shown. (C-D) OVA/CFA-immunized mice were treated with isotype control (undepleted) or RB6-8C5 (NE-depleted) antibody. On day 40 (C) (6 hours after injection of OVA) and on day 42 (D) (48 hours after injection to OVA), LN cells were processed for flow cytometry. Density plots are representative of 3 to 4 mice analyzed. (E) OVA/CFA-immunized mice treated with isotype control (undepleted) or RB6-8C5 (neutrophil-depleted) antibody were injected on day 40 in footpad with OVA, 48 hours later LN cells were removed and then cultured for 72 hours with 100 μg/mL OVA (3.5 × 105 cells/200 μL/well). The supernatants from triplicate cultures were pooled, and cytokine content was measured in triplicate by capture ELISA. *P < .005 compared with undepleted mice. One typical experiment of 3 performed is shown (n = 3-4 mice per group).
Neutrophils present in LNs mainly expressed TNF-α and their depletion resulted in an antigen-specific increase in IL-5 levels. (A-B) Mice immunized with OVA/CFA were injected with PBS or OVA, and 6 hours later LN cells and peripheral blood were obtained and analyzed for intracellular cytokines. Cells were stained with anti–TNF-α, anti–IFN-γ, anti–IL-12, anti–IL-4 (black line), or an isotype-matched control antibody (gray area) (histograms gated on Gr-1high cells). One typical experiment of 3 performed is shown. (C-D) OVA/CFA-immunized mice were treated with isotype control (undepleted) or RB6-8C5 (NE-depleted) antibody. On day 40 (C) (6 hours after injection of OVA) and on day 42 (D) (48 hours after injection to OVA), LN cells were processed for flow cytometry. Density plots are representative of 3 to 4 mice analyzed. (E) OVA/CFA-immunized mice treated with isotype control (undepleted) or RB6-8C5 (neutrophil-depleted) antibody were injected on day 40 in footpad with OVA, 48 hours later LN cells were removed and then cultured for 72 hours with 100 μg/mL OVA (3.5 × 105 cells/200 μL/well). The supernatants from triplicate cultures were pooled, and cytokine content was measured in triplicate by capture ELISA. *P < .005 compared with undepleted mice. One typical experiment of 3 performed is shown (n = 3-4 mice per group).
Discussion
In this work, we observed that soon after an antigen challenge in OVA/CFA-immunized mice, not only did OVA-FITC+ NEs appear at the injection site, but there was also an influx of NEs into secondary lymph organs, where they represented the main OVA-FITC+ cells. Although neutrophils have been generally accepted not to be involved in adaptive immune responses, over the last few years some reports have appeared that describe NE trafficking from peripheral tissue to LNs.2,15,16 In the present work, we analyzed the uptake of antigen by NEs, their arrival into LNs, and their participation as immunomodulatory cells in immune mice.
On the basis of the classic tenet that uptake and transport of antigens from the skin to lymph nodes is a function of dendritic cells and macrophages,12,13 our discovery about there being an important OVA-FITC+ NE influx into the LNs was really unexpected. NEs were the main OVA-FITC+ cells in the LNs, because we detected few OVA-FITC+ dendritic and B cells, and no OVA-FITC+ macrophages in LNs. In agreement with this, other investigators recently observed that the granulocytes were central microorganism-host cells in draining lymph nodes.16,17 Abadie et al16 showed that bacillus Calmette-Guérin (BCG) shuttles from tissue to lymph nodes via NEs. Little is known about the factors that determine which kind of cell can take up antigens in situ, but it is probable that it may be influenced by the microenvironment. Recently, we found no OVA-FITC+ CD11b+ cells in LNs from mice immunized with OVA plus synthetic oligodeoxynucleotide containing CpG motifs or OVA plus Bordetella pertussis.7 Our data and these of Abadie et al16 suggest that the selection of NEs as cells that take up antigens occurs in response to certain inflammatory signals such as BCG vaccines (containing both live and dead bacilli) or CFA (oil containing inactive bacilli), revealing the great complexity in the relationship between the immune milieu and the cells involved in the uptake of antigens.
Regarding the influx of OVA-FITC+ NEs in LNs, 2 hours after injection of OVA-FITC into the footpad, we observed that OVA-FITC+ NEs were localized in the subcapsular space, principally in the ipsilateral LNs, suggesting that NEs had reached the LNs via afferent lymphs. In addition, we showed that most of the NEs did not take OVA-FITC to the LNs, but instead arrived loaded with OVA-FITC from the skin. Nevertheless, our data do not exclude the possibility that some antigens could arrive at LNs as free OVA-FITC. In agreement with our results, it has been recently shown in mice vaccinated with BCG strains that NEs migrate via afferent lymphatics to lymphoid tissue.16 At present, the molecular mechanisms by which NEs home in on LNs via lymphatic vessels has not been explored, and subsequent experiments are needed to fully understand it. In addition, we have observed OVA-FITC+ NEs in spleen and blood after antigen injections in the skin. Probably, some OVA-FITC reaches the blood from where it is taken up by NEs and later arrives at the spleen, a secondary lymphoid organ specialized in the generation of immune responses against antigens carried by the blood.
In our model, OVA uptake by NEs is critically dependent on the presence of an OVA-specific immune response, taking into account that PBS/CFA mice have NEs in skin but do not have OVA-FITC+ cells in the skin or in LNs. Furthermore, injection of OVA-antibody complexes into these mice produces the same results observed in OVA/CFA mice injected with OVA. Although we suggest that the presence of specific antibodies is a prerequisite for NEs to be able to reach LNs, we do not discard the possibility that other signals present during the immune response may also be involved. It is well known that the recruitment of NEs into inflamed tissues is mediated by selectin. However, Coxon et al18 and Carlos and Harlan19 demonstrated that the interaction of immune complexes with Fcγ-RIII may mediate early NE recruitment in immune complex-mediated inflammations. In our model, we found that in PBS/CFA-immunized mice which did not have OVA-antibody complexes, but showed an increased number of NEs in blood similar to that of OVA/CFA-immunized mice, recruitment of NEs in skin was comparable to that observed in OVA/CFA-immunized mice. Therefore, the recruitment of NEs in skin is independent of the presence of antigen-antibody complexes. However, the uptake of an immune complex seems to be a requirement for NEs to be able to reach LNs.
NEs have been shown to influence the priming of Th1 responses, releasing immunoregulatory cytokines and chemokines.20 This effect can be direct by providing a Th1-cell cytokine environment by the production of IL-12,5,21,22 or indirect through interactions with dendritic cells.23-25 In numerous reports it has been shown that NEs triggered by different stimulus are able to produce TNF-α.23,24,26-28 Here, we show that in vivo NEs display intracellular TNF-α in LNs. A recent report described that TNF-α from an NE-conditioned medium triggers maturation of dendritic cells.24 Therefore, in our model it is probable that TNF-α produced by NEs in LNs facilitates the maturation of dendritic cells, with these cells then being the ones that participate in the support of a Th1 response. Related to this, it has been previously demonstrated that NEs promote Th1 or Th2 polarization of T-cell responses in different experimental models of infection.2-5,29-31 Also, van Gisbergen et al25 demonstrated in vitro that dendritic cells stimulated by NEs induce Th1 polarization. Accordingly, a relevant finding of our study is that in the absence of NEs in OVA/CFA mice there was an increase in the OVA-specific IL-5 secretion in LNs. Our data has demonstrated for the first time a direct correlation between NEs loaded with immune complex and the outcome of T-cell responses, supporting a new concept of NEs as cells that could help to maintain a Th1 response in the secondary phase of the immune response. The presence of NEs in the absence of an infection addresses the question about its importance during inflammatory diseases where immune complexes coexist with NEs. Of special interest are several reports from the laboratory of Matthys et al,32 who demonstrated that the development of arthritis in collagen/CFA-immunized mice is determined by CD11b+ myeloid cells. In addition, Wipke and Allen33 demonstrated that NEs play a critical role in inflammatory processes in the joint lesion. Further studies need to be addressed exploring the role played by neutrophils not only as effector cells but also as messenger cells during inflammatory processes.
In summary, the findings presented herein demonstrate that in the presence of an established immune response, NEs can capture antigen in the periphery, transport it to lymphoid organs, and provide help in sustaining the immune response. The findings described here have a particular importance for the use of NEs as a potential target in the latest therapies for inflammatory diseases.
Prepublished online as Blood First Edition Paper, July 11, 2006; DOI 10.1182/blood-2006-04-016659.
Supported by grants from the Agencia Córdoba Ciencia, Secretaría de Ciencia y Técnica de la Universidad Nacional de Córdoba, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP no. 02962). M.C.P.-P., V.G.M., and A.S.R. are career members of CONICET. D.O.A., R.P.R., and M.V.L. are recipients of graduate fellowships from CONICET.
B.A.M. and A.S.R. contributed equally to this work.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank native speaker Dr Paul Hobson, who revised the manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal