Abstract
This phase 2 study evaluated the efficacy and safety of the oral farnesyltransferase inhibitor tipifarnib in adults with refractory or relapsed acute myeloid leukemia (AML). Patients (n = 252) received tipifarnib 600 mg twice a day for 21 days in 28-day cycles. Median age was 62 years; 99 (39%) patients were 65 years or older. Eleven (4%) of 252 patients achieved complete remission (CR) or complete remission with incomplete platelet recovery (CRp; 9 CR and 2 CRp). Nineteen patients (8%), including those who achieved CR/CRp, achieved a reduction in bone marrow blasts to less than 5% blasts. Bone marrow blasts were reduced more than 50% in an additional 8 patients (total = 27; 11%). Median survival was 369 days for patients who achieved CR/CRp. Myelosuppression was the most common adverse event. The most common nonhematologic toxicities were fever, nausea, and hypokalemia. Single-agent treatment with tipifarnib induced durable CR/CRp, which was associated with prolonged survival, in some patients with refractory or relapsed AML. The response rate observed in this heavily pretreated group of patients suggests the requirement to enhance the response rate either by combining tipifarnib with other active agents or determining factors that are predictive of response.
Introduction
Tipifarnib (Zarnestra; R115777) is a selective and orally bioavailable farnesyltransferase inhibitor (FTI) that inhibits the proliferation of a variety of human tumor cell lines in vitro and in vivo.1,2 It does not appear to be a substrate for the P-glycoprotein product of the MDR1 gene.3 Observations from patient-derived samples in culture indicate that leukemic blasts are more sensitive to the cytotoxic effects of tipifarnib than are normal cells.4 The original target mediating the antiproliferative effects of FTIs was thought to be the oncogenic Ras protein,5 which requires farnesylation for proper intracellular localization and function. However, recent data demonstrate that the presence of a ras mutation is not required for the antiproliferative effects of tipifarnib.6 Nevertheless, interference with Ras signaling remains, at least in part, a possible mechanism of action. Indeed, a phase 1 study demonstrated that tipifarnib suppressed constitutively active extracellular signal regulated kinase (ERK) phosphorylation, a potential surrogate of Ras activity, in 4 of 8 patients within 1 week of starting therapy.6,7
Based on the proposed mechanism of action, tipifarnib has been tested in patients with a number of hematologic malignancies in which Ras or other farnesylated proteins play a role in the molecular basis of the disease. Complete (CR) and partial remissions (PR) have been observed in patients with high-risk myelodysplastic syndromes (16%, CR + PR),8 chronic myeloid leukemia,9 and juvenile myelomonocytic leukemia.10 In a phase 1 clinical study conducted by Karp et al,6 tipifarnib induced complete and partial remissions in 8 of 25 adults with refractory, relapsed, or untreated poor-risk acute myeloid leukemia (AML). The maximum tolerated dose was 1200 mg twice a day, but significant renal and central nervous system toxicity was observed at 900 mg twice a day. Therefore, the recommended dosing regimen for subsequent studies of patients with AML was 600 mg PO twice a day administered for 21 consecutive days in 4-week cycles. This multinational phase 2 study was conducted to extend and confirm the findings of the phase 1 study. A correlative study that identifies molecular markers predictive of response/resistance to tipifarnib is reported separately.
Patients, materials, and methods
Patients
Patients 18 years of age or older with AML (World Health Organization [WHO] classification11 ; ≥ 20% bone marrow blasts or ≥ 5% marrow blasts with evidence of increasing blast counts on 2 consecutive samples) who were refractory to or relapsed after induction therapy were enrolled. Relapsed AML was defined as the recurrence of disease > 6 months after CR. Refractory AML was defined as follows: patients > 60 years of age who failed 1 induction regimen; patients ≤ 60 years of age who failed 2 but no more than 3 regimens; or patients who had a recurrence of disease ≤ 6 months after a CR. Any white blood cell count was permitted. Control of hyperleukocytosis with hydroxyurea or plasmapheresis was permitted up to 24 hours before study entry. Patients were eligible if they had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0-2, serum creatinine and total bilirubin levels within 1.5 times the upper limit of normal, and transaminase levels within 2.5 times the upper limit of normal. Patients with an active systemic infection were eligible only if they were afebrile with stable vital signs and receiving antibiotics.
Patients were not eligible if they had active central nervous system (CNS) leukemia or uncompensated disseminated intravascular coagulation or had participated in another investigational study within 3 weeks of scheduled tipifarnib administration. Patients provided signed informed consent in accordance with the Declaration of Helsinki. The appropriate ethics committee or institutional review board, in accordance with the regulations of each participating country, approved the protocol and informed consent documents.
Study design and treatment schedule
The starting dose of tipifarnib was 600 mg administered orally twice daily for 21 consecutive days in 4-week cycles. Therapy could continue until disease progression or unacceptable toxicity. Treatment with tipifarnib was withheld for instances of treatment-related grade 2 neurologic toxicity, grade 3 or 4 nonhematologic toxicity, or grade 4 granulocytopenia or thrombocytopenia. Dose reductions of tipifarnib to 300 and 200 mg twice daily were implemented after the first and second occurrences of protocol-defined toxicity, respectively. Dose escalation of tipifarnib to 900 mg twice daily was permitted after 3 cycles of treatment for patients without significant drug-related toxicity whose best response up to that point was partial remission (PR) or stable disease (SD). A review of treatment diaries by the investigators was used to assess patient compliance.
Efficacy and safety evaluations
Complete blood counts with differential, electrolytes, aspartate aminotransferase, alanine aminotransferase, total bilirubin, and creatinine were obtained, and a physical examination was to be performed before study entry and on days 1, 8, 15, and 22 of each treatment cycle. Bone marrow aspirate and biopsy were to be obtained before study entry and every other cycle thereafter, and were reviewed by an independent hematologist.
Complete remission (CR) was defined as less than 5% bone marrow blasts with a neutrophil count of more than 1000/μL, a platelet count of more than 100 000/μL, and no extramedullary disease. CR with incomplete platelet recovery (CRp) was defined similarly except that the platelet count could be less than 100 000/μL in the setting of transfusion independence. Partial remission was defined as a 50% or better decrease in bone marrow blasts with partial neutrophil (> 500/μL) and platelet count (> 50 000/μL) recovery. Complete and partial remissions were to be confirmed at least 4 weeks after first documentation. Progressive disease was defined as a 50% or more increase in bone marrow or circulating blasts, new development of circulating blasts on at least 2 consecutive determinations, or development of extramedullary disease. To further characterize the antileukemic activity of tipifarnib, 2 additional groups of patients are reported: patients who achieved a morphologic leukemia-free state12 defined as a bone marrow blast count less than 5%; and patients with more than 50% reduction in bone marrow blast count.
The duration of CR was calculated from the first day of remission until documentation of PD. Overall survival was calculated from the first day of tipifarnib administration until death. Adverse events and laboratory values were classified according to the National Cancer Institute-Common Toxicity Criteria (version 2.0).
Pharmacokinetics
Detailed pharmacokinetics were characterized in 17 patients. Venous blood samples were collected on study day 1 immediately before and 0.5, 1, 2, 3, 5, 8, and 12 hours after tipifarnib administration. Sparse sampling was to be performed in all other patients. Population pharmacokinetics studies are published elsewhere as part of a pooled analysis from a number of tipifarnib studies.13
Statistics
The sample size was determined using CR/CRp rate as the primary efficacy end point. The protocol specifies that the per-protocol population (defined as all patients who completed at least 21 days of treatment with tipifarnib) be used for the primary analyses. A Simon 2-stage design was specified based on per-protocol patient numbers. A total sample size of 154 per-protocol patients was required. For the refractory cohort, the unacceptably low CR/CRp rate was set at 0.5%, with a target remission rate of 10% of per-protocol patients. With α at 0.05 and β at 0.1, 30 per-protocol patients were to be enrolled in the first stage. In the absence of CR, CRp, or PR, accrual was to stop. Otherwise, accrual of 55 per-protocol patients was planned. For the relapsed cohort, the corresponding CR/CRp rates were 10% and 25% of per-protocol patients, respectively. With α at 0.05 and β at 0.1, 31 per-protocol patients were to be enrolled in the first stage. Accrual was to stop if 3 or fewer CR, CRp, or PR were documented. Otherwise, accrual of 99 per-protocol patients was planned.
Two factors resulted in a higher than planned patient number. First, the protocol was amended to increase the sample size to 225 patients based on observed dropout rates (failure to complete 21 days of treatment) of 45% and 30% in the refractory and relapsed groups, respectively. In addition, presumably because of the absence of other effective therapies, patients were enrolled on the protocol at such a rapid rate that strict adherence to the pre-specified numbers did not occur. To allow data to be collected and lead investigators to evaluate responses and decide whether to enroll additional patients, enrollment was stopped completely for 3 and a half months after the first 150 patients were enrolled. The requisite number of CRs specified in the first stage of the 2-stage design (1 in the refractory cohort and 3 in the relapse cohort) was identified, and the data were reviewed with lead investigators. The decision was made not to wait for confirmation of CR, a fact that accounts for the lower number of confirmed CRs with which the study ended: a number of the CRs were never confirmed, in some cases because of progression, and in others because of patient refusal to undergo additional bone marrow examinations. When enrollment resumed, additional patients were again enrolled at a very rapid rate but very few patients in this group achieved a confirmed CR.
Although the protocol specified that the primary analysis population was the per-protocol population of patients, the analyses in this report are based on the more conservative approach of considering all treated patients in the analyses. Survival is estimated by the Kaplan-Meier method.
Results
Patients
The study was conducted between March 2001 and January 2003 in 13 countries in Europe, North America, and Asia. Two hundred fifty-two patients, 117 with refractory AML and 135 with relapsed AML, entered the study (169 patients, 78 refractory and 91 relapsed, met the per-protocol definition of having received 21 days of treatment). Table 1 summarizes baseline demographic and disease characteristics. Among the relapsed cohort, 69 patients (51%) were in first and 66 (49%) in second or subsequent relapse. Forty-four of 135 (32.6%) patients assigned to the relapsed cohort based on their response to first-line therapy were actually refractory to their most recent therapy (refractory relapse). Among 17 patients assigned to the refractory disease cohort, almost all (105; 89.7%) were refractory to their initial therapy; 41 patients never reached CR with 2 to 3 courses of induction therapy, 31 patients over the age of 60 did not achieve CR with one course of induction therapy, 7 patients relapsed rapidly after having attained CR with 1 course of induction, and 26 patients were refractory to their first induction but obtained CR with second or third induction, with rapid recurrence thereafter. The remaining 12 refractory patients attained a CR with first induction, but were refractory to subsequent induction at recurrence. The median time from diagnosis was 6.0 months for the patients with refractory disease and 19.6 months for those with relapsed disease. Approximately one quarter (26.4%) of the patients were ECOG performance status 2. Abnormalities of chromosomes 5 or 7, associated with an unfavorable prognosis, were present at diagnosis in 15.5% of patients.
Patient demographic and disease characteristics
| Parameter . | Refractory . | Relapsed . | Total . |
|---|---|---|---|
| No. patients | 117 | 135 | 252 |
| Median age (range), years | 63 (18-85) | 60 (19-82) | 62 (18-85) |
| Male, % | 59.0 | 57.8 | 58.3 |
| Female, % | 41.0 | 42.2 | 41.7 |
| Baseline ECOG performance status, % | |||
| 0 | 26.1 | 34.1 | 30.4 |
| 1 | 41.1 | 43.7 | 42.8 |
| 2 | 31.3 | 22.2 | 26.4 |
| Cytogenetics analysis | |||
| Patients, n (%) | 104 (88.9) | 107 (79.3) | 211 (83.7) |
| Normal, % | 32.5 | 37.0 | 34.9 |
| Abnormal, % | |||
| Chromosome 5, 7, or 11 abnormality | 21.4 | 10.4 | 15.5 |
| Translocation (8;21) | 4.3 | 1.5 | 2.8 |
| Inversion chromosome 16 | 1.7 | 0.7 | 1.2 |
| Other | 41.0 | 35.6 | 38.1 |
| Median time (range) from diagnosis, mo | 6.0 (0-74) | 19.6 (8-155) | 13 (0-155) |
| Prior bone marrow or stem cell transplantation for AML, n (%) | 39 (28.9) | 8 (6.8) | 47 (18.7) |
| Prior MDS, n (%) | 18 (13.3) | 32 (27.4) | 50 (19.8) |
| Parameter . | Refractory . | Relapsed . | Total . |
|---|---|---|---|
| No. patients | 117 | 135 | 252 |
| Median age (range), years | 63 (18-85) | 60 (19-82) | 62 (18-85) |
| Male, % | 59.0 | 57.8 | 58.3 |
| Female, % | 41.0 | 42.2 | 41.7 |
| Baseline ECOG performance status, % | |||
| 0 | 26.1 | 34.1 | 30.4 |
| 1 | 41.1 | 43.7 | 42.8 |
| 2 | 31.3 | 22.2 | 26.4 |
| Cytogenetics analysis | |||
| Patients, n (%) | 104 (88.9) | 107 (79.3) | 211 (83.7) |
| Normal, % | 32.5 | 37.0 | 34.9 |
| Abnormal, % | |||
| Chromosome 5, 7, or 11 abnormality | 21.4 | 10.4 | 15.5 |
| Translocation (8;21) | 4.3 | 1.5 | 2.8 |
| Inversion chromosome 16 | 1.7 | 0.7 | 1.2 |
| Other | 41.0 | 35.6 | 38.1 |
| Median time (range) from diagnosis, mo | 6.0 (0-74) | 19.6 (8-155) | 13 (0-155) |
| Prior bone marrow or stem cell transplantation for AML, n (%) | 39 (28.9) | 8 (6.8) | 47 (18.7) |
| Prior MDS, n (%) | 18 (13.3) | 32 (27.4) | 50 (19.8) |
ECOG indicates Eastern Cooperative Oncology Group; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome.
Tipifarnib treatment and exposure
The median time on study drug was 24.5 days (range, 1-296 days). One hundred thirty-seven patients (54.4%) received more than 1 cycle of therapy and 66 patients (26.2%) received 3 or more cycles. The average dose intensity was 892 mg/d, which was 99% of the target dose intensity (1200 mg/d for 21 of 28 days = 900 mg/d). Dose intensity was similar in the refractory and relapsed cohorts and across age groups (age < 65 years, 65-74 years, or age ≥ 75). Treatment delays were implemented in 41 patients (29.9%) who received more than one cycle of tipifarnib. Eighty-one patients (32.1%) had at least one dose reduction. For 24 of these patients, the dose reduction was due to a drug-related adverse event, most commonly thrombocytopenia or neutropenia. Dose escalation to 900 mg BID was implemented in 18 patients (7.1%) who did not obtain a clinical response and had no grade 3 or 4 toxicity.
Tipifarnib plasma pharmacokinetics
Full pharmacokinetic profiles after the first 600-mg dose of tipifarnib were available from 17 patients. The median tmax value of tipifarnib was 2 hours. On average, the Cmax (± SD) and AUC12h (± SD) values of tipifarnib were 1851 ± 1376 ng/mL and 9146 ± 9504 ng · h/mL, respectively. Elimination followed first-order kinetics over the 12-hour dosing interval. Figure 1 illustrates the plasma concentration-time profile of tipifarnib.
Mean (± SD) tipifarnib concentration vs time profile after 600 mg oral administration (n = 17).
Mean (± SD) tipifarnib concentration vs time profile after 600 mg oral administration (n = 17).
Efficacy
Antileukemic activity. Based on the bone marrow results reported by the independent hematologist and peripheral blood counts analyzed at the sites, CR9 or CRp2 was observed in 11 of 252 patients (7 relapsed and 4 refractory, 4%). Response was defined as the best response achieved by the patient during the course of the study. CR/CRp was confirmed at least 4 weeks later in 3 patients (1%). The median time to response was 58 days for patients who achieved a CR and 68 days (57 and 78 days) for the 2 patients who achieved a CRp. Median duration of response was 78 days for CR/CRp. Table 2 and Figure 3 lists the characteristics of the patients who achieved a CR/CRp.
Characteristics of patients who achieved a CR and CRp
| Characteristic . | CR patients . | CRp patients . | Total patients . |
|---|---|---|---|
| No. patients | 9 | 2 | 11 |
| Median age (range), y | 70 (28-79) | 60.5 (60-61) | 67 (28-79) |
| Relapsed/refractory status, no. patients | 6/3 | 1/1 | 7/4 |
| Karyotype, no. patients | |||
| Normal | 4 | 1 | 5 |
| Abnormal | 4 | 1 | 5 |
| Chromosome 5, 7, or 11 abnormality | 1 | 1 | 2 |
| Other | 3 | 0 | 3 |
| Unknown | 1 | 0 | 1 |
| Median time to response, days | 58 | 68 | 58 |
| Median duration of response, days (95% CI) | 78 (40, 107) | NAp (92+, 130+) | 78 (40, NAp) |
| Characteristic . | CR patients . | CRp patients . | Total patients . |
|---|---|---|---|
| No. patients | 9 | 2 | 11 |
| Median age (range), y | 70 (28-79) | 60.5 (60-61) | 67 (28-79) |
| Relapsed/refractory status, no. patients | 6/3 | 1/1 | 7/4 |
| Karyotype, no. patients | |||
| Normal | 4 | 1 | 5 |
| Abnormal | 4 | 1 | 5 |
| Chromosome 5, 7, or 11 abnormality | 1 | 1 | 2 |
| Other | 3 | 0 | 3 |
| Unknown | 1 | 0 | 1 |
| Median time to response, days | 58 | 68 | 58 |
| Median duration of response, days (95% CI) | 78 (40, 107) | NAp (92+, 130+) | 78 (40, NAp) |
One hundred thirty-four patients (53.2%) had a postbaseline bone marrow response. Nineteen of these patients (12 relapsed and 7 refractory), including all of those who achieved CR/CRp, achieved less than 5% bone marrow blasts; bone marrow blasts were reduced by more than 50% in 8 additional patients (6 relapsed and 2 refractory; total = 27/252; 11%).
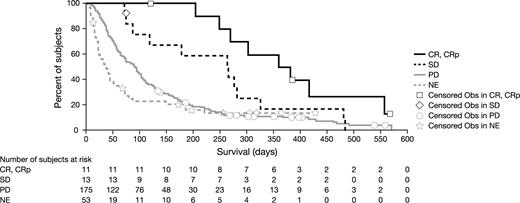
Survival. Figure 2 shows Kaplan-Meier curves for overall survival according to refractory or relapsed cohort. Median survival was 87 days for the total patient population, 65 days for the refractory cohort, and 94 days for the relapsed cohort. Table 3 show survival data by patient response to treatment. Median survival for patients with CR or CRp (n = 11) was 369 days (CR [n = 9], 386 days [range, 121-566 days]; CRp [n = 2], 324 days [268 and 380 days]).
Overall survival. Kaplan-Meier estimate of the overall population survival (n = 252). The median survival was 87 days (95% confidence interval [CI], 71-99 days). Estimated survival rate of 26% at 6 months and 12% at 12 months.
Overall survival. Kaplan-Meier estimate of the overall population survival (n = 252). The median survival was 87 days (95% confidence interval [CI], 71-99 days). Estimated survival rate of 26% at 6 months and 12% at 12 months.
Overall survival according to response
| Response . | n . | Median overall survival, days (95% CI) . |
|---|---|---|
| All treated | 252 | 87 (71-99) |
| Achieved CR/CRp | 11 | 369 (268-556) |
| CR | 9 | 386 (121-566) |
| CRp | 2 | 324 (268 380) |
| SD | 13 | 266 (119-282) |
| PD | 175 | 89 (70-102) |
| NE | 53 | 36 (22-50) |
| Response . | n . | Median overall survival, days (95% CI) . |
|---|---|---|
| All treated | 252 | 87 (71-99) |
| Achieved CR/CRp | 11 | 369 (268-556) |
| CR | 9 | 386 (121-566) |
| CRp | 2 | 324 (268 380) |
| SD | 13 | 266 (119-282) |
| PD | 175 | 89 (70-102) |
| NE | 53 | 36 (22-50) |
CI indicates confidence interval; CR, complete remission; CRp, complete remission with incomplete platelet recovery; SD, stable disease; PD, progressive disease; and NE, not evaluable.
Overall survival according to response. Kaplan-Meier estimate of overall survival as a function of response.
Overall survival according to response. Kaplan-Meier estimate of overall survival as a function of response.
Safety profile and patient hospitalization
Neutropenia and thrombocytopenia were reported frequently before and during treatment on study. The relationship of hematologic adverse events to the study drug is difficult to discern in the context of relapsed/refractory AML and should be interpreted accordingly. Grade 3 or 4 neutropenia was present in 63% of patients and grade 3 or 4 thrombocytopenia was present in 67% of patients upon entry into the study. When it occurred on treatment, the median duration of grade 3 or 4 neutropenia was 36 days, and the median duration of grade 3 or 4 thrombocytopenia was 22 days. The incidence of febrile neutropenia was 27%. When considering shifts from baseline values, 27% of patients had a worsening of at least 2 grades in neutrophil count and 23% of patients had a worsening of at least 2 grades in platelet count. Although these shifts provide a sense of changes in blood counts during the study, they do not help to distinguish the effect of the drug from that of the underlying disease.
Table 4 lists the most commonly reported drug-related nonhematologic adverse events. The most frequently reported were fever, nausea, and hypokalemia. No drug-related grade 4 vomiting or diarrhea was observed, and < 1% (0.4%) of grade 4 nausea occurred. Skin rash attributed to treatment with tipifarnib was reported in 10.7% of patients. Drug-related grade 3 or 4 rash was observed in 8 patients (3.2%).
Most common nonhematologic adverse events
| WHO preferred term . | Total, n (%) . | Drug-related, n (%) . | Grade 3/4, n (%) . |
|---|---|---|---|
| Fever | 125 (49.6) | 27 (10.7) | 35 (13.0) |
| Nausea | 113 (44.8) | 71 (28.2) | 4 (1.6) |
| Hypokalemia | 111 (44.0) | 28 (11.1) | 61 (24.2) |
| Diarrhea | 101 (40.1) | 40 (15.9) | 12 (4.8) |
| Fatigue | 93 (36.9) | 39 (15.5) | 41 (16.3) |
| Vomiting | 86 (34.1) | 48 (19.0) | 9 (3.6) |
| Anorexia | 76 (30.2) | 31 (12.3) | 18 (7.1) |
| Sepsis/infection | 75 (29.7) | 13 (5.2) | 58 (23.0) |
| Pneumonia | 66 (26.2) | 12 (4.8) | 43 (17.1) |
| Asthenia | 63 (25.0) | 20 (7.9) | 34 (13.5) |
| Skin rash | 48 (19.0) | 27 (10.7) | 11 (4.4) |
| Headache | 43 (17.1) | 12 (4.8) | 7 (2.8) |
| Creatinine increased | 40 (15.9) | 18 (7.1) | 7 (2.8) |
| Edema | 36 (14.3) | 5 (2.0) | 3 (1.2) |
| Skeletal pain | 28 (11.1) | 2 (0.8) | 10 (4.0) |
| Myalgia | 27 (10.7) | 8 (3.2) | 6 (2.4) |
| WHO preferred term . | Total, n (%) . | Drug-related, n (%) . | Grade 3/4, n (%) . |
|---|---|---|---|
| Fever | 125 (49.6) | 27 (10.7) | 35 (13.0) |
| Nausea | 113 (44.8) | 71 (28.2) | 4 (1.6) |
| Hypokalemia | 111 (44.0) | 28 (11.1) | 61 (24.2) |
| Diarrhea | 101 (40.1) | 40 (15.9) | 12 (4.8) |
| Fatigue | 93 (36.9) | 39 (15.5) | 41 (16.3) |
| Vomiting | 86 (34.1) | 48 (19.0) | 9 (3.6) |
| Anorexia | 76 (30.2) | 31 (12.3) | 18 (7.1) |
| Sepsis/infection | 75 (29.7) | 13 (5.2) | 58 (23.0) |
| Pneumonia | 66 (26.2) | 12 (4.8) | 43 (17.1) |
| Asthenia | 63 (25.0) | 20 (7.9) | 34 (13.5) |
| Skin rash | 48 (19.0) | 27 (10.7) | 11 (4.4) |
| Headache | 43 (17.1) | 12 (4.8) | 7 (2.8) |
| Creatinine increased | 40 (15.9) | 18 (7.1) | 7 (2.8) |
| Edema | 36 (14.3) | 5 (2.0) | 3 (1.2) |
| Skeletal pain | 28 (11.1) | 2 (0.8) | 10 (4.0) |
| Myalgia | 27 (10.7) | 8 (3.2) | 6 (2.4) |
WHO indicates World Health Organization.
Hypokalemia was the most common biochemical abnormality: 4.8% of patients had grade 3 drug-related abnormalities and 0.4% had grade 4. Most patients who had grades 3 or 4 toxicities were being treated or had recently been treated with diuretics, amphotericin B, or both. No grade 4 and 1.6% of grade 3 blood creatinine elevations were related to drug therapy.
Tipifarnib was often administered in the outpatient setting. Although most patients (82%) were hospitalized at least once, < 10% of study time (from enrollment until 30 days after last dose of study medication) was spent in the hospital. Disease complications were the most common reasons for hospitalization (Table 5). Fifty-eight patients (23%) were hospitalized for adverse events associated with tipifarnib treatment. Adverse events led to the termination of tipifarnib treatment in 29% of patients.
Reasons for hospitalization
| Major reasons for hospitalization . | Patients, n (%) . | Median duration, days (range) . |
|---|---|---|
| Disease-related adverse events | 111 (44.0) | 17 (1-101) |
| Treatment-related adverse events | 58 (23.0) | 14 (2-55) |
| Transfusions | 57 (22.6) | 13 (2-87) |
| Symptom control | 37 (14.7) | 12 (2-69) |
| Major reasons for hospitalization . | Patients, n (%) . | Median duration, days (range) . |
|---|---|---|
| Disease-related adverse events | 111 (44.0) | 17 (1-101) |
| Treatment-related adverse events | 58 (23.0) | 14 (2-55) |
| Transfusions | 57 (22.6) | 13 (2-87) |
| Symptom control | 37 (14.7) | 12 (2-69) |
The main cause of death during this study was leukemic disease progression. Eighteen patients (7.1%) died as a result of an adverse event associated with tipifarnib toxicity. Nine patients (3.6%) died as a result of sepsis (other causes: pneumonia and cerebral hemorrhage, 2 each; infection, hepatic failure, cardiac arrest, anemia-hyperbilirubinemia, increased creatinine-generalized edema, all 1 each). The rate of adverse event-related death on days 14 or 28 was 5% and 11%, respectively. All adverse event-related deaths are included because of the difficulty in differentiating drug-related deaths when patients are suffering from the effects of AML.
Discussion
This study assessed the efficacy of tipifarnib in 252 patients with refractory or relapsed AML and confirmed the antileukemic activity initially observed in the phase 1 setting.6 This activity was observed among refractory and relapsed patients. Tipifarnib therapy resulted in a 4% CR rate, which was associated with prolonged survival. Especially interesting is the observation of CR in 2 patients with unfavorable cytogenetics. A reduction in bone marrow blasts of at least 50% was documented in 11% of patients. We acknowledge that this was a disappointingly low response rate, which was below our expectations because, in our statistical hypothesis, the target CR rate for the refractory cohort was 10% and 25% CR/CRp for the relapsed cohort. Although it is difficult to be certain, chemotherapy may have produced a 10% to 20% response rate in this setting albeit with a much higher associated toxic death rate.
Although the response rate was low, the patients were heavily pretreated. Nonetheless, the definition of ‘refractory' did include patients who achieved CR, but relapsed within 6 months. Patients with such a short CR duration do relatively poorly, though perhaps not as badly as truly primary refractory patients. Although older patients were required to have failed just 1 regimen, it is possible a small number of such patients could have achieved a brief CR if given reinduction chemotherapy. In general, there is a relatively favorable prognosis in patients with a first CR of more than 18 months. In the relapsed cohort in this study the median duration of the first CR was 19.6 months; however, because 69 of 135 patients (51%) were over the age of 60, they did not receive more aggressive conventional chemotherapy (ie, high dose ara-C).
This study included a large population of biologically refractory AML patients. A significant number of these patients were elderly, had unfavorable karyotype, or were refractory to treatment at some point in the course of their disease. Antecedent myelodysplasia (20%) was also prevalent in these patients. These factors predict for poor response or tolerance to conventional salvage chemotherapy and justify the study of a novel targeted therapy in this patient population. Although the CR rate did not meet the prespecified target hoped for in this study, the findings suggest that tipifarnib is active against AML and that a broader view of response needs to be considered for the evaluation of targeted agents. A similar view was expressed by the investigators who reported the early results of the use of FLT3 inhibitors: these agents are considered active, because of the reported decrease in circulating and bone marrow blasts, despite the lack of any CRs with single-agent treatment.14,15 The very low clinical response rate observed in advanced previously treated AML patients does not justify the use of single agents such as FLT3 inhibitor or tipifarnib in this setting; however, they may be effective in combination with cytotoxic chemotherapy at earlier stages of the disease.
Early clinical investigations of tipifarnib focused on FTase inhibition of the product of mutated ras genes,16 found in high prevalence in pancreatic17 and colorectal cancers.18 In contrast, the rationale supporting the investigation of tipifarnib in myeloid leukemias rests on (1) the high expression of FTase in bone marrow,7 (2) the dysregulation and mutations of Ras in AML,19–22 (3) the antileukemic activity of tipifarnib in vitro at concentrations that can be achieved clinically,23 (4) the selective accumulation of tipifarnib in the bone marrow,6 (5) the dose-related incidence of myelosuppression with tipifarnib,6 and (6) the induction of complete remissions with monotherapy in a phase 1 study of patients with poor-risk leukemia.6 The latter established that tipifarnib consistently inhibits the molecular target (FTase) at the recommended dose (600 mg BID), which resulted in farnesylation inhibition of surrogate proteins (HDJ-2) and inhibition of Ras-dependent ERK pathways. Additional farnesylated proteins implicated as candidate targets that may mediate the antitumor effects of FTIs include Rho B, CENP-E and CENP-F, lamins A and B, and the protein tyrosine phosphatase PTP-CAAX. Regulation of these proteins and their downstream effectors may lead to the modulation of cell growth, proliferation, and apoptosis; therefore, it seems that FTIs may have complex inhibitory effects on several cellular events and pathways.
Tipifarnib is rapidly absorbed into the bloodstream after oral administration. The trough concentration of tipifarnib 12 hours after dosing is approximately 5- to 93-fold higher than the IC50 values for inhibition of proliferation of several AML-like cell lines. The selective accumulation of FTase in the bone marrow may explain the relative end-organ specificity and safety profile of tipifarnib. Indeed, myelosuppression was the predominant toxicity that was associated with tipifarnib in these patients. Treatment-related mortality rates observed in this study are lower than the rates (25% on average) associated with standard induction chemotherapy.24
The incidence of drug-related nonhematologic grade 4 adverse events and drug-related deaths were low in this study compared with that observed during standard induction chemotherapy.25 Fever, nausea, and hypokalemia were the most common nonhematologic adverse events in this study. The low rate of severe mucositis (2%) may explain, at least in part, the relatively low rate of septic death.
This study provides additional evidence that tipifarnib is active in patients with relapsed or refractory AML and that treatment may result in durable remission in some patients. No new side effects related to tipifarnib treatment were identified, and there was a low incidence of severe nonhematologic toxicity. Tipifarnib has demonstrated clinical efficacy (14% CR rate) in patients with previously untreated AML,26 as well as in patients with myelodysplastic syndromes, juvenile myelomonocytic leukemia,10 and chronic myeloid leukemia.9 In the context of its effect on newly diagnosed AML and other hematologic malignancies, the results from this study are encouraging. The complete remissions observed in a few patients, including 2 patients with unfavorable cytogenetics, suggest the consideration of combining tipifarnib with other antileukemic therapies, and the importance of identifying factors that will predict response or resistance to tipifarnib. A correlative study that identifies molecular markers predictive of response/resistance to tipifarnib has been reported elsewhere.27
Results from this study were previously presented at the European Hematology Association 2004 Annual Meeting as a poster discussion, at the American Society of Hematology 2003 Annual Meeting as an oral presentation, and at the American Society of Clinical Oncology 2002 Annual Meeting as a poster discussion.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Johnson & Johnson Pharmaceutical Research & Development, L.L.C., Raritan, NJ.
We gratefully acknowledge the many patients, families, and study site nurses and coordinators who made this study possible. We also acknowledge the contributions of Alain Thibault, MD, Mary Ellen Rybak, MD, Ramin Arani, PhD, and Youn Choi Park, PhD (statistical science), and Nanette Meyer and Jan Zemitis (study management).
Authorship
Contribution: J-L.H., J.L., J.R., B.L., X.T., F.H., P.F., W.R., P.D.P., and R.S. participated in designing and performing the research; S.Z. analyzed the data; all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: S.Z., P.D.P., and W.R. are employed by a company whose product was studied in the present work; the remaining authors declare no competing financial interest.
A complete list of the members of the FIGHT Acute Myeloid Leukemia Study Group appears in Document S1, available on the Blood website; see the Supplemental Document link at the top of the online article.
Correspondence: Jean-Luc Harousseau, MD, Service d'Hématologie, Hôtel Dieu, CHU de Nantes, Place Alexis Ricordeau, 44093 Nantes, France; e-mail: jean-luc.harousseau@univ-nantes.fr.


![Figure 2. Overall survival. Kaplan-Meier estimate of the overall population survival (n = 252). The median survival was 87 days (95% confidence interval [CI], 71-99 days). Estimated survival rate of 26% at 6 months and 12% at 12 months.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/12/10.1182_blood-2006-09-046144/4/m_zh80120702010002.jpeg?Expires=1765960652&Signature=saMpNc0Y7PaYeDb9TchOckKUXEOmeE8dghM-9-yIV~aZA74s-gF-~ujjDWJXraLhWU8ChEtkLbrynUNq9UREjCHQPbEDtojBvVwW7RYHjwhE7lzPndsDKy3tqVokEsyLcZrveZdiWWnwRK-wiG8uK8GZ9x3NLyq76sKxSIReDSyLPNbAaKjZYL1xloDJyRtlK83am-Jmr36jck4JdsMIEIwhDt0BBJN6~-9vFsDzLp1Gg-vSVR4iAzLoljlc~ZntCta2bgDPInva2C7XG7i4oVZENzCBgjHXqW1A2EA6JFD~D2TkwSPGAA0NuykrKxErtg8ad~PJEZ3bIYlc0GiYQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)