In this issue of Blood, Fazi and colleagues demonstrate that ligand-activated transcription via retinoic acid receptor α, which is important for proper myelomonocytic differentiation, is blocked by the AML1/ETO fusion oncoprotein.
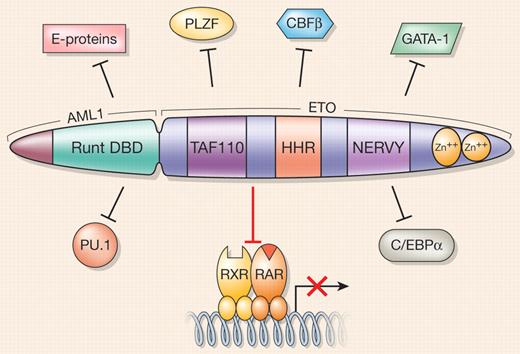
Approximately 15% of all acute myeloid leukemia (AML) cases and up to 40% of those classified by the French-American-British system as M2 subtype harbor the t(8;21) translocation that gives rise to the AML1/ETO fusion oncoprotein (also known as RUNX1/MTG8). AML1/ETO consists of the N-terminal DNA-binding domain of AML1, a transcription factor essential for definitive hematopoiesis, and almost all of ETO, a protein thought to function as a corepressor for a variety of transcription factors. Initially, a simple model of AML1/ETO function in leukemogenesis reflected only its dominant-negative effects on AML1 target genes, in large part via the aberrant recruitment of epigenetic modifiers such as histone deacetylases and DNA methyltransferases. However, phenotypic differences between the AML1-null mouse model and a knockin for AML1/ETO pointed to other activities of this oncoprotein besides deregulation of AML1 target genes. We now know that AML1/ETO impacts multiple processes involved in normal myelomonocytic development where the fusion protein interferes with multiple signal transduction pathways, promoting early myeloid cell self-renewal and interfering with proper hematopoietic differentiation.1 AML1/ETO also directly interferes with recruitment of essential cofactors by a number of crucial hematopoietic transcription factors such as C/EBPα and PU.1, thus potentially blocking their differentiation-promoting functions (see figure).
AML1/ETO promiscuously targets and blocks the activities of RXR/RARα and other hematopoietic transcription factors. Different functional domains of the AML1/ETO fusion protein are indicated as follows: the Runt DNA-binding domain (DBD) of the AML1 moiety; a region that shares homology with TAF110 and other related TAF proteins; a heptad repeat of hydrophobic amino acids (HHR); a region that shares homology with Drosophila nervy protein; and a C-terminal region containing 2 nonclassical zinc fingers. Positions of indicated transcription factors relative to AML1/ETO do not reflect regions of interaction with the fusion protein.
AML1/ETO promiscuously targets and blocks the activities of RXR/RARα and other hematopoietic transcription factors. Different functional domains of the AML1/ETO fusion protein are indicated as follows: the Runt DNA-binding domain (DBD) of the AML1 moiety; a region that shares homology with TAF110 and other related TAF proteins; a heptad repeat of hydrophobic amino acids (HHR); a region that shares homology with Drosophila nervy protein; and a C-terminal region containing 2 nonclassical zinc fingers. Positions of indicated transcription factors relative to AML1/ETO do not reflect regions of interaction with the fusion protein.
In this issue of Blood, Fazi and colleagues demonstrate that another important signaling pathway that positively regulates myelomonocytic differentiation is disrupted by AML1/ETO. They show that the AML1/ETO fusion oncoprotein recruits an array of negatively acting epigenetic factors to a model all-trans-retinoic acid (ATRA) target gene promoter, RARβ2, via direct interaction with retinoic acid receptor alpha (RARα; see figure). Of interest, siRNA knockdown of AML1/ETO relieves repression of the RARβ2 promoter, pointing to a critical role for the fusion protein in silencing RARβ2 in this context. RARα binds its cognate response element as a heterodimer with RXRα, and in the absence of ligand (ATRA) recruits a multiprotein corepressor complex to inhibit transcription. Ligand binding to RARα causes a conformational change leading to exchange of corepressor proteins for coactivator complexes and activation of gene transcription.2 The data presented by Fazi et al indicate that AML1/ETO may interfere directly with RARα function by binding to the receptor in a ligand-independent manner, thus also blocking the ability of ATRA to mediate coregulator exchange and preventing activation of RARα target gene expression. It remains to be seen whether AML1/ETO interacts with other nuclear receptors, but given its positive role in stem/progenitor cell self-renewal, it is interesting to speculate that AML1/ETO may also act upon a closely related RAR family member, RARγ, which has been shown to be critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation.3
So, what is the relevance of these findings for AML therapy? This study further underlines the importance of developing optimized combinatorial therapies that target multiple facets of the biologic activities of a given oncoprotein. In the case of AML1/ETO-associated AML, this study provides a rationale for the use of drugs such as eriocalyxin B and oridonin,4 which specifically degrade AML1/ETO, combined with differentiation therapy (ATRA and myelomonocytic growth factors). Drugs that reverse epigenetic modifications that negatively regulate gene expression, such as histone deacetylase and DNA methylation inhibitors, have been used in combination with ATRA to treat relapsed and therapy-resistant AML with encouraging results.5 This study highlights the possibility that t(8;21)-positive AML patients may represent a particularly suitable target population for this type of epigenetic/differentiation therapy and further investigation in this area is warranted.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal