Abstract
Bcl-2 plays a key role in the regulation of apoptosis. We investigated the role of a novel regulatory single-nucleotide polymorphism (−938C>A) in the inhibitory P2 BCL2 promoter in B-cell chronic lymphocytic leukemia (B-CLL). The −938C allele displayed significantly increased BCL2 promoter activity and binding of nuclear proteins compared with the A allele. Concomitantly, Bcl-2 protein expression in B cells from CLL patients carrying the −938 AA genotype was significantly increased compared with CC genotypes. Genotype distribution between 123 CLL patients (42 AA, 55 AC, 26 CC) and 120 genotyped healthy controls (36 AA, 63 AC, 21 CC) was not significantly different, suggesting that genotypes of this polymorphism do not increase the susceptibility for B-CLL. However, median time from first diagnosis to initiation of chemotherapy and median overall survival were significantly shorter in patients with −938AA genotype (38 and 199 months, respectively) compared with AC/CC genotypes (120 and 321 months, respectively; P = .008 and P = .003, respectively). Multivariable Cox regression identified the BCL2−938AA genotype as an independent prognostic factor for the time to first treatment (hazard ratio [HR] 1.9; P = .034) together with disease stage at diagnosis (HR 2.5; P = .004) and ZAP-70 status (HR 3.0; P = .001). The BCL2−938AA genotype is associated with increased Bcl-2 expression and a novel unfavorable genetic marker in patients with B-CLL.
Introduction
The hallmark of B-cell chronic lymphocytic leukemia (B-CLL) is an accumulation of B lymphocytes arrested in the G0 phase of the cell cycle.1,2 It has been suggested that the excess of B cells results more likely from decreased apoptosis and dysregulation of cell-cycle control than from an increased proliferation. In normal tissues, the delicate homeostasis between proliferation and apoptosis is controlled by a large variety of proteins of the Bcl-2 family. The Bcl-2 family of proteins consists of different apoptosis regulators that integrate diverse survival and death signals generated outside and inside the cell.3 This family is subdivided into 2 major classes: antiapoptotic members such as Bcl-2 and Bcl-xL (BCL2-like survival factors), which protect cells from apoptosis, and proapoptotic members such as Bax and Bak (BAX-like death factors).4,5,6 Due to the key role of apoptosis in the progression of B-CLL, multiple studies have investigated Bcl-2 protein expression and regulation of BCL2 gene transcription. In fact, overexpression of Bcl-2 has been demonstrated in B-CLL cells.7,8 In addition, others9 showed that a high Bcl-2/Bax ratio predicts poor clinical responsiveness in patients treated for B-CLL. Finally, Faderl et al10 demonstrated that increased Bcl-2 expression is associated with decreased survival of B-CLL patients.
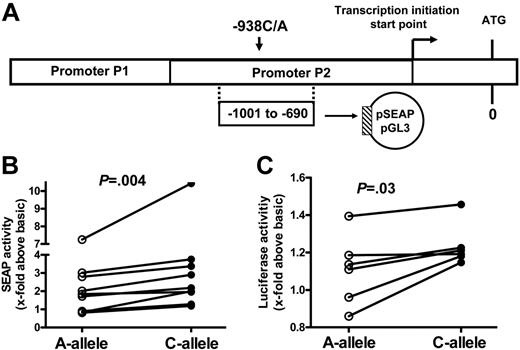
The BCL2 gene is located on chromosome 18q21.3 and consists of 3 exons and 2 promoters (Figure 1A) with different functional properties. The second promoter, P2, is located 1400-bp upstream of the translation initiation site and decreases the activity of the P1 promoter, thus functioning as a negative regulatory element.11,12 It can be speculated that functional single-nucleotide polymorphisms (SNPs) in the BCL2 gene, which alter protein function and/or expression, could exert an impact upon the delicate balance of mechanisms that regulate apoptosis. For example, a 43G>A polymorphism in exon 2 has been associated with an increased resistance to autoimmune diseases in a Japanese population,13 and this polymorphism results in a diminished antiapoptotic function in a pre–B-cell line in vitro. However, this polymorphism is rare in the white population. Recently, Park et al14 identified 6 SNPs in the BCL2 gene by direct sequencing of DNA samples from a white population. Haplotype analysis yielded a significant linkage disequilibrium between an SNP in the inhibitory P2 promoter (−938C>A) and a silent SNP in exon 1 (+21A>G). However, the potential functional implications of these alleles have not been investigated.
Activity, structure, and function of BCL2 promoter. (A) Structure of the BCL2 promoter and position of constructs for determination of transcriptional activity. Shown are the regions comprising the “stimulatory” P1 promoter and the “inhibitory” P2 promoter and the position of the (−938)C>A polymorphism. ATG is the translation initiation codon; the arrow indicates the transcription start site. The reporter construct, cloned into the pSEAP-basic vector and the pGL3-basic vector, is shown encompassing nt −690 to −1001 (ie, part of the P2 promoter). See the text for further details. (B) Genotype-dependent activity of BCL2 5′ regulatory region reporter constructs expressed in HEK293 cells. Constructs were cloned into the pSEAP-basic vector and reporter activity was quantified by measuring the concentration of secreted alkaline phosphatase in the cell culture medium (details in “Patients, materials, and methods”). In each experiment, cells were transfected in parallel with constructs harboring the A allele or the C allele. A total of 9 independent pairs of transfection experiments were conducted. The reporter activity was normalized to pSEAP-basic activity. Each symbol represents reporter activity of 1 experiment. Connecting lines indicate results from transfections performed in parallel. The P value was calculated using the Wilcoxon matched pairs test. While absolute reporter activity was variable in different experiments, reporter activity of C-allele constructs was always higher than that of A-allele constructs. (C) Genotype-dependent activity of BCL2 5′ regulatory region reporter constructs expressed in Karpas 422 cells. Constructs were cloned into the pGL3 vector and reporter activity was quantified by measuring luciferase activity (see “Patients, materials, and methods”). Reporter activities were normalized to pGL3-basic activity. In each experiment, cells were transfected in parallel with constructs harboring the A allele or the C allele. A total of 6 independent pairs of transfection experiments were conducted. Each symbol represents reporter activity of 1 experiment. Connecting lines indicate results from transfections performed in parallel. In general, reporter activity was lower than that following expression of constructs in HEK293 cells (see panel B). The P value was calculated using the Wilcoxon matched pairs test. While absolute reporter activity was variable in different experiments, reporter activity of C-allele constructs was always higher than that of A-allele constructs.
Activity, structure, and function of BCL2 promoter. (A) Structure of the BCL2 promoter and position of constructs for determination of transcriptional activity. Shown are the regions comprising the “stimulatory” P1 promoter and the “inhibitory” P2 promoter and the position of the (−938)C>A polymorphism. ATG is the translation initiation codon; the arrow indicates the transcription start site. The reporter construct, cloned into the pSEAP-basic vector and the pGL3-basic vector, is shown encompassing nt −690 to −1001 (ie, part of the P2 promoter). See the text for further details. (B) Genotype-dependent activity of BCL2 5′ regulatory region reporter constructs expressed in HEK293 cells. Constructs were cloned into the pSEAP-basic vector and reporter activity was quantified by measuring the concentration of secreted alkaline phosphatase in the cell culture medium (details in “Patients, materials, and methods”). In each experiment, cells were transfected in parallel with constructs harboring the A allele or the C allele. A total of 9 independent pairs of transfection experiments were conducted. The reporter activity was normalized to pSEAP-basic activity. Each symbol represents reporter activity of 1 experiment. Connecting lines indicate results from transfections performed in parallel. The P value was calculated using the Wilcoxon matched pairs test. While absolute reporter activity was variable in different experiments, reporter activity of C-allele constructs was always higher than that of A-allele constructs. (C) Genotype-dependent activity of BCL2 5′ regulatory region reporter constructs expressed in Karpas 422 cells. Constructs were cloned into the pGL3 vector and reporter activity was quantified by measuring luciferase activity (see “Patients, materials, and methods”). Reporter activities were normalized to pGL3-basic activity. In each experiment, cells were transfected in parallel with constructs harboring the A allele or the C allele. A total of 6 independent pairs of transfection experiments were conducted. Each symbol represents reporter activity of 1 experiment. Connecting lines indicate results from transfections performed in parallel. In general, reporter activity was lower than that following expression of constructs in HEK293 cells (see panel B). The P value was calculated using the Wilcoxon matched pairs test. While absolute reporter activity was variable in different experiments, reporter activity of C-allele constructs was always higher than that of A-allele constructs.
The aim of the present study was, therefore, to determine whether the (−938)C>A SNP, located in the negative regulatory P2 promoter, affects transcription of the BCL2 gene and whether this SNP is potentially associated with differential protein expression and disease progression and overall survival in patients with B-CLL.
Patients, materials, and methods
Subjects
Between August 2001 and October 2004, 123 consecutive white patients with CLL seeking help in the Department of Haematology were enrolled in this retrospective study and analyzed for several clinical and biologic characteristics including age, sex, Binet stage, thymidine kinase, cytogenetics, time from diagnosis to first treatment, and overall survival. Furthermore, patients transported by ambulance to the practices of other hematologists next to our hospital during the same time period were also included at random. For the latter cytogenetics, CD38 and ZAP-70 did not influence the selection of patients, since the status of these markers was unknown to the participating physicians. The characteristics of the patients were identical to those typically seen in other cohorts, with approximately 70% of patients presenting with Binet A stage at first diagnosis.15,16
For each patient, morphologic diagnosis of B-CLL was confirmed by flow cytometry,17,18 revealing a typical CD19+, CD20+, CD5+, CD23+, Ig light chain (κ or λ light chain)–restricted immunophenotype. ZAP-70 and CD38 expression was assessed by flow cytometry as described.18,19 Whole peripheral-blood samples were usually obtained during routine follow-up visits to our institutions, with all patients giving informed consent in accordance with institutional guidelines and the Declaration of Helsinki. Approval was obtained from the local Essener ethics commission institutional review board for these studies. Indications for treatment were based on standard criteria.20 The clinical and laboratory data are shown in Table 1 As control, DNA from 120 white, healthy blood donors recruited at our university hospital transfusion center was genotyped such that the median age and proportion of males were comparable between cases and control subjects (60 vs 59 years, 70% vs 67%, respectively).
Clinical and laboratory data at diagnosis in patients with BCL2-938C>A gene polymorphism
| Variable . | All patients . | BCL2-938 AA genotype . | BCL2-938 AC genotype . | BCL2-938 CC genotype . | P . |
|---|---|---|---|---|---|
| No. of patients | 123 | 42 | 55 | 26 | |
| Median age at diagnosis, y | 60 | 61 | 58 | 61 | NS |
| Male, no. (%)* | 86 (70) | 28 (67) | 42 (76) | 16 (61) | NS |
| Binet stage at diagnosis, no. (%); n = 114 | NS | ||||
| A | 80 (70) | 20 (54) | 39 (75) | 21 (84) | |
| B | 25 (22) | 13 (35) | 9 (17) | 3 (12) | |
| C | 9 (8) | 4 (11) | 4 (8) | 1 (4) | |
| CD38+ leukemia, no. (%); n = 123 | 46 (37) | 18 (43) | 18 (33) | 10 (38) | NS |
| ZAP-70+ leukemia, no. (%); n = 113 | 52 (46) | 21 (55) | 21 (41) | 10 (42) | NS |
| Thymidine kinase, IU/L; n = 72† | 19.0 ± 26.7 | 21.5 ± 30.7 | 21.0 ± 28.6 | 10.6 ± 9.5 | NS |
| Genomic aberrations, no. (%); n = 103 | NS | ||||
| Deletion 11 | 10 (10) | 4 (11) | 5 (10) | 1 (6) | |
| Deletion 17 | 6 (6) | 2 (6) | 3 (6) | 1 (6) | |
| Trisomy 12 | 11 (11) | 6 (17) | 4 (8) | 1 (6) | |
| Normal | 19 (18) | 5 (14) | 9 (18) | 5 (28) | |
| Deletion 13 | 57 (55) | 18 (51) | 29 (58) | 10 (56) | |
| Received therapy, no. (%) | 56 (46) | 26 (62) | 21 (55) | 9 (35) | .03 |
| Variable . | All patients . | BCL2-938 AA genotype . | BCL2-938 AC genotype . | BCL2-938 CC genotype . | P . |
|---|---|---|---|---|---|
| No. of patients | 123 | 42 | 55 | 26 | |
| Median age at diagnosis, y | 60 | 61 | 58 | 61 | NS |
| Male, no. (%)* | 86 (70) | 28 (67) | 42 (76) | 16 (61) | NS |
| Binet stage at diagnosis, no. (%); n = 114 | NS | ||||
| A | 80 (70) | 20 (54) | 39 (75) | 21 (84) | |
| B | 25 (22) | 13 (35) | 9 (17) | 3 (12) | |
| C | 9 (8) | 4 (11) | 4 (8) | 1 (4) | |
| CD38+ leukemia, no. (%); n = 123 | 46 (37) | 18 (43) | 18 (33) | 10 (38) | NS |
| ZAP-70+ leukemia, no. (%); n = 113 | 52 (46) | 21 (55) | 21 (41) | 10 (42) | NS |
| Thymidine kinase, IU/L; n = 72† | 19.0 ± 26.7 | 21.5 ± 30.7 | 21.0 ± 28.6 | 10.6 ± 9.5 | NS |
| Genomic aberrations, no. (%); n = 103 | NS | ||||
| Deletion 11 | 10 (10) | 4 (11) | 5 (10) | 1 (6) | |
| Deletion 17 | 6 (6) | 2 (6) | 3 (6) | 1 (6) | |
| Trisomy 12 | 11 (11) | 6 (17) | 4 (8) | 1 (6) | |
| Normal | 19 (18) | 5 (14) | 9 (18) | 5 (28) | |
| Deletion 13 | 57 (55) | 18 (51) | 29 (58) | 10 (56) | |
| Received therapy, no. (%) | 56 (46) | 26 (62) | 21 (55) | 9 (35) | .03 |
Because of rounding, percentages do not always add up to 100.
NS indicates not significant.
Percent of total number of patients.
†Mean ± standard error of the mean.
Plasmid constructions for transient transfection assays
A polymerase chain reaction (PCR) was performed on DNA using the following primers: forward primer 5′-TTGCTGTTCGGAGTTTAA-3′, and reverse primer 5′-TTCCCAGACTTCTGCTTC-3′ (10 pmol each); 10 mmol of dNTPs; 10× PCR buffer without MgCl2; 1.5 mm MgCl2; and 3 U of plaque-forming unit (PFU) high-fidelity DNA polymerase (MBI; Fermentas Roche, Mannheim, Germany). The amplification protocol consisted of 35 cycles of denaturation at 94°C for 1 minute, annealing at 50°C for 30 seconds, and amplification at 72°C for 30 seconds. The 312-bp fragments (from nucleotide [nt] −690 to nt −1001 with regard to the translation initiation site) were cloned into pGEM-T–Easy vector (Promega, Mannheim, Germany) and sequenced by an external service (GATC, Konstanz, Germany). The insert was subcloned into pSEAP-basic (Promega) and pGL3-basic vector (Promega) after digestion of the plasmids with EcoRI and XhoI, respectively. The orientation was confirmed by digestion with XhoI.
Transient transfection of HEK293 cells and SEAP reporter assay
Human embryonic kidney cells (HEK293) were maintained in a humidified atmosphere with 5% CO2 in DMEM supplemented with 5% fetal calf serum and 1% penicillin/streptomycin. Approximately 5 × 106 cells were split onto 6-well plates, grown to 60% to 70% confluency, and transiently transfected by lipofectamine 2000 with 4 μg BCL2–alkaline phosphatase reporter constructs in pSEAP-basic, essentially as recommended by the manufacturer. The activity of the BCL2 gene promoter constructs was quantified using a GreatEscAPe SEAP fluorescence detection kit (CLONTECH, Heidelberg, Germany) in the supernatant of the cell cultures after 24 hours. A plasmid containing the SV40 promoter and enhancer in pSEAP vector served as a positive control, and a promoterless pSEAP vector was used as a negative control. Secreted alkaline phosphatase activity was measured according to the manufacturer's protocol using a Lumat LB 9501 Luminometer (Berthold, Bad Wildbach, Germany). In all experiments, constructs with the A allele and the C allele were transfected in parallel into the same batch of cells. The activities of the constructs were expressed as the percentage of the basic promoter activity. Data were obtained from 9 independent transfection experiments. Transfection efficiency was estimated from transient transfection of the pEGFP-C1 vector containing the CMV promoter in front of the eGFP gene (Promega) and was found to average 90%. Raw data for reporter gene activity were corrected for transfection efficiency.
Transient transfection of Karpas 422 cells and luciferase reporter assay
Human follicular lymphoma cells (Karpas 422) were maintained in a humidified atmosphere with 5% CO2 in RPMI 1640 supplemented with 5% fetal calf serum and 1% penicillin/streptomycin. Karpas 422 cells were transfected with the Amaxa Nucleofector system (Koeln, Germany) according to the manufacturer's instruction. In brief, 5 μg of luciferase reporter constructs, cloned in the pGL3 vector, were transfected into 2 × 106 cells in cell-line solution V, no. VCA-1003. Plasmid DNA was introduced into the nucleus of the cells using an Amaxa Nucleofector set at program T-01. Transfection efficiency of Karpas 422 cells was determined using Amaxa's pmaxGFP plasmid and found to be in the range of 65% to 75% (ie, somewhat lower than that of HEK293 cells). Cells were harvested after 8 hours and centrifuged for 10 minutes at 400g. Cellular pellets were stored at −80°C until assayed for luciferase activity. Luciferase activity was measured according to the manufacturer's protocol (Promega Luciferase Dual Glo Luciferase Assay System) using a Lumat LB 9501 Luminometer (Berthold). In all experiments, constructs with the A allele and the C allele were transfected in parallel into the same batch of cells. The activities of the constructs were expressed as the percentage of the basic promoter activity. Data were obtained from 6 independent transfection experiments. Raw data for reporter gene activity were corrected for transfection efficiency.
Nuclear extracts and electrophoretic mobility shift assay
Nuclear extracts from human follicular lymphoma cells (Karpas 422) were prepared using the NuCLEAR Extraction Kit (Sigma, Deisenhofen, Germany) as recommended by the manufacturer. Briefly, Karpas 422 cells were washed twice in ice-cold PBS, subsequently lysed in ice-cold lysis buffer (0.1 M dithiothreitol [DTT], protease inhibitors), and allowed to swell on ice for 15 minutes. After adding 10% IGEPAL to the mixture and shaking for 10 seconds, the mixture was centrifuged for 30 seconds at 10 000g at 4°C. The supernatant was discarded, the nuclear pellets were resuspended in ice-cold extraction buffer (0.1 M dithiothreitol [DTT], 0.1 M protease-inhibitors), and the mixture was vigorously shaken at 4°C for 30 minutes. The nuclear debris was discarded following centrifugation for 5 minutes at 10 000g and the extracts were aliquoted and stored at −80°C until used.
Electrophoretic mobility shift assays (EMSAs) were performed with 10 μg of nuclear extracts and double-stranded oligonucleotides with the following sequences: sense, 5′-CTTCATCGTCCC C/A TCTCCCCTGTCT-3′; antisense, 5′-AGACAGGGGAGA T/G GGGACGATGAAG-3′.
Oligonucleotide probes for EMSAs were digoxigenin-labeled according to the manufacturer's instructions (Gel Shift kit; Roche Applied Sciences, Indianapolis, IN). For the supershift assay, protein-DNA complexes were incubated with 2 μg of antibody Sp-1 (Santa Cruz Biotechnology, Santa Cruz, CA) for 16 hours at 4°C before electrophoresis. Labeled oligonucleotides were incubated with nuclear lysates for 20 minutes at room temperature and separated by electrophoresis on a 6% nondenaturing polyacrylamide gel with 0.5-fold TBE running buffer (45 mM Tris; 45 mM boric acid; 1 mM EDTA, pH 8.0). DNA-protein complexes were electroblotted onto a positively charged nylon membrane, probed with an anti–digoxigenin-peroxidase antibody, and developed by chemiluminescence. Controls contained free probe alone or a molar excess of unlabeled probe.
Western-blot analysis
Mononuclear cells of untreated B-CLL patients were isolated from whole blood by centrifugation on a Ficoll/Hypaque gradient and cryopreserved in liquid nitrogen. Cells were thawed, washed twice in ice-cold PBS, and subsequently lysed in ice-cold lysis buffer (0.1 M dithiothreitol [DTT]; 0.3 M benzamidine; 1 mg/mL trypsin inhibitor; 0.5 M EDTA; 1 M Tris, pH 7.5; 150 mM NaCl; 1% NP-40) for 30 minutes. Debris was sedimented by centrifugation for 20 minutes at 13 000g. Protein concentration was determined according to Bradford.21 After mixing with 3× Laemmli buffer and denaturation for 1 minute at 95°C, sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis was performed with the use of equal amounts of the supernatant whole-cell lysate (30 μg), followed by transfer to nitrocellulose filters. After blocking the reaction with 5% milk powder and incubation with the primary antibodies, the nitrocellulose sheet was further incubated with a rabbit or mouse peroxidase–conjugated secondary antibody and developed using an enhanced chemiluminescence system (ECS) according to the manufacturer's instructions (Western Lightning, PerkinElmer Life Sciences, Shelton, CT). To detect different proteins using the same blot, the blot was stripped twice for 30 minutes in strip buffer (0.2 M glycine, 0.1% SDS, 10 mL Tween 20 in 1 L distilled water.) at room temperature. Thereafter, the blot was washed 3 times and subsequently blocked and probed with primary antibody. Films were scanned and densitometry was used to quantify the immunoblot signals (NIH Image; Scion, Frederick, MD). To compare protein expression between the different genotypes, the average intensity of the signal was multiplied by the number of pixels in that area and normalized to actin expression. The following antibodies were used: rabbit polyclonal anti-BCL2 antibody (Santa Cruz Biotechnology), mouse monoclonal antiactin antibody (Chemicon International, Temecula, CA), rabbit peroxidase–conjugated secondary antibody (Sigma), and mouse peroxidase–conjugated secondary antibody (Sigma).
Determination of BCL2−938 genotypes
Genomic DNA was extracted using the QIAamp blood kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. Genotypes for the −938C>A polymorphism were determined by the “slowdown” PCR, which allows the amplification of GC-rich targets.22 The following primers were used: forward primer, 5′-CAGCAGCTTTTCGGAAAATG-3′; and biotinylated reverse primer, 5′-TATCCACGGGACCGCTTCAC-3′. The slowdown PCR was performed using Eppendorf Taq PCR Mastermix (Eppendorf, Hamburg, Germany) by 48 cycles with 30 seconds denaturation at 95°C, 30 seconds annealing with a progressively lowered temperature from 70°C to 53°C at a rate of 1°C every third cycle, and a primer extension of 40 seconds followed by 15 additional cycles with an annealing temperature of 58°C. Slowdown PCR was used with a generally reduced ramp rate at 2.5°C and an especially small cooling rate for reaching annealing temperature at 1.5°C.
The 134-bp PCR products were analyzed by pyrosequencing using the primer 5′-TCCCCGGCTCCTTCATCGTC-3′ on the PSQ96 system according to the manufacturer's instructions (Biotage, Uppsala, Sweden). Results were analyzed using PSQ96 SNP software.
Detection of genomic aberrations by fluorescence in situ hybridization
In order to detect prognostically relevant anomalies of chromosomal regions 11q, 13q, 17p, and of chromosome 12, the following fluorescent-labeled DNA probes were used in interphase cytogenetic analyses: LSI ATM (11q23), LSI D13S319 (13q14), LSI p53 (17p13.1), cep12 (centromere 12; all probes purchased from Abbot Vysis, Wiesbaden, Germany). Sample preparation, fluorescence in situ hybridization (FISH), counterstaining with 4,6-diamidino-2-phenylindole dihydrochloride, and analysis were performed as reported.23,24
Statistical analysis
We used Kaplan-Meier survival analysis and the log-rank test to investigate the prognostic importance of the SNP in the BCL2 gene. The time to first therapy was calculated from the date of first diagnosis until first therapy. Overall survival was calculated from the date of first diagnosis to confirmed death from B-CLL. Comparison of clinical and laboratory parameters between patient subgroups was performed using Mann-Whitney U test for continuous variables and the χ2 test for categoric data. We used the Wilcoxon matched pairs test to investigate the statistical difference between the 2 different allele-dependent reporter activities. To test whether reporter activities were significantly different from one (ie, the basic vector activity), we applied the Wilcoxon signed rank test. To test for genotype-dependent differences in protein expression (Western-blot analysis), we performed pairwise comparisons using Student t test. In addition, assuming a gene-dose effect on protein expression, we used the linear analysis of variance (ANOVA) test. Differences were regarded significant at P values less than .05.
To study the prognostic influence for a first-line therapy, a multivariable forward stepwise regression analysis (Cox proportional hazards model) was performed. The results were analyzed using SPSS for Windows 11.0 (SPSS, Chicago, IL).
Results
Effects of the BCL2−938C>A polymorphism on promoter activity, binding of nuclear proteins, and Bcl-2 protein expression in B-CLL cells
To determine a potential impact of the BCL2−938C>A polymorphism upon promoter activity, we cloned fragments of the known BCL2 promoter region encompassing nt −690 to −1001 with regard to the translation initiation site and that carried either the C allele or the A allele into the pSEAP-basic reporter vector (Figure 1A). We pairwise transfected these constructs into otherwise identical batches of HEK293 cells, quantified secreted alkaline phosphatase, and corrected these values for transfection efficacy (Figure 1B). It can be seen that reporter activity was variable, but in 9 independently conducted paired experiments the activity associated with the C allele was always higher than that of the A allele. Normalized reporter activity increased by a factor of 3.2 ± 2.8 (mean ± SD) over baseline upon transfection of the BCL2 construct with the C allele, whereas the construct with the A allele increased reporter activity by only a factor of 2.3 ± 2.0 (mean ± SD; P = .004; Wilcoxon matched pairs test). Thus, in this assay the C allele was associated with a significantly higher activity, approximately 40%, compared with the A allele. To confirm these results we repeated these experiments in Karpas 422 cells, a human follicular B-cell line (Figure 1C), using a different vector (pGL3) and reporter assay based on the luciferase system. In these experiments, reporter activity of the A allele was not significantly different from baseline (1.1 ± 0.19; mean ± SD; P = .22, Wilcoxon signed rank test). In contrast, we measured a significant activity associated with the C allele (1.3 ± 0.11; mean ± SD) that was statistically significantly different from baseline (P = .004; 1-sample t test) and significantly higher than that of the A allele (P = .03; Wilcoxon matched pairs test). Collectively, these data suggested an increased activity of the inhibitory P2 promoter associated with the C allele. This is suggestive of an overall reduced transcriptional activity of the BCL2 promoter in the presence of a C allele.
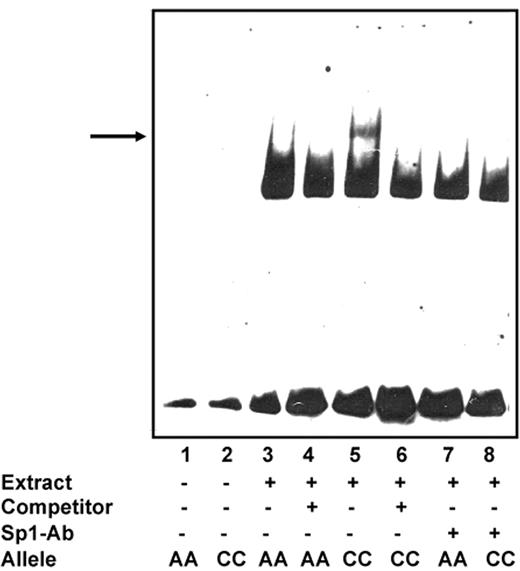
Because these differences in promoter activity suggested potentially different binding of transcription factors to the C and A alleles, respectively, we subsequently compared differential DNA-protein binding by means of electrophoretic mobility shift assays (EMSAs). Results are displayed in Figure 2 When oligonucleotides were incubated with nuclear proteins from Karpas 422 cells, we observed a significantly stronger band indicative of DNA-protein binding for the C allele compared with the A allele. Specificity was confirmed through experiments in which a 100-fold molar excess of unlabeled nucleotide competed these bands away. Addition of an Sp-1 antibody competed this band almost completely away, indicating that this band was specific for Sp-1 binding sites. Missing a supershift is allegeable with the increased detection limit (lower sensitivity) of the digoxigenin (DIG)–labeled double-stranded oligonucleotides compared with radioactive oligonucleotides, and has been described previously.25
Genotype-dependent binding of nuclear proteins to the human BCL2 promoter. Electrophoretic mobility shift assays (EMSAs) were performed using nuclear extracts (10 μg) from human follicular lymphoma cells (Karpas 422). Nuclear extracts were incubated with DIG-labeled oligonucleotides. Assays were performed in the absence (−) or presence (+) of 100-fold molar excess of unlabeled oligonucleotide. Arrow indicates a specifically retarded band observed upon addition of nuclear extracts from Karpas cells. These extracts contain nuclear proteins that specifically bind to the construct with the BCL2−938C allele compared with the BCL2−938A allele. Moreover, addition of an Sp-1 antibody competed this band almost completely away, suggesting that this band was specific for Sp-1 binding sites.
Genotype-dependent binding of nuclear proteins to the human BCL2 promoter. Electrophoretic mobility shift assays (EMSAs) were performed using nuclear extracts (10 μg) from human follicular lymphoma cells (Karpas 422). Nuclear extracts were incubated with DIG-labeled oligonucleotides. Assays were performed in the absence (−) or presence (+) of 100-fold molar excess of unlabeled oligonucleotide. Arrow indicates a specifically retarded band observed upon addition of nuclear extracts from Karpas cells. These extracts contain nuclear proteins that specifically bind to the construct with the BCL2−938C allele compared with the BCL2−938A allele. Moreover, addition of an Sp-1 antibody competed this band almost completely away, suggesting that this band was specific for Sp-1 binding sites.
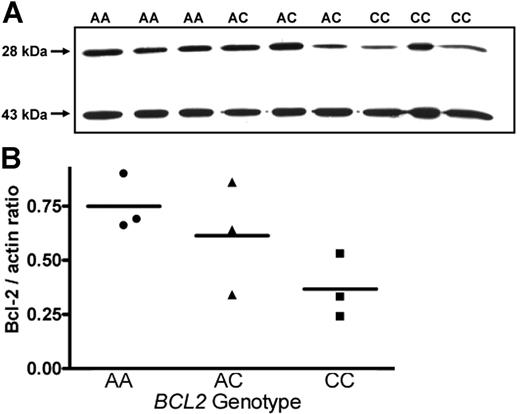
One consequence of the increased activity of the inhibitory BCL2 promoter associated with the C allele should be a concomitant reduction in Bcl-2 protein expression in cells from C-allele carriers. To confirm this hypothesis, we investigated potential genotype-dependent Bcl-2 protein expression in B lymphocytes from patients with B-CLL. As chemotherapeutic agents may down-regulate Bcl-2 expression,26,27 samples were exclusively taken from patients who had never received antileukemic therapy. As shown in Figure 3A, expression of Bcl-2 in cells from 3 patients with −938AA genotype was increased compared with that in cells from 3 patients with CC genotypes. Protein expression associated with the AC genotype was somewhat more variable, showing low expression in 1 specimen comparable to that seen in CC genotypes, intermediate expression in another sample, and higher expression in 1 sample resembling the AA genotype. To correct for variations in total protein, blots were also probed with an antiactin antibody. After densitometry of Bcl-2– and actin-specific bands, the ratios of Bcl-2/actin were calculated and associated with BCL2 genotypes (Figure 3B). The ratios BCL2/actin were almost 2-fold higher comparing AA (0.75 ± 0.13) with CC genotypes (0.36 ± 0.14; P = .028; Student t test), whereas intermediate ratios were found associated with the AC genotype (0.61 ± 0.16). Assuming a gene-dose effect, expression levels across genotypes were also analyzed by linear ANOVA and found to be borderline significantly different (P = .048). Thus, an increased activity of the inhibitory P2 promoter appears to result in an accordingly reduced bcl-2 protein expression associated with the CC genotype.
Genotype-dependent expression of Bcl-2 protein in B cells from CLL patients. (A) Western-blot analysis of B cells from patients with genotype BCL2−938AA showed increased expression of Bcl-2 protein (28 kDa) compared with those from patients with BCL2−938AC and BCL2−938CC genotypes. Actin (43 kDa) was used as a standard to allow for normalization to differential protein loading. (B) Quantitative analysis of protein expression shown in panel A. Densitometry was performed using Scion Image statistical analysis, as described in “Western-blot analysis.” Statistical analysis was performed using the linear ANOVA test. Horizontal bars represent the mean value of the three values of each genotype.
Genotype-dependent expression of Bcl-2 protein in B cells from CLL patients. (A) Western-blot analysis of B cells from patients with genotype BCL2−938AA showed increased expression of Bcl-2 protein (28 kDa) compared with those from patients with BCL2−938AC and BCL2−938CC genotypes. Actin (43 kDa) was used as a standard to allow for normalization to differential protein loading. (B) Quantitative analysis of protein expression shown in panel A. Densitometry was performed using Scion Image statistical analysis, as described in “Western-blot analysis.” Statistical analysis was performed using the linear ANOVA test. Horizontal bars represent the mean value of the three values of each genotype.
BCL2–938C>A genotype distributions in cases and controls
We analyzed 123 white patients with B-CLL and 120 white, healthy blood donors for the BCL2−938A>C polymorphism. The genotype distributions in B-CLL patients (42 AA, 55 AC, 26 CC) and in healthy controls (36 AA, 63 AC, 21 CC) were in accordance with Hardy-Weinberg equilibrium. As both genotype distributions and allele frequencies were not significantly different in patients (A-allele frequency: 0.57) and controls (A-allele frequency: 0.56), there is no evidence for an association of BCL2 938 genotypes with an increased risk to develop B-CLL.
Correlation of the BCL2−938C>A polymorphism with clinical and laboratory data in CLL patients
Clinical and laboratory data of all patients and upon stratification by BCL2 genotypes are displayed in Table 1. We found no association with sex or age at first diagnosis. Moreover, genotypes were similarly distributed among different Binet stages, in CD38+ and CD38− patients, and in patients with ZAP-70–positive or –negative disease.
Correlation of BCL2−938C>A polymorphism with time to first treatment
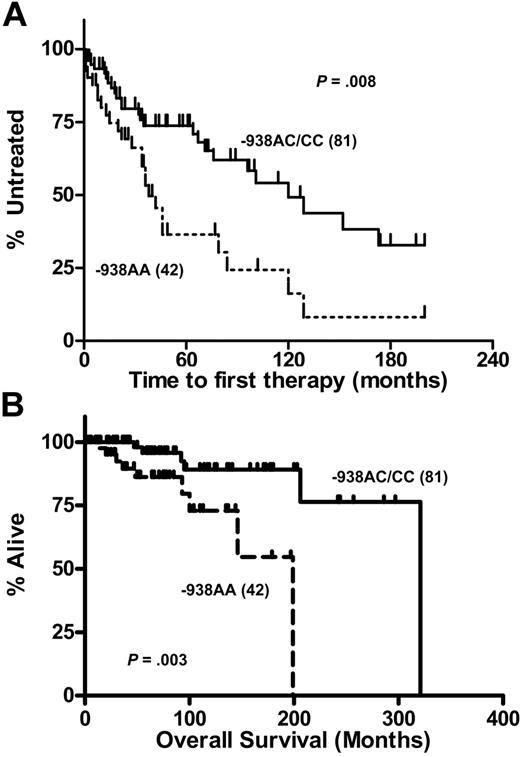
Subsequently we investigated BCL2 genotype-dependent differences in disease progression as indicated by different times to first therapy. Analysis of Kaplan-Meier estimates in which AA, AC, and CC genotypes were regarded separately yielded an overall significant difference (P = .028) for time to first therapy. However, since the curves for AC and CC genotypes were identical (not shown), we pooled C-allele carriers and compared them with patients carrying the AA genotype (Figure 4A) The median time to first therapy was significantly longer in the patients with the BCL2−938CC or AC genotype (120 months) compared with patients with AA genotype (38 months, P = .008).
Genotype-dependent disease progression in patients with B-CLL. Probability of disease progression as indicated by the time to first therapy (A) and overall survival (B) in 123 CLL patients displaying the BCL2−938CC or AC genotypes (n = 81) versus BCL2−938AA (n = 42) genotypes. Statistical analysis was performed using the log-rank test.
Genotype-dependent disease progression in patients with B-CLL. Probability of disease progression as indicated by the time to first therapy (A) and overall survival (B) in 123 CLL patients displaying the BCL2−938CC or AC genotypes (n = 81) versus BCL2−938AA (n = 42) genotypes. Statistical analysis was performed using the log-rank test.
Thus, reduced expression of Bcl-2 protein associated with the CC and eventually AC genotypes appears to be associated with an increased time to first therapy interval in B-CLL. During the period of observation, a total of 56 patients (46%) ultimately required first-line chemotherapy: 9 (35%) with BCL2−938CC genotype, 21 (38%) in the heterozygous group, and 26 (62%) in the group of patients with BCL2−938AA genotype.
Correlation of BCL2−938C>A polymorphism with overall survival
Fifteen (83%) of the 18 deaths were attributable to B-CLL (disease progression, infection). One patient died of an additional acute myelogenous leukemia (AC genotype), 1 patient of an unnatural death (CC genotype), and the other patient suffered from a hepatic metastatic urothelial carcinoma 24 years after first diagnosis of CLL (AC genotype). No patient died of lethal toxicities of therapy for CLL. Because of these 3 leukemia-independent deaths, we conducted a loss–to–follow-up analysis.
The median survival time for the entire group was 267 months. The median survival times from the date of diagnosis for BCL2−938C allele carriers was 321 months and for BCL2−938AA genotypes, 199 months (P = .003; Figure 4B). A total of 18 of 123 patients had died after a median follow-up of 63 months, whereas the median overall survival for the remaining CLL patients was 92 months.
Multivariable analysis for disease progression
In order to test the established prognostic factors and the novel factor BCL2 genotype for independency, the BCL2−938AA genotype, age (cutoff level: 60 years), Binet stage (A versus B versus C), CD38 status, and ZAP-70 status (cutoff level: 20% positive cells) were investigated in multivariable forward stepwise regression analysis using Cox proportional hazards model. In addition to Binet stage and ZAP-70 expression, the BCL2−938AA genotype was identified as an independent factor predicting disease progression as indicated by the time to first therapy (Table 2) The hazard ratio for CLL patients with the BCL2−938AA genotype receiving first-line therapy was nearly 2 compared with BCL2−938AC or CC patients (HR 1.9; P = .034). Only expression of the tyrosine kinase ZAP-70, known as one of the most predictive markers, and the stage at first diagnosis were associated with higher hazard ratios.
Stepwise forward multivariable Cox regression analysis model for the time to first treatment in 104 CLL patients
| Variable . | Hazard ratio . | CI (95%) . | P . |
|---|---|---|---|
| Step 1 | |||
| Disease stage at diagnosis | 3.26 | 1.8–5.8 | <.001 |
| Step 2 | |||
| Disease stage at diagnosis | 2.95 | 1.7–5.3 | <.001 |
| ZAP-70 status | 3.09 | 1.7–5.8 | <.001 |
| Step 3 | |||
| Disease stage at diagnosis | 2.46 | 1.3–4.5 | .004 |
| ZAP-70 status | 3.03 | 1.6–5.7 | .001 |
| BCL2 status, −938AA vs AC/CC | 1.90 | 1.1–3.5 | .034 |
| Variable . | Hazard ratio . | CI (95%) . | P . |
|---|---|---|---|
| Step 1 | |||
| Disease stage at diagnosis | 3.26 | 1.8–5.8 | <.001 |
| Step 2 | |||
| Disease stage at diagnosis | 2.95 | 1.7–5.3 | <.001 |
| ZAP-70 status | 3.09 | 1.7–5.8 | <.001 |
| Step 3 | |||
| Disease stage at diagnosis | 2.46 | 1.3–4.5 | .004 |
| ZAP-70 status | 3.03 | 1.6–5.7 | .001 |
| BCL2 status, −938AA vs AC/CC | 1.90 | 1.1–3.5 | .034 |
The cutoff levels used in the analysis were as follows: age, 60 years; disease stage at diagnosis, Binet stage A versus B and C; ZAP-70 or CD38 status, 20% positive cells. Because of missing data in a substantial proportion of patients, the prognostic marker thymidine kinase and genomic aberrations (Table 1) were not included in the analysis.
CI indicates confidence interval.
Because of missing data in a substantial proportion of patients, the prognostic marker “genomic aberrations” (Table 1) was not included in this analysis. When the genomic aberrations, divided into groups (good risk, deletion 13q; intermediate risk, normal, and trisomy 12; poor risk, deletion 17p, and del 11q) were included in multivariable analysis (79 patients), BCL2 genotype was next to the ZAP-70 status (HR 3.5; P < .001) as the best independent prognostic marker (HR 2.6; P = .004). Thus, genomic aberrations did not relevantly change the impact of the genotype status.
Multivariable Cox-regression analysis for overall survival revealed that ZAP-70 status (HR 4.8; P = .014) was the only independent factor. However, the BCL2−938AA was the next best factor with a trend for significance (HR 2.8; P = .06).
Discussion
Genetic host factors may influence not only the risk of developing B-CLL but also the natural course of this disorder. Recent efforts have identified polymorphisms in drug-metabolizing genes, including the glutathione S-transferase genes, which modulate the risk of B-CLL.28 Genetic variations in other pathways, which, eg, contribute to DNA repair or regulate apoptosis, may also modulate the prognosis of B-CLL. Starczynski et al29 showed an association of a novel polymorphism in the promoter region of the BAX gene (−248G>A) with treatment and overall survival in patients with B-CLL. Recently, we associated the 2 common genetic MTHFR polymorphisms (677C>T and 1298A>C) with progression-free survival and different spontaneous apoptosis rate of B-CLL cells in vitro.30 We also showed an association of the CC genotype of the 825C>T polymorphism of the gene GNB3, which encodes the β3 subunit of heterotrimeric G proteins, with a high relapse rate in patients with CLL.31
Here we show for the first time that a common −938C>A polymorphism has a statistically significant impact on transcriptional activity of a region of the BCL2 gene that inhibits promoter activity. EMSA experiments revealed differential binding of nuclear extracts to the C allele and the A allele that may underlie or contribute to the observed changes in promoter activity. It is well established that the 5′ regulatory sequences of genes harbor binding sites for transcription factors that control constitutive gene expression. We therefore examined the putative binding sites in the BCL2 promoter region containing the −938C>A polymorphism. AliBaba2.1 (an online database)35 analysis predicted BCL2−938 to lie within recognition sequences for an important transcription factor. The BCL2−938C allele contains a putative binding site for Sp-1, which plays an important role in the transcription of numerous genes and which is abolished in the BCL2−938A allele. Addition of an Sp-1 antibody competed this band almost completely away, suggesting that this band could be specific for Sp-1 binding sites. Subsequently, we could confirm genotype-dependent Bcl-2 protein expression in B cells from treatment-naive CLL patients. Cells from patients homozygous for the 938A allele displayed an increased Bcl-2 protein expression compared with those from patients with −938CC genotypes. This may appear counterintuitive at first glance, as reporter activity was highest in constructs with the C allele. As mentioned in “Introduction,” 2 promoters, P1 and P2, control Bcl-2 transcription. The predominant promoter, P1, plays a significant role in the control of Bcl-2 expression, whereas the second promoter, P2, decreases expression mediated by the BCL2 P1 promoter and acts as a negative regulatory element in the BCL2 5′-untranslated region. Transfection analysis of P1 and P2 constructs in DHL9 cells have already confirmed this negative control of transcription by the second promoter, P2.11,32 The novel regulatory polymorphism BCL2−938C>A is located within this second promoter. This may suggest that the significantly higher reporter activity of the C allele may more efficiently decrease activation of the predominant promoter, P1. Therefore, reduced expression of the Bcl-2 protein associated with the C allele in comparison with the A allele appears sensible.
As increased Bcl-2 expression in cells from CLL patients has been frequently observed and since high Bcl-2 expression correlates with unfavorable prognosis and disease stage as well as resistance to chemotherapy,1,10,33 we investigated both association with CLL and relation to disease progression and overall survival. While genotypes showed no association with an increased risk for B-CLL if compared with healthy controls, we found a significantly more aggressive disease analyzing the time to first treatment and overall survival in patients with BCL2−938AA genotypes compared with those with BCL2−938AC or CC genotypes (Figure 4A-B).
After having confirmed the prognostic value of the ZAP-70 status as well as the stage of disease at time of diagnosis in our cohort, multivariable analysis revealed the BCL2−938C>A polymorphism to be an independent prognostic marker for time to first treatment. The hazard ratio for CLL patients with the BCL2−938AA genotype receiving first-line therapy was nearly 2 compared with BCL2−938AC or CC patients (Table 2).
The fact that this genetic host factor plays a role especially in the favorable prognostic group of B-CLL patients (CD38− and ZAP-70 negative) is in line with the hypothesis that such genetic polymorphisms influence the course of disease mainly in localized and not advanced stages (data not shown; P = .040 and P = .032, respectively).
To the best of our knowledge, this is the first report demonstrating the influence of a novel regulatory polymorphism in the BCL2 gene upon the progression of B-CLL, which, of course, requires confirmation from independent studies. On the other hand, the results presented here appear plausible with regard to differences in promoter activity, binding of nuclear proteins, protein level depending on genotype, and the known impact of Bcl-2 on disease progression and overall survival. Thus, our report exceeds other genetic association studies in that a mechanistic link between genotype and observed phenotype can be proposed.
The novel BCL2 antisense oligonucleotide (oblimersen) shows promising effects in clinical trials by increasing the complete remission rates in combination with fludarabine and cyclophosphamide, which again confirms the important role of the Bcl-2 protein in B-CLL.33,34 It would, therefore, be interesting to investigate whether this polymorphism could also serve as a marker to predict responders/nonresponders to the BCL2 antisense therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: H.N., U.H.F., U.D., and W.S. designed research; H.N., U.H.F., M.B., L.S., J.D., and J.S. performed research; and H.N., U.H.F., K.-H.J., and W.S. analyzed data.
Acknowledgments
This work was supported by the Interne Forschungsförderung Essen (IFORES) program of the University of Essen (H.N., L.S.) and the Deutsche Forschungsgemeinschaft (Ku 1315/2-2; J.S.). We are indebted to numerous colleagues for generously contributing information on the clinical course and treatment histories of the study patients. This paper is dedicated to Professor Günter Brittinger on the occasion of his 75th birthday.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal