Abstract
We studied T-cell reconstitution in 31 primary T-cell–immunodeficient patients who had undergone hematopoietic stem-cell transplantation (HSCT) over 10 years previously. In 19 patients, there was no evidence of myeloid chimerism because little or no myeloablation had been performed. Given this context, we sought factors associated with good long-term T-cell reconstitution. We found that all patients having undergone full myeloablation had donor myeloid cells and persistent thymopoiesis, as evidenced by the presence of naive T cells carrying T-cell receptor excision circles (TRECs). In 9 patients with host myeloid chimerism, sustained thymic output was also observed and appeared to be associated with γc deficiency. It is therefore possible that the complete absence of thymic progenitors characterizing this condition created a more favorable environment for thymic seeding by a population of early progenitor cells with the potential for self-renewal, thus enabling long-term (> 10 years) T-cell production.
Introduction
Primary T-cell immunodeficiencies (PIDs) comprise a heterogeneous group of genetic disorders characterized by profound impairment of T-lymphocyte differentiation.1–3 This is always associated with a direct or indirect deficiency in B-cell immunity.1–3 Consequently, in the absence of treatment, patients are highly susceptible to infections caused by microorganisms within the first year of life. In this context, allogeneic hematopoietic stem-cell transplantation (HSCT) constitutes life-saving therapy and is highly successful when a related, HLA-genoidentical donor is available.4 However, in many cases, HLA-identical donors are not available and HSCs from a haploidentical donor must be used. In this situation, the bone marrow inoculum must be strictly T-cell depleted in order to prevent lethal graft-versus-host disease (GvHD). Pretransplantation myeloablation may be used to increase the engraftment rate and to improve T- + B-cell immune reconstitution (particularly in natural killer–positive [NK+] severe combined immunodeficiencies [SCIDs]), though at the cost of increased early toxicity.4–9 T-cell reconstitution and detection of de novo thymopoiesis requires a 3- to 6-month period after haploidentical HSCT. However, there is little published information on the long-term outcomes of HSCT in SCID. Patel et al10 and Sarzotti et al11 have shown that within 10 to 12 years, the thymus' ability to support T-cell development fell to zero following haploidentical transplantation in SCID patients. This was based on quantification of TREC+ cells, reflecting emigration of newly formed T cells from the thymus. It is unclear whether this decline in thymic function is due to the inability of a SCID patient's hypoplastic thymus to sustain long-term thymopoiesis or whether donor stem-cell engraftment was inadequate (and thus prevented the thymus from being further seeded with progenitor cells for 10 or more years)
Thymic output can be assessed in vivo by surface immunophenotyping of CD45RA+ naive T cells in the peripheral blood. However, these cells can proliferate via an antigen-independent pathway and may persist in the circulation for a long time before converting to a memory phenotype.12 In parallel, many investigators have used the episomal DNA circles generated during rearrangement of gene segments encoding the T-cell receptor (TCR) to monitor thymic function following HSCT.13 In order to assess post-HSCT thymic output capacity, we monitored T-cell reconstitution in a cohort of 31 PID patients starting 10 years after HSCT, as assessed by determination of TREC levels and CD31+CD45RA+CD4+ T-cell counts.14 We also analyzed associated factors that could be able to predict the quality of T-cell reconstitution.
Patients, materials, and methods
Patients
Between 1971 and 1995, 88 PID patients underwent allogeneic HSCT in the Paediatric Immunology and Haematology Unit at Necker Hospital in Paris. Fifty-five individuals were still alive at the time of this study and 31 of these (followed up for ≥ 10 years) were evaluated. Patient characteristics are shown in Table 1. Eleven patients had a γc deficiency, 3 had a JAK-3 deficiency, 1 had an IL7-Rα deficiency, 3 had either a Rag-1 (n = 1) or Rag-2 (n = 2) deficiency, 2 had an Artemis deficiency, 5 had Omenn syndrome (caused by Rag-1 deficiency in 4 cases, with the fifth case undetermined), 2 had molecularly undetermined T− B− NK+ SCID, 2 had an HLA class II expression deficiency phenotype, and 2 had an undetermined functional T-cell immunodeficiency (T-cell ID).
Clinical and immunologic characteristics of patients 10 years or more after HSCT
| Group and UPN . | Diagnosis* . | Recipient age at HSCT, mo . | Conditioning regimen, mg/kg† . | T-cell depletion‡ . | Donor . | HLA compatibility§ . | IV Ig . | Years after HSCT . | Myeloid chimerism (%) . | TRECs/105 PBMCs‖ . | CD31+ CD45RA+ CD4+/μL¶ . | CD3/μL# . | CD4/μL** . | CD8/μL†† . | PHA, × 103 cpm . | Tetanus toxoid, × 103 cpm . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | ||||||||||||||||
| 221 | Jak-3 | 11 | Bu 8, Cy 200 | + | Father | 2 | − | 14 | D (100) | 5666 | 222 | 1505 | 794 | 711 | 73 | 7 |
| 216 | Rag-2 | 1 | Bu 16, Cy 200 | + | Mother | 2 | − | 14 | D (100) | 2252 | 415 | 2108 | 1012 | 984 | 76 | 18 |
| 48 | Omenn (Rag-1) | 8 | Bu 16, Cy 200 | + | Father | 2 | − | 22 | D (40) | 2149 | 129 | 1462 | 680 | 748 | 26 | 1 |
| 100 | Omenn (ND) | 6 | 0 | Brother | 1 | − | 16 | D (88) | 916 | 697 | 1778 | 793 | 771 | 152 | 137 | |
| 36 | γc | 6 | 0 | − | Sister | 1 | + | 20 | D (18) | 824 | ND | 1449 | 682 | 733 | 373 | 18 |
| 231 | T-cell ID | 23 | Bu 16, Cy 200 | + | Father | 2 | − | 13 | D (100) | 782 | 108 | 1234 | 542 | 748 | 100 | 19 |
| 264 | T-cell ID | 17 | Bu 20, Cy 200 | + | Father | 2 | − | 12 | D (100) | 693 | 257 | 1541 | 828 | 736 | 88 | 36 |
| 257 | HLA II | 42 | Bu 20, Cy 200 | + | Mother | 2 | − | 13 | D (100) | 606 | 58 | 1890 | 486 | 1323 | 24 | 8 |
| 133 | HLA II | 24 | Bu 16, Cy 200 | − | Brother | 1 | − | 16 | D (100) | 313 | 51 | 1206 | 468 | 702 | 52 | 5 |
| 123 | T−B−NK+ | 6 | 0 | − | Mother | 1 | − | 18 | D (7) | 156 | 92 | 960 | 576 | 396 | 66 | 58 |
| 186 | Omenn (Rag-1) | 5.5 | Bu 16, Cy 200 | + | Mother | 2 | − | 15 | D (100) | 116 | 61 | 780 | 559 | 481 | 29 | 45 |
| 1B | ||||||||||||||||
| 116 | γc | 1 | 0 | − | Brother | 1 | − | 13 | H | 2190 | ND | ND | ND | ND | 67 | 34 |
| 261 | γc | 7 | 0 | − | Mother | 1 | − | 12 | H | 1449 | 235 | 2279 | 735 | 1446 | 76 | 42 |
| 188 | Omenn (Rag-1) | 5 | Bu 8, Cy 200 | − | Mother | 1 | + | 13 | H | 1264 | 249 | 2093 | 1385 | 805 | 71 | 7 |
| 308 | γc | 9 | Bu 8, Cy 200 | + | Father | 2 | + | 11 | H | 712 | 423 | 2079 | 783 | 1134 | 188 | 17 |
| 277 | γc | 9 | 0 | + | Father | 2 | + | 12 | H | 406 | 231 | 5335 | 660 | 4510 | 37 | 5 |
| 348 | γc | 1 | Bu 8, Cy 200 | + | Father | 2 | + | 10 | H | 322 | 95 | 1659 | 378 | 1197 | 75 | 9 |
| 303 | γc | 1 | Bu 8, Cy 200 | + | Father | 2 | − | 10 | H | 259 | 85 | 1519 | 449 | 942 | 74 | 4 |
| 325 | γc | 14 | 0 | + | Father | 2 | + | 10 | H | 220 | 306 | 1566 | 612 | 792 | 65 | 1 |
| 17 | γc | 6 | 0 | − | Aunt | 1 | + | 27 | H | 112 | ND | ND | ND | ND | ND | ND |
| 2 | ||||||||||||||||
| 86 | Rag-2 | 5 | 0 | − | Brother | 1 | − | 16 | D (28) | < 100 | 5 | 800 | 480 | 300 | 73 | 36 |
| 54 | Jak-3 | 13 | Bu 8, Cy 200 | + | Father | 2 | − | 21 | H | < 100 | 54 | 1168 | 318 | 737 | 21 | 6 |
| 97 | Rag-1 | 10 | Bu 8, Cy 200 | + | Father | 2 | + | 17 | H | < 100 | 13 | 925 | 442 | 509 | 44 | 45 |
| 144 | Omenn (Rag-1) | 6 | Bu 8, Cy 200 | − | Brother | 1 | + | 17 | H | < 100 | 5 | 480 | 252 | 216 | 8 | 42 |
| 168 | γc | 2 | Bu 8, Cy 200 | + | Mother | 2 | + | 16 | H | < 100 | 21 | 3564 | 689 | 2633 | 19 | 13 |
| 164 | Artemis | 15 | 0 | + | Mother | 2 | + | 13 | H | < 100 | 44 | 443 | 337 | 155 | 21 | 8 |
| 249 | Artemis | 6.5 | 0 | + | Mother | 2 | + | 13 | H | < 100 | 4 | 2105 | 391 | 1649 | 16 | 3 |
| 51 | γc | 2 | 0 | + | Father | 2 | + | 21 | H | < 100 | 1 | 362 | 114 | 184 | 17 | 11 |
| 57 | Jak-3 | 9 | 0 | + | Father | 2 | − | 20 | H | < 100 | 38 | 882 | 225 | 549 | 29 | 5 |
| 158 | IL-7Rα | 3.5 | Bu 8, Cy 200 | + | Mother | 2 | − | 15 | H | < 100 | 13 | 288 | 132 | 180 | 35 | 16 |
| 201 | T−B−NK++ | 5 | 0 | − | Father | 1 | − | 14 | H | < 100 | 17 | 759 | 418 | 308 | 20 | 17 |
| Group and UPN . | Diagnosis* . | Recipient age at HSCT, mo . | Conditioning regimen, mg/kg† . | T-cell depletion‡ . | Donor . | HLA compatibility§ . | IV Ig . | Years after HSCT . | Myeloid chimerism (%) . | TRECs/105 PBMCs‖ . | CD31+ CD45RA+ CD4+/μL¶ . | CD3/μL# . | CD4/μL** . | CD8/μL†† . | PHA, × 103 cpm . | Tetanus toxoid, × 103 cpm . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | ||||||||||||||||
| 221 | Jak-3 | 11 | Bu 8, Cy 200 | + | Father | 2 | − | 14 | D (100) | 5666 | 222 | 1505 | 794 | 711 | 73 | 7 |
| 216 | Rag-2 | 1 | Bu 16, Cy 200 | + | Mother | 2 | − | 14 | D (100) | 2252 | 415 | 2108 | 1012 | 984 | 76 | 18 |
| 48 | Omenn (Rag-1) | 8 | Bu 16, Cy 200 | + | Father | 2 | − | 22 | D (40) | 2149 | 129 | 1462 | 680 | 748 | 26 | 1 |
| 100 | Omenn (ND) | 6 | 0 | Brother | 1 | − | 16 | D (88) | 916 | 697 | 1778 | 793 | 771 | 152 | 137 | |
| 36 | γc | 6 | 0 | − | Sister | 1 | + | 20 | D (18) | 824 | ND | 1449 | 682 | 733 | 373 | 18 |
| 231 | T-cell ID | 23 | Bu 16, Cy 200 | + | Father | 2 | − | 13 | D (100) | 782 | 108 | 1234 | 542 | 748 | 100 | 19 |
| 264 | T-cell ID | 17 | Bu 20, Cy 200 | + | Father | 2 | − | 12 | D (100) | 693 | 257 | 1541 | 828 | 736 | 88 | 36 |
| 257 | HLA II | 42 | Bu 20, Cy 200 | + | Mother | 2 | − | 13 | D (100) | 606 | 58 | 1890 | 486 | 1323 | 24 | 8 |
| 133 | HLA II | 24 | Bu 16, Cy 200 | − | Brother | 1 | − | 16 | D (100) | 313 | 51 | 1206 | 468 | 702 | 52 | 5 |
| 123 | T−B−NK+ | 6 | 0 | − | Mother | 1 | − | 18 | D (7) | 156 | 92 | 960 | 576 | 396 | 66 | 58 |
| 186 | Omenn (Rag-1) | 5.5 | Bu 16, Cy 200 | + | Mother | 2 | − | 15 | D (100) | 116 | 61 | 780 | 559 | 481 | 29 | 45 |
| 1B | ||||||||||||||||
| 116 | γc | 1 | 0 | − | Brother | 1 | − | 13 | H | 2190 | ND | ND | ND | ND | 67 | 34 |
| 261 | γc | 7 | 0 | − | Mother | 1 | − | 12 | H | 1449 | 235 | 2279 | 735 | 1446 | 76 | 42 |
| 188 | Omenn (Rag-1) | 5 | Bu 8, Cy 200 | − | Mother | 1 | + | 13 | H | 1264 | 249 | 2093 | 1385 | 805 | 71 | 7 |
| 308 | γc | 9 | Bu 8, Cy 200 | + | Father | 2 | + | 11 | H | 712 | 423 | 2079 | 783 | 1134 | 188 | 17 |
| 277 | γc | 9 | 0 | + | Father | 2 | + | 12 | H | 406 | 231 | 5335 | 660 | 4510 | 37 | 5 |
| 348 | γc | 1 | Bu 8, Cy 200 | + | Father | 2 | + | 10 | H | 322 | 95 | 1659 | 378 | 1197 | 75 | 9 |
| 303 | γc | 1 | Bu 8, Cy 200 | + | Father | 2 | − | 10 | H | 259 | 85 | 1519 | 449 | 942 | 74 | 4 |
| 325 | γc | 14 | 0 | + | Father | 2 | + | 10 | H | 220 | 306 | 1566 | 612 | 792 | 65 | 1 |
| 17 | γc | 6 | 0 | − | Aunt | 1 | + | 27 | H | 112 | ND | ND | ND | ND | ND | ND |
| 2 | ||||||||||||||||
| 86 | Rag-2 | 5 | 0 | − | Brother | 1 | − | 16 | D (28) | < 100 | 5 | 800 | 480 | 300 | 73 | 36 |
| 54 | Jak-3 | 13 | Bu 8, Cy 200 | + | Father | 2 | − | 21 | H | < 100 | 54 | 1168 | 318 | 737 | 21 | 6 |
| 97 | Rag-1 | 10 | Bu 8, Cy 200 | + | Father | 2 | + | 17 | H | < 100 | 13 | 925 | 442 | 509 | 44 | 45 |
| 144 | Omenn (Rag-1) | 6 | Bu 8, Cy 200 | − | Brother | 1 | + | 17 | H | < 100 | 5 | 480 | 252 | 216 | 8 | 42 |
| 168 | γc | 2 | Bu 8, Cy 200 | + | Mother | 2 | + | 16 | H | < 100 | 21 | 3564 | 689 | 2633 | 19 | 13 |
| 164 | Artemis | 15 | 0 | + | Mother | 2 | + | 13 | H | < 100 | 44 | 443 | 337 | 155 | 21 | 8 |
| 249 | Artemis | 6.5 | 0 | + | Mother | 2 | + | 13 | H | < 100 | 4 | 2105 | 391 | 1649 | 16 | 3 |
| 51 | γc | 2 | 0 | + | Father | 2 | + | 21 | H | < 100 | 1 | 362 | 114 | 184 | 17 | 11 |
| 57 | Jak-3 | 9 | 0 | + | Father | 2 | − | 20 | H | < 100 | 38 | 882 | 225 | 549 | 29 | 5 |
| 158 | IL-7Rα | 3.5 | Bu 8, Cy 200 | + | Mother | 2 | − | 15 | H | < 100 | 13 | 288 | 132 | 180 | 35 | 16 |
| 201 | T−B−NK++ | 5 | 0 | − | Father | 1 | − | 14 | H | < 100 | 17 | 759 | 418 | 308 | 20 | 17 |
UPN indicates unique patient number; IVIg, immunoglobulin substitution; PBMC indicates peripheral blood mononuclear cell; PHA, phytohemagglutinin A; cpm, counts per minute of3H thymidine incorporation during PBMC proliferation after stimulation with PHA or tetanus toxoid; JAK-3, Janus kinase-3 deficiency; D, donor chimerism as a percentage of donor myeloid cells; Rag −1/−2, recombination-activating gene deficiency; ND, not determined; γc, common gamma chain deficiency; T-cell ID, T-cell immunodeficiency; HLA II, human leukocyte antigen class II deficiency; T−B−NK+, molecularly undetermined T−B−NK+ SCID phenotype; H, host; and IL-7Rα, interleukin 7 receptor α deficiency.
Type of SCID.
Total busulphan dose, in mg/kg of recipient's bodyweight and total cyclophosphamide dose in mg/kg; 0, absence of treatment or cyclophosphamide 200 mg/kg alone.
T-cell depletion of transplant after alemtuzumab treatment, E Rosetting, or positive selection for CD34+ cells.
HLA A, B, and DR antigen compatibility between donor and recipient, where 1 indicates matched and 2 indicates mismatched.
Normal range between 10 and 20 years of age, 150 to 10 000/μL.
Absolute count of CD31+ among CD45RA+CD4+ T lymphocytes per μL of peripheral blood; normal count, 60 to 800/μL.
Absolute CD3+ T lymphocytes counts per μL of peripheral blood; normal range between 10 and 16 years of age, 800 to 3500/μL; and for adults, 700 to 2100/μL.
Absolute counts of CD4+ T lymphocytes per μL of peripheral blood; normal range between 10 and 16 years of age, 400-1200/μL; and for adults, 300 to 1400/μL.
Absolute counts of CD8+ T lymphocytes per μL of peripheral blood; normal range between 10 and 16 years of age, 200-1200/μL; and for adults, 200 to 900/μL.
Eleven patients received an HSC transplant from an HLA-identical donor (with 6 from a genoidentical donor and 5 from a phenoidentical related donor). When patients lacked T cells completely, a nonmanipulated marrow sample was used without a preconditioning regimen. Twenty patients underwent haploidentical HSCT following T-cell depletion in order to prevent GvHD. Seven of the latter individuals received a fully myeloablative conditioning regimen (CR) consisting of busulphan (Bu; a total dose of 16 or 20 mg/kg) and, depending on whether T cells were present, cyclophosphamide (Cy; 200 mg/kg). A second group of 10 patients was conditioned with a lower dose of busulphan (8 mg/kg) in combination with cyclophosphamide (200 mg/kg), in accordance with the European Society for Immunodeficiencies/The European Group for Blood and Marrow Transplantation (ESID/EBMT) guidelines on PID. Bone marrow harvests were depleted of T cells by E-rosetting (n = 13), alemtuzumab antibody treatment (n = 2), or selection of CD34+ cells (n = 5), depending on when HSCT had been performed. Thirteen of the 31 patients had at least grade II acute GvHD (8 with grade II and 5 with grade III). Informed consent for this study was obtained from parents and/or patients, depending on the individual's age, in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review board at the Hospital Necker–Enfants Malades.
Flow-cytometry analysis
Over the period from 2003 to 2005, blood samples were obtained from 31 patients at different time points after HSCT (10 to 27 years). Phenotype analysis of blood lymphocytes was performed using 3-color immunofluorescence flow cytometry. The following conjugated monoclonal antibodies were used: CD3-APC, CD45RA-PE, CD4-FITC or APC, CD8-PE, and CD31-FITC. Data were acquired with a FACSCalibur and analyzed using Cell Quest Software (Becton Dickinson Systems, Heidelberg, Germany). The percentage of CD31+CD45RA+ cells in the CD4+ T-cell population was determined as a guide to the proportion of recent thymic emigrants in the CD4 T-cell subset.14 We decided not to assess naive CD8 T cells by immunophenotyping, as CD31 cannot be considered to be an unequivocal marker of naive CD8 T cells. Results are presented as absolute counts for each analyzed subset (see “T-cell phenotype determination”). Lymphocyte proliferation was determined by the amount of 3H-thymidine incorporated into peripheral-blood mononuclear cells (PBMCs) after stimulation with phytohemagglutinin (PHA) or tetanus toxoid (TT), following an appropriate immunization schedule. Each study was run in parallel with samples from healthy adult controls.
Quantitative analyses of thymic function
PBMCs were obtained using Ficoll density gradient centrifugation on a lymphocyte separation medium (Eurobio, Courtaboeuf, France). Signal joint TRECs (sjTRECs; δRec-ϕjα) were determined using real-time quantitative polymerase chain reaction (PCR), as described previously.15,16 The TREC content was expressed as the number of TREC copies per 1 × 105 PBMCs. The control value (established by assessing 20 healthy individuals) was between 150 and 2500 copies/1 × 105 PBMCs.
Chimerism analysis
CD3+, CD19+, and CD15+ populations were isolated by cell sorting (purity, > 95%). Two methods were used to analyze the chimerism of T and B lymphocytes and monocytes; one method was based on detection of variable number of tandem repeats (VNTR) markers (with a sensitivity of 1%) and the second used fluorescence in situ hybridization (FISH) with PDMX1 Red and pLAY 113.5 green fluorescence probes to detect locus DXZ1 (Xp11.1-q11.1) on the X chromosome and locus DYZ1 (Yq12) on the Y chromosome (with a sensitivity of 5%).
Statistical analysis
Due to the small sample size, we analyzed correlations between TREC values and other continuous variables. Naive CD4 T cells, CD3+ T cells, CD4+ T cells, CD8+ T cells, and mitogen- and antigen-induced proliferation were investigated using Spearman correlation coefficient. Intergroup comparisons of continuous variables (namely host/donor status, busulphan status, and γc status) were performed with the Wilcoxon rank sum test or the Kruskal-Wallis test, depending on the number of groups analyzed. Fisher exact test was used to compare categoric variables. A 2-sided P value of .05 or less was considered to indicate statistical significance. Statistical analyses were conducted with R software (a language and environment for statistical computing), version 2.0.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
T-cell phenotype determination
Absolute counts of CD3, CD4, CD8, and recent thymic emigrants (as defined by naive CD4 T cells expressing CD31) and the results of mitogen- or tetanus toxoid–induced T-cell proliferation are shown in Table 1. In 26 of the 31 patients, T-cell counts were within the age-matched normal range (0.8 × 109/L to 3.5 × 109/L [800-3500/μL]). CD4, CD8, and naive CD31+CD45RA+CD4+ T-cell counts were within the normal range (0.4 × 109/L to 2.1 × 109/L [400-2100/μL]; 0.2 × 109/L to 1.2 × 109/L [200-1200/μL]; 0.06 × 109/L to 0.36 × 109/L [60-360/μL]) in 23, 26, and 18 of the 31 patients, respectively. Mitogen-induced T-cell proliferation within the normal range was found in 30 patients (> 25 000 counts per minute [cpm]). T-cell proliferation in response to tetanus toxoid (TT) was also within the normal range (> 10 000 cpm) in 17 patients and was detectable (> 5000 cpm) in another 10. These data attest to the persistence of functional naive and memory T cells over a 10- to 27-year period after HSCT.
TREC quantification was used to analyze the population of naive T cells that had not undergone cell division in the periphery. TREC+ cell counts were in the normal range in 18 of the 31 patients and were detectable in 2 other individuals. We used a cutoff value of 1 × 102 TRECs/105 PBMCs to distinguish patients with TREC+ cells from those lacking them. The first group consisted of 20 patients (Table 1) and the second comprised 11. A very strong correlation was found between TREC+ cell numbers and the absolute counts of naive CD31+CD45RA+CD4+T cells (Table 1; Figure 1; r = 0.84; P < .001).
Correlation between TREC levels and CD31+CD45RA+CD4+ naive T-cell status. TREC copies/1 × 105 PBMCs (on the y-axis) were plotted against the absolute numbers of CD31+CD45RA+CD4+ (on the x-axis). The chosen cutoff TREC value was 100/1 × 105 PBMCs and the cutoff for CD31+CD45RA+CD4+ naive T-cell value was 0.06 × 109/L [60/μL]. The positive correlation between these 2 markers is significant at P < .001, r = 0.848.
Correlation between TREC levels and CD31+CD45RA+CD4+ naive T-cell status. TREC copies/1 × 105 PBMCs (on the y-axis) were plotted against the absolute numbers of CD31+CD45RA+CD4+ (on the x-axis). The chosen cutoff TREC value was 100/1 × 105 PBMCs and the cutoff for CD31+CD45RA+CD4+ naive T-cell value was 0.06 × 109/L [60/μL]. The positive correlation between these 2 markers is significant at P < .001, r = 0.848.
TREC+ naive T cells in patients with donor myeloid chimerism
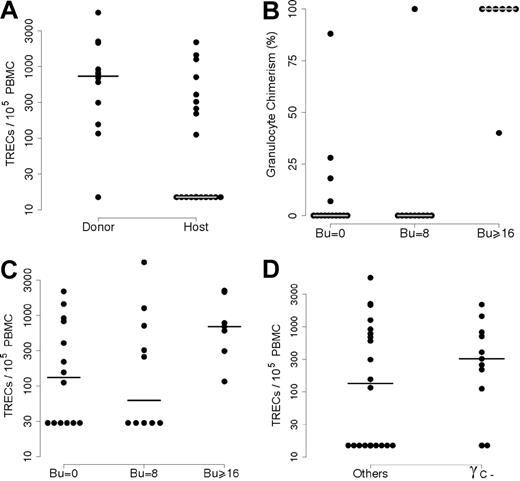
Several parameters were studied in order to discover possible correlations with the presence of TREC+ naive T cells. In order to assess myeloid chimerism, relationships were tested by determining the frequency of donor-derived cells in sorted granulocytes. In all 31 cases, the T-cell population was of donor origin (including those patients who still had host T cells prior to HSCT), whereas B-cell chimerism correlated with myeloid chimerism. This prompted us to split the population with detectable TRECs and naive T cells into 2 subgroups (1A and 1B; Table 1) according to their granulocyte chimerism. In 11 of the 20 patients (subgroup 1A), granulocytes were partly or exclusively of donor origin, whereas they were exclusively of host origin in 9 individuals (subgroup 1B), taking into account the method's sensitivity (see “Patients, materials, and methods”). In contrast, no donor granulocytes could be detected (group 2) in all but 1 patient in whom no TREC+/naive T cells could be detected (n = 11) 10 years or more after HSCT. As shown in Figure 2A, there was a significantly higher mean TREC+ level in patients with donor-derived granulocytes than in individuals with host-derived granulocytes (P = .014).
Correlation between TRECs and either donor myeloid chimerism or diagnosis. (A) Patients were separated into 2 groups according to their myeloid chimerism: group 1 with donor myeloid chimerism and group 2 with host myeloid chimerism. TREC analysis showed significantly higher values in the “Donor” group than in the “Host” group (P = .014). (B) The relationship between myeloid chimerism and the conditioning regimen. Percentages of donor myeloid cells (on the y-axis) were plotted against the busulphan dose (Bu = 0; Bu = 8 mg/kg or Bu > 16 mg/kg). There was a significantly higher number of patients with donor myeloid cells in the group having received full myeloablative treatment (Bu = 16 or 20 mg/kg; P < .001). (C) TREC values (on the y-axis) were analyzed as a function of the intensity of the conditioning regimen (Bu = 0; Bu = 8 mg/kg or Bu > 16 mg/kg). The differences between the 3 groups were not significant (P = .15). (D)l The relationship between TREC+ T-cell status and diagnosis. Patients who did not receive a fully myeloablative CR (n = 24) were separated into 2 groups according to their genetic defect: γc deficiency and others (γc+). The difference between the 2 groups was not significant (P = .06).
Correlation between TRECs and either donor myeloid chimerism or diagnosis. (A) Patients were separated into 2 groups according to their myeloid chimerism: group 1 with donor myeloid chimerism and group 2 with host myeloid chimerism. TREC analysis showed significantly higher values in the “Donor” group than in the “Host” group (P = .014). (B) The relationship between myeloid chimerism and the conditioning regimen. Percentages of donor myeloid cells (on the y-axis) were plotted against the busulphan dose (Bu = 0; Bu = 8 mg/kg or Bu > 16 mg/kg). There was a significantly higher number of patients with donor myeloid cells in the group having received full myeloablative treatment (Bu = 16 or 20 mg/kg; P < .001). (C) TREC values (on the y-axis) were analyzed as a function of the intensity of the conditioning regimen (Bu = 0; Bu = 8 mg/kg or Bu > 16 mg/kg). The differences between the 3 groups were not significant (P = .15). (D)l The relationship between TREC+ T-cell status and diagnosis. Patients who did not receive a fully myeloablative CR (n = 24) were separated into 2 groups according to their genetic defect: γc deficiency and others (γc+). The difference between the 2 groups was not significant (P = .06).
Conversely, the patients lacking donor-derived granulocytes (n = 19) could be separated into 2 groups according to the presence or absence of TREC+ T cells (n = 9 and n = 10, respectively; Table 1). As expected, detection of donor-derived granulocytes was highly correlated with fully myeloablative CR, since all 7 patients who received this treatment had donor granulocytes 10 years or more after HSCT versus just 1 of the 10 who had undergone a partially myeloablative CR (busulphan 8 mg/kg, Cy 200 mg/kg) and 4 of 14 who had not undergone a CR at all (P < .001; Figure 2B). The latter 2 groups did not differ statistically (P = .38). Interestingly, all 7 patients having received a fully myeloablative CR belong to group 1A (ie, individuals with detectable TREC+ naive T cells; Table 1; Figure 2C). In contrast, only 13 of the 24 patients who underwent only partially myeloablative CR or no CR whatsoever had detectable TREC+ cells in their blood. Patient status in terms of myeloablative CR versus partially myeloablative CR had no influence on TREC+ cell counts (P = .97).
Factors potentially influencing the persistence of TREC+ naive T cells
We analyzed several pre-/post-HSCT parameters in an attempt to identify factors influencing the persistence of donor-derived TREC+/naive T cells. It has been shown that SCID patients who receive transplants during the neonatal period exhibit a higher survival rate and a faster T-cell reconstitution than those who received a transplant later.17 We therefore compared the patients' median age at transplantation with their status in terms of the presence or absence of detectable TREC+ T cells (6.5 and 6 months, respectively) but did not find a statistically significant difference. By considering only patients who did not receive a fully myeloablative CR, there was also no detectable difference (data not shown). Equally, the occurrence of either acute and/or chronic GvHD had no influence (data not shown).
The post-HSCT time interval did not significantly influence TREC level determination, since the follow-up period of TREC+ patients lacking donor myeloid chimerism (median, 13 years) was only slightly shorter than that of TREC− patients (median, 16 years).
TREC levels and SCID diagnosis
We next sought to determine whether the presence of NK cells prior to HSCT might influence the persistence of TREC+ naive T cells. In fact, no difference between TREC levels in NK− SCID patients who received transplants (n = 14) versus NK+ patients who received transplants (n = 17) was observed (P = .4). Equally, no difference was found when considering patients who received a haploidentical HSC transplant without myeloablative CR, a setting in which host NK cells could reduce donor cell engraftment (data not shown; P = .4). When analyzing data from patients who did not undergo a fully myeloablative CR (n = 24), we found that TREC+ cells were detected in 9 of 11 patients with a γc deficiency SCID versus only 4 of 13 γc+ SCID individuals (P = .06; Figure 2D). It is particularly striking to note that 8 of the 9 SCID patients who exhibited TREC+ T cells without detectable myeloid chimerism had a γc deficiency (group 1B), whereas only 2 of the 11 individuals in group 2 lacking TREC+ cells and host myeloid cells had a γc deficiency (P < .05). In addition, it is worth noting that although 2 of the 3 patients with JAK-3 deficiency (an immunodeficiency similar to that caused by γc deficiency) belong to group 2, they had a few CD31+CD45RA+CD4+ naive T cells (0.054 × 109/L [54/μL] and 0.038 × 109/L [38/μL], respectively), unlike the other group 2 patients (Table 1).
Correlation between the presence of TREC+ naive T cells and overall T-cell reconstitution
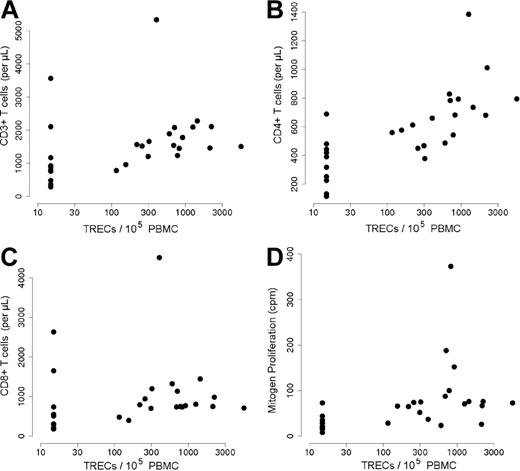
The presence of TREC+/naive T cells was associated with significantly higher T-cell counts (1.8 × 109/L ± 0.969 × 109/L vs 1.133 × 109/L ± 0.997 × 109/L T cells [1800 ± 969 vs 1133 ± 997 T cells/μL], respectively; P = .003; r = 0.55; Figure 3A). Total CD4+ and CD8+ T-cell counts were significantly lower in patients with no detectable TRECs (P < .001, r = 0.79; P = .02, r = 0.43, respectively; Figure 3B,C). Lastly, the T-cell population in patients with low TREC+ cell counts also displayed a lower mitogen-induced proliferative capacity than those in group 1 (P < .001, r = 0.68; Figure 3D), as well as a lower antigen-induced proliferation (even though the difference with group 1 was not statistically significant; P = .82, r = 0.04). However, there was no detectable difference in terms of the frequency of B-cell function reconstitution, as evidenced by IgIV therapy dependence; 7 of the 20 patients with detectable TREC+ cells required IgIV therapy compared with 6 of the 11 TREC− patients (Table 1).
T-cell numbers and function according to TREC+ T-cell status. (A-C) Absolute counts of CD3+ (A), CD4+ (B), and CD8+ (C) (on the y-axis) were plotted against TREC values (copies/1 × 105 PBMCs; on the x-axis). There was a significant correlation between CD3+ T cells and TRECs (P = .003, r = 0.55); CD4+ and TRECs (P < .001, r = 0.79); and CD8+ and TRECs (P = .02, r = 0.43). (D) Counts per minute (cpm) of tritium-labeled thymidine incorporated into proliferating lymphocytes in response to phytohemagglutinin (PHA) stimulation (y-axis) were plotted against the TREC values (copies/1 × 105 PBMCs; x-axis). There was a significant correlation between T-cell proliferation and TREC values (P < .001, r = 0.68).
T-cell numbers and function according to TREC+ T-cell status. (A-C) Absolute counts of CD3+ (A), CD4+ (B), and CD8+ (C) (on the y-axis) were plotted against TREC values (copies/1 × 105 PBMCs; on the x-axis). There was a significant correlation between CD3+ T cells and TRECs (P = .003, r = 0.55); CD4+ and TRECs (P < .001, r = 0.79); and CD8+ and TRECs (P = .02, r = 0.43). (D) Counts per minute (cpm) of tritium-labeled thymidine incorporated into proliferating lymphocytes in response to phytohemagglutinin (PHA) stimulation (y-axis) were plotted against the TREC values (copies/1 × 105 PBMCs; x-axis). There was a significant correlation between T-cell proliferation and TREC values (P < .001, r = 0.68).
Clinical status of patients
Within the group of patients with detectable TREC+ cells, 60% (n = 12) did not present any clinical manifestations, whereas 30% had upper respiratory tract infections (URTIs; 15%, n = 3) or chronic warts (15%, n = 3). Two patients had immunologic complications (autoimmune hemolytic anemia in 1 case and chronic pulmonary disease in the other). In the group of patients lacking detectable TREC+ cells, 45% (n = 5) did not display any clinical manifestations and 38% (n = 4) had infective complications (URTIs in 2 cases, recurrent pneumonitis in 1 case, and severe chronic warts in the other). Two patients in the latter group suffered from chronic immunopathologic conditions, including hepatitis and colitis.
Discussion
We analyzed T-cell immunity in 31 PID patients between 10 to 27 years after HSCT in order to identify parameters determining the long-term correction of T-cell immunodeficiency. We found that the persistence of naive T cells is (i) positively correlated with overall T-cell reconstitution, (ii) mostly associated with myeloid chimerism, and (iii) possibly influenced by diagnosis. Based on TREC levels and naive CD4 T-cell counts, patients were classified into 2 groups. Patients from the group with a high sjTREC content exhibited higher CD3, CD4, and CD8 T-cell counts and greater lymphocyte proliferation in response to mitogen and tetanus toxoid than those displaying low TREC levels. These differences in T-cell phenotype and functional characteristics can probably be attributed to a persistent thymic output. Patel et al10 and Sarzotti et al11 have reported that 10 to 12 years after transplantation in SCID patients, phenotypically naive T-cell and TREC levels fall to very low values and that T-cell diversity decreases concomitantly. Our observation of positive TREC levels in 20 of 31 patients somewhat contrasts with those authors' findings. However, it should be noted that none of patients in the Duke report (Patel et al10 and Sarzotti et al11 ) underwent a myeloablative conditioning regimen before transplantation, whereas this was the case for 7 patients in our series. Our results agree in part with those recently published by Borghans et al,18 indicating that 11 of 19 SCID patients displayed good-quality, long-term T-cell reconstitution after HSCT.
The presence of TREC+ T cells was found not to correlate with a better clinical outcome. It should be emphasized, however, that our patient numbers were perhaps too small to provide this sort of evidence. It is also possible that a better, TREC-associated immune status will only translate into clinical consequences following a longer period of time than that monitored here, especially since memory T cells are long lived. This warrants careful follow-up of patients over an extended period of time. TREC+ T-cell status was also found not to be associated with B-cell function in patients with host myeloid and B-cell chimerism. It is noteworthy that the partially myeloablative CR (Bu = 8 and Cy = 200) underwent by several of the patients was, in most cases, not sufficient to achieve myeloid/B-cell chimerism, as previously reported.19
In our series, TREC+ cell status correlated strongly with myeloid chimerism. In addition, we found a correlation between the use of a myeloablative conditioning regimen and myeloid chimerism. It should be noted that myeloid donor chimerism was detected in 2 patients who did not undergo conditioning, as has occasionally been reported in nonconditioned HSCT for SCID.8 Muller et al9 have reported that intrathymic T-cell maturation kinetics are similar in patients who receive either HLA-identical or haploidentical HSC transplants. Likewise, we did not observe any difference between these 2 types of HSCT in terms of long-term thymic output.
Given the very short lifespan of donor-derived granulocytes, detection of the latter reflects the persistence of donor HSCs. It is thus likely that in this setting, there is a persistent flow of T-cell progenitors capable of differentiating into T cells in the thymus over time. The situation is less clear for patients who have a selective T-cell chimerism, since some (n = 9 of 20) still displayed TREC+ cells (subgroup 1B), whereas 11 had no longer detectable TREC+ cells 10 to 27 years after HSCT. One cannot completely exclude some bias, since the follow-up period of TREC+ patients without donor myeloid chimerism was slightly shorter (13 years) than that of TREC− patients (16 years). However, it is highly unlikely that this 3-year difference accounts for the overall disparity in TREC counts.
Failure to detect sustained TREC levels could be related to the loss of T-cell progenitors in the absence of donor stem cells. It could also be due to the pre-HSCT absence of thymopoiesis in PID, as well as a possible post-HSCT lesion caused by infections or GvHD.8,17,20–22 It is noteworthy that we were able to exclude a role for GvHD in inducing thymic lesions, since the TREC+ and TREC− groups did not differ in terms of GvHD incidence and severity.23–26 Age at transplantation was also analyzed, since it has clearly been shown that SCID patients who receive transplants during the first months of life exhibit faster, better T-cell reconstitution, as evidenced by higher numbers of CD3+ and naive T cells and increased mitogen-triggered lymphocyte proliferation during the 3 years following transplantation.17 The favorable outcome of early transplantation has been attributed to the absence of detrimental effects (due to infections or other comorbid factors) on thymic function. However, in the present study, we did not detect a reliable, age-related factor. A high hematopoietic stem-cell dose has been shown to correlate with better immune reconstitution after HSCT.27,28 Whether increased numbers of engrafted stem cells could result in long-term thymic production is, however, unknown. In our study, we were not able to test the correlation between the number of injected CD34+ cells and long-term TREC detection. Of note is the recent observation in mice by Bhattacharya et al29 indicating that an excess of donor HSCs over a certain threshold cannot further displace the endogenous HSCs from their niches in an unconditioned host, since only some bone marrow niches are available for engraftment.
The relatively poor engraftment in patients with T−/B−SCID has been attributed to the presence of NK cells able to mediate donor HSC rejection.30,31 In our cohort, however, we did not find any difference in long-term T-cell reconstitution between patients who had NK+ and NK− PID phenotypes. One possible explanation (related to the genetic defect itself and its phenotypic consequences) could be related to the competition in the intrathymic stromal niches between donor progenitors and endogenous, immature, double-negative (DN) thymocytes previously found in certain SCID conditions. This is supported by the data of Prockop et al,32 who demonstrated that Rag-1–deficient mice exhibit high numbers of DN2/DN3 cells and are refractory to thymic reconstitution following HSCT without conditioning because of competition for thymic niches seeding. In contrast, γc-deficient mice (in which DN T cells cannot proliferate) were well reconstituted. Strikingly, all but 1 of our patients with good thymic function (but no myeloid engraftment) displayed a γc deficiency. It is therefore possible that the low level of thymic progenitors that characterizes this SCID condition had allowed thymic seeding by a population of very early progenitors (possibly with some self-renewal capacity), thus enabling sustained T-cell differentiation. The assumption is also supported by recent data from Weerkamp et al33 who isolated a very immature, multilineage progenitor from the human thymus. Whether these CD34+ thymocytes represent true HSCs is still subject to debate, since the cells failed to repopulate nonobese diabetic (NOD)–SCID mice. However, it is possible that the fraction of this population that retains the ability to self-renew is too small to be detected.33 Thymocyte-derived signals are necessary for maturation of the thymic microenvironment and maintenance of thymic epithelial cell organization. It is possible that occupation of the stromal niches by the DN2/DN3 cells in Rag-1/-2 or Artemis deficiencies prevented full replenishment of thymic tissue by normal precursors able to differentiate and then to provide stromal cells with all the required growth signals derived from the different cell subsets. In this respect, the γc deficiency background might be more favorable for maintaining full lymphostromal interactions after transplantation.34,35
Further study of larger cohorts of HSCT patients with molecularly characterized T-cell immunodeficiencies is now warranted. Our study nevertheless shows that a combination of factors determines the long-term outcome of T-cell reconstitution following HSCT in SCID patients. In the future, these results could lead to better-adapted approaches to HSCT and/or gene therapy for T-cell–immunodeficient patients.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Salima Hacein-Bey-Abina, Hôpital Necker–Enfants Malades, 149 rue de Sèvres, F-75015 Paris, France; e-mail: salima.hacein-bey@nck.ap-hop-paris.fr.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from INSERM, AP-HP, EC contract CONSERT no. LSHB-CT-2004-005242 (coordinator: G. Wagemaker), and the Agence Nationale de la Recherche (ANR). We wish to thank Ms I. Hirsch for data collection, Ms M. Sifouane for skilful secretarial assistance, and Dr D. Fraser for rereading the manuscript.
Current address for F.L.: Dept de Microbiologie et d'Immunologie, Hôpital Sainte Justine, Montreal, QC, Canada.

![Figure 1. Correlation between TREC levels and CD31+CD45RA+CD4+ naive T-cell status. TREC copies/1 × 105 PBMCs (on the y-axis) were plotted against the absolute numbers of CD31+CD45RA+CD4+ (on the x-axis). The chosen cutoff TREC value was 100/1 × 105 PBMCs and the cutoff for CD31+CD45RA+CD4+ naive T-cell value was 0.06 × 109/L [60/μL]. The positive correlation between these 2 markers is significant at P < .001, r = 0.848.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/10/10.1182_blood-2006-07-029090/4/m_zh80100700900001.jpeg?Expires=1769152157&Signature=G-49-Ba7p0xkLFY-aBz1qC5icZSzWsF1XInbvaNY--zuR0e-4goRBly~4afJLD4v0EnipQjwgJzBr9o~fNsPKe9XUNXaWZ1uEhEpvJznJC~tBzWYo9NGuNSApQzHfdVyvyUKSolhiBjy94R2sDeROjFIvTpdoBRel6mAr-f4HjE9G5YTVZcXZZtuqIXFBaKZ5n10jENTQuy95konOvUmtDG01u9cmt70JyWHVbzrIM7I1WjGdRlVD0IRuJzDoTjIswiDYntpGxukDM~KhT-NIQ4Fz94BsKXseWGZK3NGx~-UBdk7X8l~E8fVcx3vOGKiCX86OmTSyJPXF6lxYDUh2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal