Abstract
Two unusual but similar cases of very high serum iron, extremely high transferrin concentrations (5.4 to 6.5 g/L), and increased transferrin saturation are reported. No anemia or microcytosis was observed. The ferritin concentration remained within the normal range, and no iron overload was observed. In one case, the in vivo half-life of 59Fe-labeled transferrin was shown to be prolonged (206 minutes versus 75 minutes for controls). In both patients, a monoclonal IgG was observed in the serum. The association between serum transferrin and monoclonal IgG was demonstrated by Western blot analysis and affinity chromatography. We suggest that the transferrin bound to the monoclonal IgG molecule has a prolonged half-life in the circulation, leading to high transferrin concentrations, and that the increased serum iron values prevent the onset of anemia. The antitransferrin activity of monoclonal antibody should be added to the list of situations accounting for elevated serum iron with elevated transferrin saturation.
Introduction
Plasma iron is normally bound to transferrin and taken up by most tissues by interaction with specific membranebound receptors present on the cell surface. Erythroid precursors of the bone marrow have a high density of these receptors and rely entirely on the transferrin uptake pathway for iron acquisition and heme synthesis.1 About 20 to 25 mg of iron is recycled every day by tissue macrophages following destruction of senescent red blood cells, bound to plasma transferrin, and delivered to the bone marrow. Impairment of this recycling as can be seen in inflammatory conditions invariably leads to the onset of anemia.2
Transferrin is mostly synthesized by hepatocytes and to a lesser extent in testis and brain. Plasma transferrin levels are increased following iron deficiency and reduced in hemochromatosis.3 Although transferrin exhibits substantial molecular variability, the effects of transferrin variants on iron metabolism in the body are absent or minor.4-6
In this paper, we report 2 unusual cases of acquired hypersideremia without iron overload due to the presence of a monoclonal antitransferrin immunoglobulin.
Case 1
A 55-year-old woman was referred to us in 1997 on suspicion of hemochromatosis based on the repeated observations of unusually high serum iron and transferrin saturation (Table 1). There was no family history or any clinical symptoms. Serum ferritin levels were normal, as was HFE genotyping. The only biologic abnormality was a monoclonal IgG2-κ at 14.6 g/L. Bone marrow aspiration was normal for all lineages. Perls staining showed a normal number of sideroblasts and normal iron deposits in the macrophages. The diagnosis was made of a monoclonal gammopathy of unknown significance (MGUS). After a 4-year follow-up, serum iron slowly returned to a near normal level, as did transferrin saturation. There was a simultaneous reduction of the IgG2-κ levels to 4g/L, suggesting that there might be a relationship between the abnormal serum iron indices and the gammopathy.
Serum iron indices in the 2 patients
| . | Serum iron level,μM . | Transferrin level,g/L . | TIBC,μM . | % Tf saturation . | Ferritin level,μg/L . | IgG-κ level,g/L . | Hb level,g/L . |
|---|---|---|---|---|---|---|---|
| Patient 1* | 120 | 5.4 | 135 | 89 | 55 | 14.6 | 141 |
| Patient 1† | 36.2 | 3.36 | 84 | 43 | 93 | 4 | ND |
| Patient 2 | 145 | 6.38 | 159 | 91 | 200 | 8.4 | 155 |
| Normal range | 12-25 | 2-3.5 | 43-90 | 30-40 | 50-250 | NA | 110-150 |
| . | Serum iron level,μM . | Transferrin level,g/L . | TIBC,μM . | % Tf saturation . | Ferritin level,μg/L . | IgG-κ level,g/L . | Hb level,g/L . |
|---|---|---|---|---|---|---|---|
| Patient 1* | 120 | 5.4 | 135 | 89 | 55 | 14.6 | 141 |
| Patient 1† | 36.2 | 3.36 | 84 | 43 | 93 | 4 | ND |
| Patient 2 | 145 | 6.38 | 159 | 91 | 200 | 8.4 | 155 |
| Normal range | 12-25 | 2-3.5 | 43-90 | 30-40 | 50-250 | NA | 110-150 |
TIBC indicates total iron-binding capacity; Tf, transferrin; Hb, hemoglobin; ND, not done; and NA, not applicable.
1997.
2001.
Case 2
A healthy 53-year-old man consulted a general practitioner for a routine medical checkup. He had no specific complaints and no recurrent infections. Initial laboratory examination revealed extremely elevated serum iron and transferrin concentration (Table 1), with normal serum ferritin concentration. Further investigations revealed the presence of a monoclonal immunoglobulin IgG-κ at 19.8 g/L. Blood count and red cell indices were normal. Bone marrow aspiration showed a normal plasmocyte count. X-ray examination of the total skeleton revealed no osteolytic lesions, and abdominal ultrasound examination found no evidence of liver cirrhosis. Based on these findings, a diagnosis of MGUS is put forward.
The institutional review board approval was not required because all the clinical and biologic tests performed were performed for the benefit of the patient and not for research purposes. Both patients signed informed consent for the studies.
Patients, materials, and methods
Protein A affinity chromatography
Serum samples were applied on a column packed with protein A–Sepharose CL-4B (Amersham Biosciences, Roosendaal, The Netherlands). Non-IgG constituents were eluted with phosphate-buffered saline (0.1 M, pH 7.0), and IgG was eluted using 0.5 mL fractions of 0.1 M citric acid, pH 3, for biochemical analysis or 1 mL of 0.1 M glycine, pH 2.7, for Western blot analysis. After elution the fractions were neutralized to pH 7.4. Transferrin, iron, IgG, and κ-chains concentrations were measured in the different fractions using routine assays, or the protein content was measured using the optical density at 280 nm.
Western blot
Protein A–purified proteins and diluted serum from patients or control were analyzed by Western blotting with polyclonal peroxidase-labeled goat antibodies (Rockland, Gilbertsville, PA) directed against either human IgG (diluted 1:50 000) or human transferrin (diluted 1:4000). Human holotransferrin (Sigma, St Quentin Fallavier, France) was run in parallel as a control.
In vivo iron kinetics
Plasma labeling was performed by incubating 8.5 mL of the patient's plasma with 7.4 KBq/kg 59Fe-citrate (CIS bio International, Gif/Yvette, France) for 15 minutes at room temperature. A total of 333 KBq was injected at T0, as previously described.7 Radioactivity in the plasma was measured over a 6-hour period using a gamma counter.
Results and discussion
The 2 patients reported here both had unusually elevated serum iron concentrations (5 to 6 times the upper reference concentration) at presentation associated with elevated serum transferrin concentrations, leading to transferrin saturations of about 90% (Table 1) By contrast, serum ferritin values were within the normal range, suggesting the absence of tissue iron overload. There was no sign of anemia, with normal hemoglobin concentrations (140-150 g/L) and mean corpuscular volumes (91 fL) as well as soluble transferrin receptor concentrations within reference range (case 2: 1.57 mg/L; normal, 0.5-1.7 mg/L).
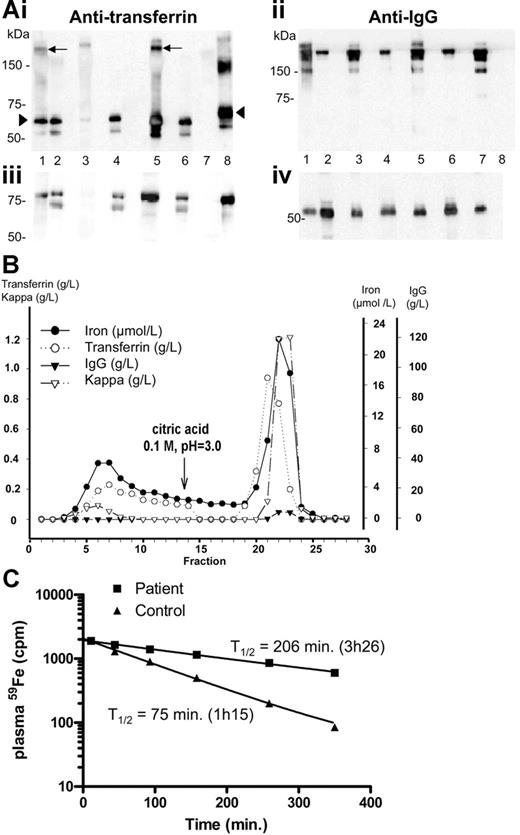
Because both patients had a minor gammopathy and because in case 1 a spontaneous parallel decrease in both the monoclonal component and the transferrin level was observed, we tested the hypothesis that the monoclonal immunoglobulin could have an antitransferrin activity. Indeed, Western blot analysis of the immunoglobulins purified on protein A–Sepharose from both sera revealed that the fraction retained on the column contained both transferrin (Figure 1Ai, lanes 1 and 5) and IgG (Figure 1Aii, lanes 1 and 5), whereas transferrin was absent from the purified fraction from a control serum (Figure 1Ai, lane 3) and from the serum of a myeloma patient (Figure 1Ai, lane 7). These results have also been confirmed by enzyme-linked immunosorbent assay (ELISA) (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). Protein A affinity chromatography of the serum from patient 2 showed that that some transferrin is free and not retained on the column (Figure 1B, fractions 1 to 14), whereas most of it is found in the IgG fractions (acid elution, fractions 14 to 29). Iron coelutes with transferrin in both unbound and bound fractions. When the same experiment was performed on the serum from patient 1, at a stage where the IgG-κ levels were greatly reduced (4 g/L), most transferrin was found in the unbound fraction (not shown). These experiments confirm the presence of IgG-transferrin complexes, together with free transferrin in the serum of both patients. In patient 1, in vivo iron kinetic studies (Figure 1C) demonstrated a prolonged T1/2 of plasma iron (T1/2, 206 minutes; normal, 70 to 110 minutes), a value comparable to what is usually observed in patients with erythroid aplasia. However, because the patient had a 2-fold increase in plasma iron levels (28 μM at the time of the ferrokinetics measurement), it is likely that plasma iron turnover, which is inversely proportional to the plasma T1/2 but directly proportional to plasma iron level,8 was not modified. This result is in agreement with the observation that both patients had normal red cell indices, reflecting sufficient iron-transferrin uptake in the bone marrow to support erythropoiesis. By contrast, a patient described in the literature with autoantibody associated with transferrin had a more drastically increased plasma iron clearance (T1/2, 540 minutes, as opposed to 206 minutes in patient 1), associated with iron overload as shown by liver biopsy and with iron deficiency anemia.9,10 The deficiency in iron supply for erythropoiesis probably triggered enhanced intestinal absorption and onset of iron overload, as it is frequently observed in iron-loading anemias.11 This did not occur in our 2 patients, and differences in the affinity of the monoclonal antibodies against transferrin might account for these phenotypic differences. Taken together, these data highlight the fine-tuning between the concentration of available monoferric or diferric transferrin and intestinal iron absorption. This is in good agreement with a model proposed recently in which changes in transferrin saturation are detected by transferrin receptor 1 on developing erythroid cells or by transferrin receptor 2 on hepatocytes,12 resulting in the regulation of hepcidin gene expression and intestinal iron absorption. It is also noteworthy that transferrin synthesis itself is up-regulated to maintain sufficient non-IgG–bound transferrin for normal iron delivery to developing erythroid cells.
Analysis of the transferrin-IgG complex and ferrokinetics measurements. (A) Identification of an IgG-transferrin complex in the serum of both patients by Western blot. A 5 μL sample of diluted serum (1:1000) or 1 μg of protein A–purified proteins from the 2 patients, from 1 healthy individual, and from 1 IgG-κ myeloma were boiled in 1% SDS loading buffer without (i, ii) or with (iii, iv) 5% β-mercaptoethanol, subjected to 1% SDS–7.5% polyacrylamide gel electrophoresis, and transferred on Hybond ECL Nitrocellulose Membrane (Amersham, Saclay, France). Following blocking with 3% nonfat milk, the membrane was incubated with polyclonal anti–human transferrin (i, iii) or anti–human IgG (ii, iv) antibodies. The purified fraction (lane 1) and the serum (lane 2) of patient 1 were run in parallel with the purified fraction (lane 3) or the serum (lane 4) from a control individual, the purified fraction (lane 5) and the serum (lane 6) of patient 2, and the purified fraction from a monoclonal IgG2 (lane 7). Human transferrin (lane 8) was used as a control. The transferrin-IgG complex is indicated by an arrow and free transferrin by an arrowhead. (B) Protein A chromatography. A 300 μL serum sample from patient 2 was applied on a (5 × 0.5 cm) column packed with protein A–Sepharose 4B and eluted as indicated. IgG, transferrin, and iron were measured in the various fractions. Transferrin was in both unbound (1 to 14) and bound fractions (15 to 29). IgG was only demonstrated in the bound fractions. (C) In vivo plasma clearance of 59Fe. Radioactivity in the plasma was measured at different time intervals following injection of 59Fe-labeled autologous plasma. The reference curve established from the ferrokinetics measurement obtained for 30 healthy controls7 shows that the normal half-life is 75 minutes (range, 70-110 minutes). The half-life of the radioactivity in the patient's plasma was 206 minutes, 2.75 times longer than normal half-life.
Analysis of the transferrin-IgG complex and ferrokinetics measurements. (A) Identification of an IgG-transferrin complex in the serum of both patients by Western blot. A 5 μL sample of diluted serum (1:1000) or 1 μg of protein A–purified proteins from the 2 patients, from 1 healthy individual, and from 1 IgG-κ myeloma were boiled in 1% SDS loading buffer without (i, ii) or with (iii, iv) 5% β-mercaptoethanol, subjected to 1% SDS–7.5% polyacrylamide gel electrophoresis, and transferred on Hybond ECL Nitrocellulose Membrane (Amersham, Saclay, France). Following blocking with 3% nonfat milk, the membrane was incubated with polyclonal anti–human transferrin (i, iii) or anti–human IgG (ii, iv) antibodies. The purified fraction (lane 1) and the serum (lane 2) of patient 1 were run in parallel with the purified fraction (lane 3) or the serum (lane 4) from a control individual, the purified fraction (lane 5) and the serum (lane 6) of patient 2, and the purified fraction from a monoclonal IgG2 (lane 7). Human transferrin (lane 8) was used as a control. The transferrin-IgG complex is indicated by an arrow and free transferrin by an arrowhead. (B) Protein A chromatography. A 300 μL serum sample from patient 2 was applied on a (5 × 0.5 cm) column packed with protein A–Sepharose 4B and eluted as indicated. IgG, transferrin, and iron were measured in the various fractions. Transferrin was in both unbound (1 to 14) and bound fractions (15 to 29). IgG was only demonstrated in the bound fractions. (C) In vivo plasma clearance of 59Fe. Radioactivity in the plasma was measured at different time intervals following injection of 59Fe-labeled autologous plasma. The reference curve established from the ferrokinetics measurement obtained for 30 healthy controls7 shows that the normal half-life is 75 minutes (range, 70-110 minutes). The half-life of the radioactivity in the patient's plasma was 206 minutes, 2.75 times longer than normal half-life.
Although these cases are probably extremely rare, clinicians should look for monoclonal immunoglobulin when facing this peculiar profile of highly elevated serum iron and transferrin saturation contrasting with a normal ferritin level.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: M.A.A., Y.T., M.B., B.S., and P.A. designed and performed in vitro experiments; J.D.R. conducted in vivo ferrokinetics measurements; J.D. and B.V. followed patients, discussed the results, and wrote the paper; and C.B. designed experiments and wrote the paper.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal