Abstract
RabGEF1/Rabex-5, a guanine nucleotide exchange factor (GEF) for the endocytic pathway regulator, Rab5, contains a Vps9 domain, an A20-like zinc finger (ZnF) domain, and a coiled coil domain. To investigate the importance of these domains in regulating receptor internalization and cell activation, we lentivirally delivered RabGEF1 mutants into RabGEF1-deficient (−/−) mast cells and examined FcϵRI-dependent responses. Wild-type RabGEF1 expression corrected phenotypic abnormalities in −/− mast cells, including decreased basal FcϵRI expression, slowed FcϵRI internalization, elevated IgE + Ag–induced degranulation and IL-6 production, and the decreased ability of −/− cytosol to support endosome fusion. We showed that RabGEF1's ZnF domain has ubiquitin ligase activity. Moreover, the coiled coil domain of RabGEF1 is required for Rabaptin-5 binding and for maintaining basal levels of Rabaptin-5 and surface FcϵRI. However, mutants lacking either of these domains normalized phenotypic abnormalities in IgE + antigen–activated −/− mast cells. By contrast, correction of these −/− phenotypes required a functional Vps9 domain. Thus, FcϵRI-mediated mast cell functional activation is dependent on RabGEF1's GEF activity.
Introduction
Mast cells are critical effector cells in IgE-associated immediate hypersensitivity and other allergic disorders.1–4 IgE primes mast cells for antigen (Ag)–specific activation by binding to FcϵRI, a member of the immune receptor superfamily that is expressed on the mast cell surface.1–4 Cross-linking of FcϵRI-bound IgE by multivalent Ag initiates a complex cascade of signaling events involving the activation and recruitment of signaling and scaffolding proteins including Lyn, Syk, Ras, and Gab2.3–6 The functional consequences of IgE + Ag–induced mast cell activation include the release of proinflammatory mediators (eg, histamine and β-hexosaminidase) stored in the cells' cytoplasmic granules, in a process called degranulation, the secretion of lipid mediators, and the release of cytokines, chemokines, and growth factors.1–4
Because cell activation through specific receptors can initiate a variety of responses, the appropriate regulation of receptors and their downstream signaling pathways is critical for cells to interpret properly and respond to their environment, and perturbations in these processes can have diverse and dramatic effects.7 Members of the Rab family of small GTPases are essential regulators of membrane trafficking events including receptor endocytosis, vesicle sorting, and receptor recycling.8–11 Rab5 is a major regulator of early endocytic events.10,12–14 Like other GTPases, Rab5 is activated by guanine nucleotide exchange factors (GEFs), which catalyze the release of GDP; subsequent GTP binding induces a conformational change that allows Rab5 to interact with downstream effectors.10,15
RabGEF1 (aka, Rabex-5), initially characterized as a GEF for Rab5,16 forms a stable complex with the Rab5 effector, Rabaptin-5,16–18 thus coupling Rab5 activation and effector recruitment. This interaction also increases the GEF activity of RabGEF1 in vitro.16,18 Of interest, the yeast ortholog of RabGEF1, vacuolar sorting protein 9 (Vps9p),16,19 contains, in addition to a GEF-encoding Vps9 domain, a C-terminal CUE domain that binds to monoubiquitin and is required for the coupled monoubiquitination of Vps9p.20–24 Because monoubiquitination is an important regulatory signal in membrane trafficking events,20,25 RabGEF1 may be involved in both Rab5- and ubiquitin (Ub)–mediated regulation of membrane trafficking. Indeed, RabGEF1 recently was shown to have E3 Ub ligase activity26 and to bind Ub via 2 distinct domains.27,28
We recently reported that RabGEF1 negatively regulates the activation of mast cells initiated by aggregation of the cells' high-affinity receptor for IgE, FcϵRI; IgE + Ag–induced degranulation and cytokine release were elevated in bone marrow–derived cultured mast cells (BMCMCs) from Rabgef1−/− (−/−) versus +/+ mice.29 RabGEF1 also plays an important role in the regulation of mast cell signaling and functional responses mediated through the growth factor receptor, c-Kit.30
By amino acid sequence analysis, RabGEF1 contains an N-terminal A20-like zinc finger (ZnF) domain, a central Vps9 domain, and a C-terminal coiled coil domain. To understand the roles of RabGEF1's domains in regulating FcϵRI-mediated mast cell activation, we studied a panel of RabGEF1 mutants in vitro and in lentivirally infected −/− BMCMCs. We found that even though the ZnF domain has Ub ligase activity and the coiled coil domain is necessary for Rabaptin-5 binding and for maintaining basal levels of both endogenous Rabaptin-5 and surface expression of FcϵRI in mast cells, only the Rab5-activating Vps9 domain is required for RabGEF1 to restore FcϵRI internalization and IgE + Ag–induced functional activation in −/− BMCMCs to +/+ levels.
Materials and methods
Detailed methods are provided in Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article.
Cell culture
BMCMCs were generated and cultured as described.29
RabGEF1 mutants
The coding region of full-length or mutant mouse RabGEF1 cDNA was subcloned into pRRLsin-18.PPT.PGK.MCS.IRES.GFP.pre,30 pGEX-4T2 (Amersham, Piscataway, NJ) or pEGFP-N1 (Clontech, Mountain View, CA).
Lentivirus production and BMCMC infection
RabGEF1 +/+ and −/− BMCMCs were infected with active lentiviral stocks as described.30
Immunoprecipitation and Western blot analysis
For immunoprecipitation studies, 2 × 106 BMCMCs were solubilized with 1% Triton X-100 + phosphorylation solubilization buffer31 and subjected to immunoprecipitation with the indicated antibodies. A spontaneously derived −/− BMCMC line was used for anti-RabGEF1 and anti-HA immunoprecipitation studies.
Mediator release
Degranulation assays and IL-6 enzyme-linked immunosorbent assays (ELISAs) were performed as described.29
Endosome fusion assay
Endosome fusion activity was assessed as described.32
Flow cytometry
BMCMCs were stained with the indicated antibodies and analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Data were analyzed with FlowJo software (Treestar, Ashland, OR).
FcϵRI internalization
IgE-sensitized BMCMCs were labeled (4°C) with biotinylated anti-IgE Abs (clone R35-72), placed at 37°C for 0, 30, or 60 minutes, then incubated (4°C) with APC-conjugated streptavidin (BD PharMingen, San Diego, CA).
Fusion proteins
Rab5a and WT and mutant RabGEF1 GST fusion proteins were extracted with B-PER II (Pierce, Rockford, IL) and purified with glutathione sepharose 4B (Amersham).
Microscopy
RabGEF1−/− BMCMCs transiently transfected with the indicated GFP-fusion constructs were induced to adhere to fibronectin-coated coverslips (25 μg/mL) by treatment with SCF, then imaged by confocal microscopy at 37°C.
GDP release assay
GDP release was assessed as described.16
Immobilized DNP assays
DNP-HSA (Sigma, St Louis, MO) was immobilized to EIA/RIA Stripwell plates (Costar, Corning, NY) for 16 hours at 4°C. Sensitized BMCMCs were added to the wells for 6 hours and IL-6 levels assessed by ELISA.
Ubiquitination assays
WT or mutant RabGEF1 GST fusion protein was incubated with 1 × energy regenerating solution, 0.1 μg E1, 0.5 μg UbcH5a, and 1 μg Ub (Boston Biochem, Cambridge, MA).
Pull-down assays
For Ub pull-down assays, the indicated RabGEF1 GST fusion protein was incubated with polyubiquitin chains (Ub1-7, K48-linked). For rabaptin-5 pull-down assays, −/− BMCMCs were lysed in 1% Triton X-100 and incubated with WT or CT2 RabGEF1 GST fusion protein.
Reverse-transcription–polymerase chain reaction (RT-PCR)
RNA was reverse transcribed using Omniscript reverse transcriptase (Qiagen, Valencia, CA), and Rabaptin-5 cDNA was amplified by PCR.
Statistics
Results
Expression of wild-type RabGEF1 in −/− BMCMCs restores FcϵRI aggregation-induced responses to +/+ levels
We first tested whether expression of full-length wild-type (WT) RabGEF1 in −/− BMCMCs could repair their phenotypic abnormalities. We used lentiviruses for this work because they can infect slowly dividing or nondividing cells, such as BMCMCs, and they permit stable gene expression and incorporation of bicistronic markers (eg, GFP) for purification of infected cells.33,34 Consistent with our prior findings,30 RabGEF1 expression levels in WT-infected −/− BMCMCs were similar to endogenous levels in +/+ BMCMCs (Figure 1A). We reported that surface FcϵRI expression is lower on −/− versus +/+ BMCMCs,29 and we found that expression of WT RabGEF1 in −/− BMCMCs restored basal FcϵRI expression to +/+ levels (Figure 1B).
Expression of WT RabGEF1 in −/− BMCMCs normalizes basal FcϵRI surface expression, FcϵRI internalization, and IgE + Ag–induced degranulation and IL-6 production. (A) RabGEF1 +/+ and −/− BMCMCs were infected with control lentiviral vector (empty) or the lentiviral vector containing RabGEF1 cDNA (WT), then sorted by GFP expression. Total cell lysates were analyzed by Western blot using α-HA and α-RabGEF1 Abs. Blots were reprobed with α-GAPDH Abs to show loading. (B) Basal FcϵRI surface expression was analyzed on the indicated mast cell populations by flow cytometry. Data represent mean fluorescent intensity (MFI) of the indicated BMCMC population divided by MFI of the corresponding −/− empty vector control–treated BMCMCs (× 100%) pooled from 4 separate experiments + SEM (n = 4). (C) Infected BMCMCs generated as in panel A were sensitized for 16 hours in DMEM + 10% FCS containing 2 μg/mL IgE, then stimulated with biotinylated α-IgE Abs for the indicated times. Surface α-IgE was assessed by streptavidin (SA)–APC fluorescence and analyzed by flow cytometry. Gray represents SA-APC only. Histograms (top panels) are representative of results obtained in 5 separate experiments. The bar graph (bottom panel) shows the mean + SEM of percentage FcϵRI internalization determinations from 5 separate batches of BMCMCs. Percentage FcϵRI internalization was calculated by subtracting mean fluorescence intensity at 30 or 60 minutes from mean fluorescence intensity at 0 minute and dividing this number by mean fluorescence intensity at 0 minute (× 100%). (D-E) Infected BMCMCs generated as in panel A were sensitized for 16 hours with 2 μg/mL IgE, then (D) degranulation was quantified by measuring release of β-hexosaminidase (β-Hex) 1 hour after challenge with the indicated concentrations of DNP, and (E) IL-6 production was quantified 6 hours after challenge with 20 ng/mL DNP. Results in panels D-E are the mean + SEM of 9 separate determinations from 3 batches of BMCMCs. (B) * indicates P < .05 versus assigned −/− value of 100%. (C-E) + indicates P < .05; ++, P < .01; +++, P < .001 versus corresponding +/+ “empty” values; * indicates P < .05; **, P < .01; ***, P < .001 versus corresponding −/− “empty” values.
Expression of WT RabGEF1 in −/− BMCMCs normalizes basal FcϵRI surface expression, FcϵRI internalization, and IgE + Ag–induced degranulation and IL-6 production. (A) RabGEF1 +/+ and −/− BMCMCs were infected with control lentiviral vector (empty) or the lentiviral vector containing RabGEF1 cDNA (WT), then sorted by GFP expression. Total cell lysates were analyzed by Western blot using α-HA and α-RabGEF1 Abs. Blots were reprobed with α-GAPDH Abs to show loading. (B) Basal FcϵRI surface expression was analyzed on the indicated mast cell populations by flow cytometry. Data represent mean fluorescent intensity (MFI) of the indicated BMCMC population divided by MFI of the corresponding −/− empty vector control–treated BMCMCs (× 100%) pooled from 4 separate experiments + SEM (n = 4). (C) Infected BMCMCs generated as in panel A were sensitized for 16 hours in DMEM + 10% FCS containing 2 μg/mL IgE, then stimulated with biotinylated α-IgE Abs for the indicated times. Surface α-IgE was assessed by streptavidin (SA)–APC fluorescence and analyzed by flow cytometry. Gray represents SA-APC only. Histograms (top panels) are representative of results obtained in 5 separate experiments. The bar graph (bottom panel) shows the mean + SEM of percentage FcϵRI internalization determinations from 5 separate batches of BMCMCs. Percentage FcϵRI internalization was calculated by subtracting mean fluorescence intensity at 30 or 60 minutes from mean fluorescence intensity at 0 minute and dividing this number by mean fluorescence intensity at 0 minute (× 100%). (D-E) Infected BMCMCs generated as in panel A were sensitized for 16 hours with 2 μg/mL IgE, then (D) degranulation was quantified by measuring release of β-hexosaminidase (β-Hex) 1 hour after challenge with the indicated concentrations of DNP, and (E) IL-6 production was quantified 6 hours after challenge with 20 ng/mL DNP. Results in panels D-E are the mean + SEM of 9 separate determinations from 3 batches of BMCMCs. (B) * indicates P < .05 versus assigned −/− value of 100%. (C-E) + indicates P < .05; ++, P < .01; +++, P < .001 versus corresponding +/+ “empty” values; * indicates P < .05; **, P < .01; ***, P < .001 versus corresponding −/− “empty” values.
Because c-Kit internalization is delayed in the absence of RabGEF1,30 we examined whether FcϵRI internalization might be similarly affected. Indeed, FcϵRI internalization was markedly delayed in −/− versus +/+ BMCMCs after exposure to anti-IgE antibodies (Abs) (Figure 1C). Percent FcϵRI internalization was 41.5 ± 0.6 versus 29.8 ± 2.3 (n = 5, P < .01) at 30 minutes and 67.8 ± 2.3 versus 43.5 ± 3.5 (n = 5, P < .001) at 60 minutes after stimulation in +/+ versus −/− BMCMCs, respectively. To investigate whether slowed FcϵRI internalization might be associated with changes in downstream signaling, we compared IgE + Ag–induced tyrosine phosphorylation events in RabGEF1 +/+ versus −/− BMCMCS. Total cellular tyrosine phosphorylation events (Figure S1A), as well as phosphorylation of the FcϵRI β and γ subunits, were prolonged in −/− versus +/+ BMCMCs (Figure S1B). These findings are consistent with the hypothesis that delayed FcϵRI internalization contributes, at least in part, to increased IgE + Ag–induced signals and responses in −/− BMCMCs. Of importance, expression of WT RabGEF1 in −/− BMCMCs normalized FcϵRI internalization to +/+ levels (Figure 1C).
Once an activated receptor is internalized, endosome-endosome fusion occurs in a Rab5-dependent manner.10,12,13 We compared endosome fusion activity in BMCMCs using an in vitro assay; cytosolic extracts were added to labeled endosomes, and the extent of fusion activity was measured.32 We found that mast cell cytosol supported endosome fusion in a concentration-dependent manner and that the addition of cytosol from −/− BMCMCs resulted in less fusion activity than cytosol from +/+ BMCMCs (Figure S2A). Moreover, cytosol from WT-expressing −/− BMCMCs was more fusogenic than cytosol from the corresponding parental −/− BMCMCs (Figure S2B).
Finally, we found that expression of WT RabGEF1 in −/− BMCMCs restored levels of IgE + Ag–induced degranulation (Figure 1D) and IL-6 production (Figure 1E) to +/+ levels. Thus, the decreased basal FcϵRI expression, delayed FcϵRI internalization, decreased endosome fusion, and enhanced IgE + Ag–induced mediator release observed in −/− BMCMCs can be rescued by the expression of WT RabGEF1.
Generation and intracellular localization of RabGEF1 mutants
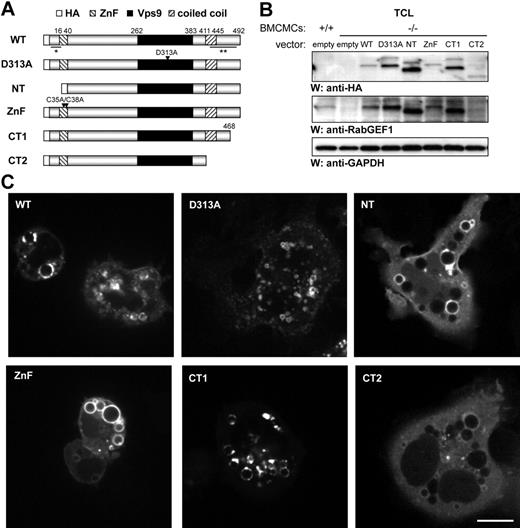
We generated a panel of RabGEF1 mutants (Figure 2A) to assess which domains are required for RabGEF1 to rescue abnormalities in −/− BMCMCs. RabGEF1 contains 3 modular domains: an N-terminal A20-like zinc finger (ZnF) domain (amino acids 16-40); a central Vps9 domain (amino acids 262-383); and a C-terminal coiled coil domain (amino acids 411-445).
RabGEF1 mutants can be expressed in mast cells. (A) Schematic of the RabGEF1 mutants used in this study, with the ZnF, Vps9, and coiled coil domains indicated. Numbering corresponds to amino acid residues in the mouse RabGEF1 sequence. Arrows indicate point mutations. All constructs were HA-tagged at the N-terminus. * indicates α-RabGEF1 (Ac-KSER) Ab epitope; **, α-RabGEF1 (BD Biosciences) Ab epitope. (B) Total cell lysates from +/+ and −/− BMCMCs infected with the indicated constructs were analyzed for RabGEF1 expression by Western blot using α-HA and α-RabGEF1 Abs. The blot was reprobed with α-GAPDH Abs to show loading. Results are representative of those obtained in 4 or more separate experiments. (C) RabGEF1−/− BMCMCs cells transiently transfected (24 hours) with the indicated GFP constructs were induced to adhere to fibronectin-coated coverslips and imaged in real time by spinning-disc confocal microscopy. Bar (for scale of all images in C) represents 10 μm. Cells were imaged by spinning disc confocal Axiovert 200M microscope (Zeiss, Germany) with a 63× oil objective and CoolSNAP HQ CCD camera. Images were collected using slidebook software (v. 4.1.15; 3I, Denver, CO) and exported into Adobe Photoshop (v. 7.0), where signals were balanced and cropped.
RabGEF1 mutants can be expressed in mast cells. (A) Schematic of the RabGEF1 mutants used in this study, with the ZnF, Vps9, and coiled coil domains indicated. Numbering corresponds to amino acid residues in the mouse RabGEF1 sequence. Arrows indicate point mutations. All constructs were HA-tagged at the N-terminus. * indicates α-RabGEF1 (Ac-KSER) Ab epitope; **, α-RabGEF1 (BD Biosciences) Ab epitope. (B) Total cell lysates from +/+ and −/− BMCMCs infected with the indicated constructs were analyzed for RabGEF1 expression by Western blot using α-HA and α-RabGEF1 Abs. The blot was reprobed with α-GAPDH Abs to show loading. Results are representative of those obtained in 4 or more separate experiments. (C) RabGEF1−/− BMCMCs cells transiently transfected (24 hours) with the indicated GFP constructs were induced to adhere to fibronectin-coated coverslips and imaged in real time by spinning-disc confocal microscopy. Bar (for scale of all images in C) represents 10 μm. Cells were imaged by spinning disc confocal Axiovert 200M microscope (Zeiss, Germany) with a 63× oil objective and CoolSNAP HQ CCD camera. Images were collected using slidebook software (v. 4.1.15; 3I, Denver, CO) and exported into Adobe Photoshop (v. 7.0), where signals were balanced and cropped.
To study the ZnF domain, we made both an N-terminal truncation mutant (NT) lacking the first 40 amino acids of RabGEF1, including the entire ZnF domain, and a point mutant (ZnF) in which 2 conserved cysteines were mutated to alanines (C35A/C38A). Mutation analysis of the human RabGEF1 Vps9 domain demonstrated that a conserved aspartic acid residue is necessary for Rab5 GEF activity in vitro14 ; thus, we generated a RabGEF1 point mutant (D313A) with this aspartic acid mutated to alanine. To analyze the C-terminal region and coiled coil motif, we made 2 C-terminal RabGEF1 truncation mutants (CT1 and CT2). We generated GST fusion proteins of these mutants for in vitro studies, GFP fusion proteins for localization studies, and lentiviral-packaged constructs for stable expression in BMCMCs. Mutants were N-terminally HA-tagged in the lentiviral and pEGFP-N1 vectors (Figure 2A). Infection of −/− BMCMCs with each of these lentiviral vectors yielded stable expression of the corresponding mutant protein (Figure 2B), and we consistently observed a higher molecular weight band with anti-HA and anti-RabGEF1 reactivity by Western blot analysis in BMCMCs.
We assessed the subcellular distribution of RabGEF1 in −/− BMCMCs transiently transfected with GFP-tagged RabGEF1 mutants by confocal microscopy. All mutants localized to vesicular structures (Figure 2C); however, localization of the NT, CT2, and, to a lesser degree, ZnF mutants was more diffuse when compared to the other RabGEF1 proteins. Also, overexpression of each mutant except for D313A appeared to result in enlarged vesicular structures. Studies with the fluid-phase endocytic marker, Dextran, provided evidence that WT RabGEF1 localized to endocytic compartments in BMCMCs (Figure S3).35,36 We decided to confirm these findings in fibroblasts, a cell type in which Rab5 and early endosome antigen 1 (EEA1) localization have been well characterized.37,38 In −/− fibroblasts transiently transfected with GFP-tagged RabGEF1 constructs, all RabGEF1 GFP-fusion proteins localized to punctuate structures that partially colocalized with Rab5 and EEA1 (Figure S4); however, the degree of colocalization varied among the different mutants. For example, we observed relatively poor colocalization of CT1 and CT2 with Rab5 and EEA1. Moreover, localization of the NT mutant and, to a lesser degree, the ZnF mutant was more diffuse when compared to the other mutant or WT RabGEF1 proteins.
Both D313A-expressing −/− BMCMCs (Figure 2C) and D313A-expressing −/− fibroblasts (Figure S4–S5) appeared to have vesicles of relatively small size. When we measured the size of EEA1-positive endosomes in fibroblasts, we found that RabGEF1 deficiency had no detectable effect on endosome size in primary fibroblasts (data not shown), but that overexpression in −/− fibroblasts of each RabGEF1 GFP fusion protein except for D313A resulted in enlarged EEA1-positive endosomes, like those observed in fibroblasts expressing constitutively active Rab5 (Rab5:Q79L) (Figure S5).
Thus, although we observed relatively small vesicles in D313A-expressing −/− mast cells and fibroblasts, all RabGEF1 GFP-fusion proteins examined in this study localized to vesicular structures in both cell types.
Effects of RabGEF1 domains on surface FcϵRI expression
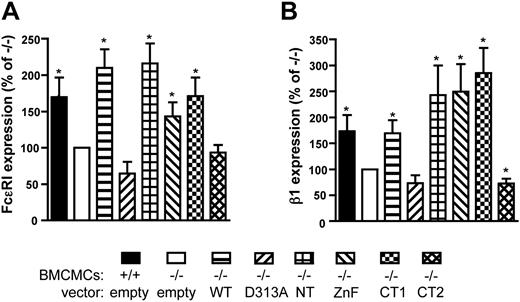
The regulation of surface receptor levels is a dynamic process involving targeting the receptors to the cell surface, followed by their fluid phase removal and possible recycling.10,39 Because expression of WT RabGEF1 restored basal FcϵRI receptor levels in −/− BMCMCs to +/+ levels (Figure 1B), we examined the roles of the various RabGEF1 domains in the regulation of surface FcϵRI expression. Expression of each RabGEF1 mutant in −/− BMCMCs, with the exception of D313A and CT2, restored basal FcϵRI surface expression to +/+ levels (Figure 3A).
RabGEF1's coiled coil and Vps9 domains regulate basal surface expression of FcϵRI and β1 integrin. Basal surface expression levels of (A) FcϵRI and (B) β1 integrin were analyzed on the indicated mast cell populations by flow cytometry, as described in Figure 1B. Values were pooled from 4 separate experiments. * indicates P < .05 versus assigned −/− value of 100%.
RabGEF1's coiled coil and Vps9 domains regulate basal surface expression of FcϵRI and β1 integrin. Basal surface expression levels of (A) FcϵRI and (B) β1 integrin were analyzed on the indicated mast cell populations by flow cytometry, as described in Figure 1B. Values were pooled from 4 separate experiments. * indicates P < .05 versus assigned −/− value of 100%.
Surface expression of β1 integrin, a component of the fibronectin receptor, is also lower on −/− versus +/+ BMCMCs.30 In accord with our FcϵRI results, we found that each of the mutants, except for CT2 and D313A, restored basal β1 integrin surface expression in −/− BMCMCs to +/+ levels (Figure 3B). By contrast, surface expression of the growth factor receptor, c-Kit, was not affected by the absence of RabGEF130 or by the expression of the various RabGEF1 mutants in −/− BMCMCs (data not shown).
These data demonstrate that the coiled coil and Vps9 domains of RabGEF1 contribute significantly to the regulation of basal surface expression for a subset of mast cell receptors.
A functional Vps9 domain is required to correct FcϵRI aggregation-induced −/− phenotypes
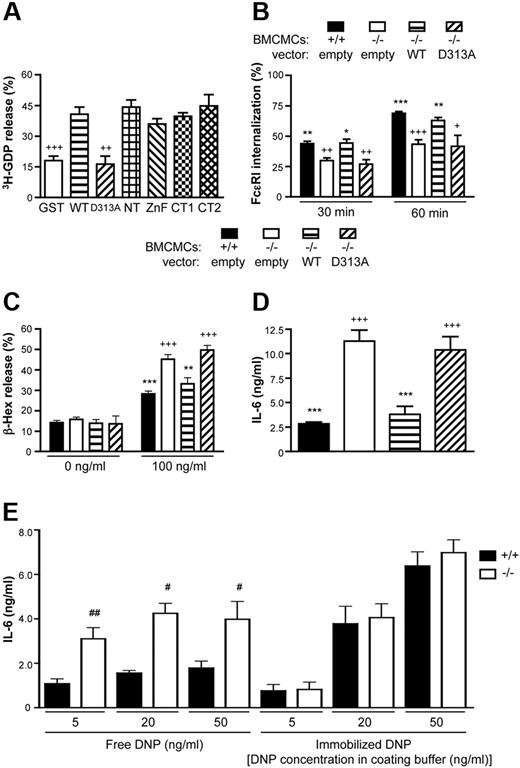
The central Vps9 domain of RabGEF1 activates Rab5 in vitro,10 and this GEF activity likely is important for Rab5 cellular function.15 We assessed whether our RabGEF1 GST fusion proteins could stimulate GDP release from Rab5a in vitro. All fusion proteins containing an intact Vps9 domain stimulated 3H-GDP release from Rab5a by 30 minutes (Figure 4A), whereas the Vps9 domain point mutant, D313A, exhibited no enhancement in 3H-GDP release when compared to that observed with GST alone.
The Vps9 domain of RabGEF1 is critical for GEF activity and the correction of −/− phenotypes. (A) Release of 3H-GDP (%) from Rab5 after a 30 minute incubation of 3H-GDP–loaded Rab5 with the indicated GST fusion proteins, as an assessment of GEF activity in vitro. Data shown are the mean + SEM from 3 separate experiments. ++ indicates P < .01; +++, P < .001 versus WT. (B) Percentage FcϵRI internalization (mean + SEM), determined as described in Figure 1C, from 5 (empty and WT) or 3 (D313A) separate batches of BMCMCs infected with the indicated lentiviral vectors. (C-D) IgE + Ag–induced (C) degranulation or (D) IL-6 production, determined as described in Figure 1D-E (mean + SEM of 9 separate determinations from 3 batches of BMCMCs). (E) IL-6 production by RabGEF1 +/+ or −/− BMCMCs sensitized for 16 hours in DMEM + 10% FCS containing 2 μg/mL IgE and stimulated for 6 hours in wells containing free or immobilized DNP at the indicated starting concentrations. (B-D) + indicates P < .05; ++, P < .01; +++, P < .001 versus corresponding +/+ “empty” values; * indicates P < .05; **, P < .01; ***, P < .001 versus corresponding −/− “empty” values. (E) # indicates P < .05; ##, P < .01 vs corresponding +/+ values. P < .01 vs corresponding +/+ values.
The Vps9 domain of RabGEF1 is critical for GEF activity and the correction of −/− phenotypes. (A) Release of 3H-GDP (%) from Rab5 after a 30 minute incubation of 3H-GDP–loaded Rab5 with the indicated GST fusion proteins, as an assessment of GEF activity in vitro. Data shown are the mean + SEM from 3 separate experiments. ++ indicates P < .01; +++, P < .001 versus WT. (B) Percentage FcϵRI internalization (mean + SEM), determined as described in Figure 1C, from 5 (empty and WT) or 3 (D313A) separate batches of BMCMCs infected with the indicated lentiviral vectors. (C-D) IgE + Ag–induced (C) degranulation or (D) IL-6 production, determined as described in Figure 1D-E (mean + SEM of 9 separate determinations from 3 batches of BMCMCs). (E) IL-6 production by RabGEF1 +/+ or −/− BMCMCs sensitized for 16 hours in DMEM + 10% FCS containing 2 μg/mL IgE and stimulated for 6 hours in wells containing free or immobilized DNP at the indicated starting concentrations. (B-D) + indicates P < .05; ++, P < .01; +++, P < .001 versus corresponding +/+ “empty” values; * indicates P < .05; **, P < .01; ***, P < .001 versus corresponding −/− “empty” values. (E) # indicates P < .05; ##, P < .01 vs corresponding +/+ values. P < .01 vs corresponding +/+ values.
Given Rab5's important role in the regulation of receptor internalization,12,13 we assessed FcϵRI internalization in D313A-expressing −/− BMCMCs. Although FcϵRI internalization was normalized in WT-expressing −/− BMCMCs, D313A-expressing −/− BMCMCs exhibited delayed internalization (41.5 ± 0.6% vs 26.9 ± 3.7% for +/+ vs D313A after 30 minutes; n = 5 or n = 4, respectively; P < .01), with an internalization rate similar to that of the empty vector–treated −/− BMCMCs (Figure 4B). Similarly, the D313A mutant did not normalize c-Kit internalization (Figure S6). D313A-expressing −/− BMCMCs also exhibited significantly elevated levels of IgE + Ag–induced degranulation (Figure 4C) and IL-6 production (Figure 4D) vs +/+ BMCMCs. Of importance, we did not observe any significant differences in PMA/ionophore-induced degranulation between +/+ and −/− BMCMCs or among −/− BMCMCs infected with WT or any of the mutant RabGEF1 lentiviral vectors used in this study (data not shown).
These data indicate that an intact Vps9 domain is crucial for cellular functions of RabGEF1 in mast cells stimulated through FcϵRI aggregation.
Interference with FcϵRI internalization increases IL-6 production in IgE + Ag–stimulated +/+ BMCMCs to −/− levels
To investigate whether slowed receptor internalization per se might contribute to the elevated IL-6 release observed in RabGEF1−/− BMCMCs, we artificially interfered with FcϵRI internalization by immobilizing the Ag, DNP, to the stimulus chamber and assessed IL-6 production. In agreement with our previous findings,29 IL-6 release was markedly elevated in −/− versus +/+ BMCMCs when the cells were placed in wells with free Ag; however, with immobilized Ag, the difference in IL-6 production between +/+ and −/− mast cells was essentially eliminated (Figure 4E). Furthermore, IL-6 production was elevated when +/+ BMCMCs were stimulated with plate-bound Ag derived from solutions containing 20 or 50 ng/mL DNP, compared to results obtained with the corresponding concentrations of Ag free in solution (Figure 4E). The latter findings with +/+ BMCMCs, together with the results obtained with D313A-expressing −/− BMCMCs, support the hypothesis that slowed receptor internalization can contribute to enhanced cytokine release. However, an alternative explanation of the findings obtained with plate-bound Ag is that Ag immobilization, by increasing stimulus strength, might thereby mask the negative regulatory control of RabGEF1 (or other molecules) on mast cell cytokine production.
RabGEF1's ZnF domain has E3 Ub ligase activity in vitro but is not required to correct FcϵRI aggregation-induced −/− phenotypes
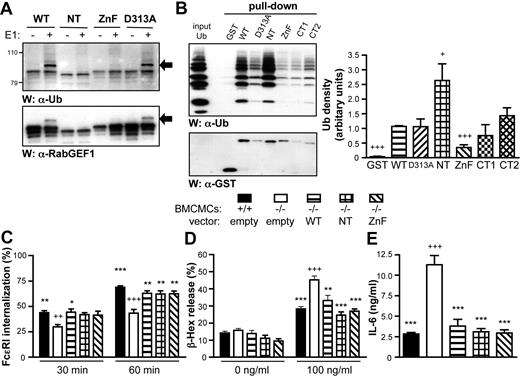
A Cys2/Cys2 ZnF domain in the C-terminal region of A20 has E3 Ub ligase activity.40 To assess whether RabGEF1's ZnF domain has Ub ligase activity, we conducted in vitro ubiquitination assays using our RabGEF1 GST fusion proteins. WT or D313A RabGEF1 catalyzed ubiquitination in the presence of Ub-activating enzyme (E1) and the Ub-conjugating (Ubc) enzyme (E2), UbcH5a. Western blot analysis revealed a band of approximately 94 kDa with strong anti-Ub and anti-RabGEF1 reactivity in ubiquitination assays with WT or D313A fusion proteins, suggesting autoubiquitination of these proteins (Figure 5A). RabGEF1 autoubiquitination was observed in the presence of UbcH3, 5a or 5b (Figure S7A). E3 ligase activity was also observed with the C-terminal–truncated fusion proteins, CT1 and CT2 (Figure S7B). However, neither NT nor ZnF RabGEF1 displayed significant E3 ligase activity (Figure 5A). These results indicate that the ZnF domain is required for the Ub ligase activity of RabGEF1 in vitro, confirming recently reported findings.26
The ZnF domain of RabGEF1 mediates ubiquitination in vitro, but is not required for the correction of FcϵRI aggregation-induced −/− phenotypes. (A) In vitro ubiquitination assays with WT or mutant RabGEF1 GST fusion proteins were performed + Ub and UbcH5a (E2), and ± Ub-activating enzyme (E1), as indicated. Samples were analyzed by Western blot using α-Ub and α-RabGEF1 Abs. Arrows indicate autoubiquitinated RabGEF1. (B) In vitro Ub pull-down assays (left panel) with WT or mutant RabGEF1 GST fusion proteins were performed in the presence of poly-Ub chains (Ub1-7, K48-linked) and analyzed by Western blot using α-Ub and α-GST Abs. Results in panels A and B are representative of those obtained in 4 or more separate experiments. Signals from 4 separate experiments were quantified by densitometric scanning and corrected for loading (B, right panel); data shown are mean + SEM. + indicates P < .05, +++, P < .001 versus WT. (C) Percentage FcϵRI internalization (mean + SEM), determined as described in Figure 1C, from 5 (empty and WT) or 3 (NT and ZnF) separate batches of BMCMCs infected with the indicated lentiviral vectors. (D-E) IgE + Ag–induced (D) degranulation or (E) IL-6 production, determined as described in Figure 1D-E (mean + SEM of 9 separate determinations from 3 batches of BMCMCs). (C-E) ++ indicates P < .01; +++, P < .001 versus corresponding +/+ “empty” values; * indicates P < .05; **, P < .01; ***, P < .001 versus corresponding −/− “empty” values.
The ZnF domain of RabGEF1 mediates ubiquitination in vitro, but is not required for the correction of FcϵRI aggregation-induced −/− phenotypes. (A) In vitro ubiquitination assays with WT or mutant RabGEF1 GST fusion proteins were performed + Ub and UbcH5a (E2), and ± Ub-activating enzyme (E1), as indicated. Samples were analyzed by Western blot using α-Ub and α-RabGEF1 Abs. Arrows indicate autoubiquitinated RabGEF1. (B) In vitro Ub pull-down assays (left panel) with WT or mutant RabGEF1 GST fusion proteins were performed in the presence of poly-Ub chains (Ub1-7, K48-linked) and analyzed by Western blot using α-Ub and α-GST Abs. Results in panels A and B are representative of those obtained in 4 or more separate experiments. Signals from 4 separate experiments were quantified by densitometric scanning and corrected for loading (B, right panel); data shown are mean + SEM. + indicates P < .05, +++, P < .001 versus WT. (C) Percentage FcϵRI internalization (mean + SEM), determined as described in Figure 1C, from 5 (empty and WT) or 3 (NT and ZnF) separate batches of BMCMCs infected with the indicated lentiviral vectors. (D-E) IgE + Ag–induced (D) degranulation or (E) IL-6 production, determined as described in Figure 1D-E (mean + SEM of 9 separate determinations from 3 batches of BMCMCs). (C-E) ++ indicates P < .01; +++, P < .001 versus corresponding +/+ “empty” values; * indicates P < .05; **, P < .01; ***, P < .001 versus corresponding −/− “empty” values.
Several endocytic proteins contain Ub-binding domains (eg, UIM [Ub-interacting motif] and CUE domains).20 Based on sequence homology, it was proposed that RabGEF1 contains a C-terminal–modified CUE domain (amino acids 445-481).20,21 To test RabGEF1's ability to bind Ub, we performed in vitro Ub pull-down experiments using WT and mutant RabGEF1 GST fusion proteins. All fusion proteins bound directly to Ub in vitro (Figure 5B); however, NT displayed a stronger affinity for Ub than WT RabGEF1, whereas ZnF bound Ub with weaker affinity. CT2 also bound Ub, indicating that the putative CUE domain is not required for Ub binding, a result concordant with findings in 2 recent papers.27,28
Despite the loss of Ub ligase activity in the NT and ZnF mutants, expression of either mutant in −/− BMCMCs restored the rate of anti–IgE-induced FcϵRI internalization to levels seen in +/+ or WT-expressing −/− BMCMCs (Figure 5C). Similarly, levels of IgE + Ag–induced degranulation (Figure 5D) and cytokine release (Figure 5E) in NT- or ZnF-expressing −/− BMCMCs were very similar to those observed in +/+ cells. These results indicate that the E3 Ub ligase activity of RabGEF1 is not required for the regulation of IgE + Ag–induced functional activation in mast cells.
Rabaptin-5 levels are decreased in the absence of RabGEF1
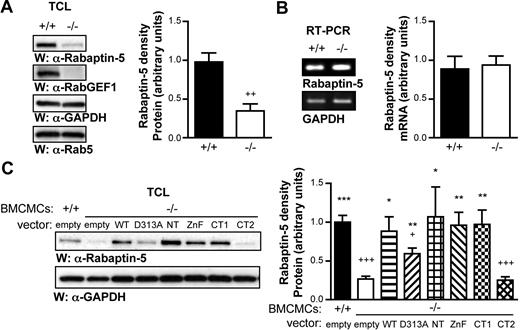
RabGEF1 forms a complex with Rabaptin-5,16 and we consistently observed that Rabaptin-5, but not Rab5, protein levels are decreased in −/− versus +/+ BMCMCs (Figure 6A). Decreased Rabaptin-5 levels were also observed in −/− spleen lysates or dermal fibroblasts (data not shown). This decrease in Rabaptin-5 levels appears to occur at the protein level, since, as assessed by semiquantitative RT-PCR, levels of Rabaptin-5 mRNA are similar in −/− versus +/+ BMCMCs (Figure 6B and Figure S8).
Rabaptin-5 levels are decreased in the absence of RabGEF1. (A) Total cell lysates from RabGEF1 +/+ and −/− BMCMCs were analyzed by Western blot using α–Rabaptin-5, α-RabGEF1, and α-Rab5 Abs (left panel). The blot was reprobed with α-GAPDH Abs to show loading. Results are representative of those obtained in 6 or more experiments. Rabaptin-5 signals from 6 separate experiments were quantified by densitometric scanning and corrected for loading (right panel). (B) Rabaptin-5 mRNA levels in +/+ and −/− BMCMCs were analyzed by semiquantitative RT-PCR (28 cycles; left panel). Signals from 3 separate experiments were quantified by densitometric scanning and corrected for loading (right panel). (C) Total cell lysates from Figure 2 B (RabGEF1 +/+ and −/− BMCMCs as well as −/− BMCMCs expressing the indicated RabGEF1 mutants) were analyzed by Western blot using α–Rabaptin-5 Abs (left panel). The blot was reprobed with α-GAPDH Abs to show loading. Rabaptin-5 signals from 4 (NT and D313A) or 5 separate experiments were quantified by densitometric scanning and corrected for loading (right panel). (A-C; right panels) Data shown are the mean + SEM. (A) ++ indicates P < .01 versus +/+. (C) + indicates P < .05; +++, P < .001 versus +/+ “empty” values; * indicate P < .05; **, P < .01; ***, P < .001 versus−/− “empty” values.
Rabaptin-5 levels are decreased in the absence of RabGEF1. (A) Total cell lysates from RabGEF1 +/+ and −/− BMCMCs were analyzed by Western blot using α–Rabaptin-5, α-RabGEF1, and α-Rab5 Abs (left panel). The blot was reprobed with α-GAPDH Abs to show loading. Results are representative of those obtained in 6 or more experiments. Rabaptin-5 signals from 6 separate experiments were quantified by densitometric scanning and corrected for loading (right panel). (B) Rabaptin-5 mRNA levels in +/+ and −/− BMCMCs were analyzed by semiquantitative RT-PCR (28 cycles; left panel). Signals from 3 separate experiments were quantified by densitometric scanning and corrected for loading (right panel). (C) Total cell lysates from Figure 2 B (RabGEF1 +/+ and −/− BMCMCs as well as −/− BMCMCs expressing the indicated RabGEF1 mutants) were analyzed by Western blot using α–Rabaptin-5 Abs (left panel). The blot was reprobed with α-GAPDH Abs to show loading. Rabaptin-5 signals from 4 (NT and D313A) or 5 separate experiments were quantified by densitometric scanning and corrected for loading (right panel). (A-C; right panels) Data shown are the mean + SEM. (A) ++ indicates P < .01 versus +/+. (C) + indicates P < .05; +++, P < .001 versus +/+ “empty” values; * indicate P < .05; **, P < .01; ***, P < .001 versus−/− “empty” values.
Because Rabaptin-5 levels are altered in the absence of RabGEF1, we compared Rabaptin-5 protein levels in −/− BMCMCs expressing the various RabGEF1 mutants. Figure 6C shows a representative Western blot from one batch of BMCMCs (left panel) and Rabaptin-5 protein levels quantified by densitometry and corrected for loading in 4 or 5 separate batches of BMCMCs (right panel). Rabaptin-5 protein levels in CT2-expressing −/− cells were comparable to those observed in −/− BMCMCs. Rabaptin-5 levels in all other RabGEF1 mutant–expressing −/− BMCMCs were significantly higher than −/− levels and similar to +/+ levels, with the exception of the D313A-expressing cells, in which Rabaptin-5 levels were significantly higher than −/− and lower than +/+ levels.
RabGEF1's coiled coil domain is required for Rabaptin-5 binding but not for the correction of FcϵRI aggregation-induced −/− phenotypes
We used the panel of RabGEF1 mutants to map the Rabaptin-5–binding site. Immunoprecipitation studies revealed that the CT2 mutant, which lacks the coiled coil domain, did not bind to Rabaptin-5 (Figure 7A). All other mutants formed complexes with Rabaptin-5. We used anti-RabGEF1 (Ac-KSER) antibodies to immunoprecipitate all the RabGEF1 mutants except NT, which does not have the consensus site for this antibody (Figure 2A); anti-HA antibodies were used to immunoprecipitate NT. To rule out the possibility that the inability of CT2 to bind Rabaptin-5 simply reflects the decreased levels of Rabaptin-5 in CT2-expressing BMCMCs (Figure 6C), we used pull-down assays to show that, unlike the WT fusion protein, the CT2 RabGEF1 GST fusion protein did not bind Rabaptin-5 from −/− BMCMC lysates (Figure 7B). These results indicate that the coiled coil domain is required for Rabaptin-5 binding, confirming findings of Mattera et al26 and extending that work by showing that such binding to RabGEF1 can regulate Rabaptin-5 levels in the cell.
The coiled coil domain of RabGEF1 mediates Rabaptin-5 binding, but is not required for the correction of FcϵRI aggregation-induced −/− phenotypes. (A) RabGEF1 +/+ and −/− BMCMCs infected with the indicated constructs were subjected to immunoprecipitation with α-RabGEF1 (Ac-KSER) Abs (left panels) or α-HA Abs (right panels) and Western blot analysis with α–Rabaptin-5, α-HA, or α-RabGEF1 Abs, as indicated. (B) Pull-down assays on RabGEF1−/− BMCMC lysates with WT or CT2 RabGEF1 GST fusion proteins were analyzed by Western blot using α-Rabaptin-5, α-GST, and α-GAPDH Abs, as indicated. Results in panels A-B are representative of those obtained in 3 or more separate experiments. (C) Percentage FcϵRI internalization (mean + SEM), determined as described in Figure 1C, from 5 (empty and WT) or 3 (CT1 and CT2) separate batches of BMCMCs infected with the indicated lentiviral vectors. (D-E) IgE + Ag–induced (D) degranulation or (E) IL-6 production, determined as described in Figure 1D-E (mean + SEM of 9 separate determinations from 3 batches of BMCMCs). (C-E) ++ indicates P < .01; +++, P < .001 versus corresponding +/+ “empty” values; * indicates P < .05; **, P < .01; ***, P < .001 versus corresponding −/− “empty” values.
The coiled coil domain of RabGEF1 mediates Rabaptin-5 binding, but is not required for the correction of FcϵRI aggregation-induced −/− phenotypes. (A) RabGEF1 +/+ and −/− BMCMCs infected with the indicated constructs were subjected to immunoprecipitation with α-RabGEF1 (Ac-KSER) Abs (left panels) or α-HA Abs (right panels) and Western blot analysis with α–Rabaptin-5, α-HA, or α-RabGEF1 Abs, as indicated. (B) Pull-down assays on RabGEF1−/− BMCMC lysates with WT or CT2 RabGEF1 GST fusion proteins were analyzed by Western blot using α-Rabaptin-5, α-GST, and α-GAPDH Abs, as indicated. Results in panels A-B are representative of those obtained in 3 or more separate experiments. (C) Percentage FcϵRI internalization (mean + SEM), determined as described in Figure 1C, from 5 (empty and WT) or 3 (CT1 and CT2) separate batches of BMCMCs infected with the indicated lentiviral vectors. (D-E) IgE + Ag–induced (D) degranulation or (E) IL-6 production, determined as described in Figure 1D-E (mean + SEM of 9 separate determinations from 3 batches of BMCMCs). (C-E) ++ indicates P < .01; +++, P < .001 versus corresponding +/+ “empty” values; * indicates P < .05; **, P < .01; ***, P < .001 versus corresponding −/− “empty” values.
Despite decreased basal levels of FcϵRI surface expression on CT2-expressing mast cells (Figure 3A) and the inability of CT2 to bind Rabaptin-5, CT2-expressing −/− BMCMCs exhibited a rate of FcϵRI internalization similar to that observed in +/+ BMCMCs or WT-expressing −/− BMCMCs (Figure 7C). Similarly, CT2-expressing −/− BMCMCs exhibited levels of IgE + Ag–induced degranulation (Figure 7D) and IL-6 production (Figure 7E) similar to those in +/+ cells. CT1-expressing −/− BMCMCs exhibited phenotypes after FcϵRI aggregation that were similar to those of +/+ BMCMCs or WT-expressing −/− BMCMCs in all assays (Figure 7). These results indicate that the coiled coil domain of RabGEF1 mediates Rabaptin-5 binding and RabGEF1 regulates cellular levels of Rabaptin-5, but Rabaptin-5 binding is not required for RabGEF1-mediated regulation of IgE + Ag–induced functional activation of BMCMCs.
Discussion
RabGEF1 Vps9 domain links endocytosis to functional activation
Receptor-mediated endocytosis and signal transduction are tightly coupled, however, the functional relationship between these 2 complex processes is not fully understood. We demonstrate that a catalytically active Vps9 domain is required for RabGEF1-mediated regulation of early endocytic events in mast cells (ie, FcϵRI internalization upon receptor aggregation) and for the ability of RabGEF1 to correct the abnormally enhanced levels of IgE + Ag–induced mediator release observed in −/− BMCMCs (Figure 4). Similarly, expression of the catalytically dead D313A mutant in −/− BMCMCs failed to restore basal surface expression of FcϵRI and β1 to +/+ levels (Figure 3). Thus, GEF activity is critical for several of RabGEF1's functions within the cell. Of importance, even though the D313A point mutation disrupted the Rab5 GEF activity of RabGEF1, this mutation did not detectably alter the protein's subcellular localization, Ub ligase activity, or Rabaptin-5 binding ability.
In addition to detecting enhanced IgE + Ag–induced mediator release in −/− and D313A-expressing −/− BMCMCs, we found that interference with FcϵRI internalization in +/+ BMCMCs increased IgE + Ag–induced cytokine production and reduced the differences in cytokine production between +/+ and −/− BMCMCs (Figure 4E). Taken together, these data suggest that delayed FcϵRI internalization upon receptor aggregation can contribute to the hyperresponsive phenotypes of RabGEF1−/− BMCMCs.
Work by others offers additional support for this hypothesis. Overexpression of CIN85, a scaffolding protein that binds Cbl and regulates clathrin-mediated endocytosis, accelerated FcϵRI internalization and dampened functional activation in a mast cell line.41 Further supporting the critical role of the endocytic pathway in regulating signal transduction in other cell contexts, Rab5 was recently identified as a negative regulator of Wnt signaling42 and inhibition of TLR4 internalization or endosomal trafficking potentiated LPS-induced signaling.43
We reported that RabGEF1 can both bind to Ras and negatively regulate FcϵRI-induced Ras activation in mast cells.29 Of note, all of the RabGEF1 mutants we examined bound to active Ras, including D313A (Figure S9). This raises the possibility that the negative effects of RabGEF1 on Ras activity in IgE + Ag–29 or c-Kit–30 stimulated BMCMCs are related to RabGEF1's role in receptor endocytosis. However, it remains to be determined whether RabGEF1-mediated endocytosis is potentiated by activated Ras, as has been shown for Rin1.44
RabGEF1 was recently identified as an inhibitor of NFκB in a genome-wide survey of monocyte adherence-induced genes.45 In agreement with these findings, we observe increased IgE + Ag–induced phosphorylation of NFκB in RabGEF1−/− versus +/+ BMCMCs (J.K. and S.J.G., unpublished observations, February 2006). Since IgE + Ag–induced IL-6 production in BMCMCs is dependent on NFκB activation46 and IL-6 production is elevated in −/− BMCMCs and D313A-expressing −/− BMCMCs (Figure 4C), it is likely that RabGEF1 negatively regulates NFκB activity through its effects on endocytosis and endosomal sorting. Supporting this hypothesis, inhibition of either endocytosis or endosomal sorting has recently been shown to increase LPS-induced activation of NFκB.43
Several Rab5 GEFs have been identified in addition to RabGEF1, including Rin1,44 RME-6,47 and ALS2,48 but the potential redundancy for Rab activation is only beginning to be explored. For example, bulk endocytosis in C elegans cells was dramatically reduced in the absence of RME-6, and RNAi knockdown of the RabGEF1 ortholog in these cells diminished endocytosis further.47 Our results indicate that RabGEF1 plays a significant and largely nonredundant role in regulating receptor endocytosis in mast cells.
RabGEF1 and ubiquitin
The ZnF domain in A20 with highest homology to the RabGEF1 ZnF domain has E3 ligase activity.40 We found that RabGEF1's ZnF domain expresses E3 ligase activity in vitro (Figure 5A), confirming findings that were reported while our work was in progress.26,28 In contrast to A20,40 we observed RabGEF1 autoubiquitination, but not polyubiquitin chain formation, in in vitro ubiquitination assays.
E3 ligases provide specificity to ubiquitination reactions by regulating the timing and substrate selection of these processes.20,49 ZnF domain E3s are structurally related to RING (really interesting gene)–finger domains, which coordinate Ub-loaded E2s with specific substrates and promote the direct transfer of Ub from E2 to the substrate.50 Although ZnF domain E3s probably function in a manner similar to RING-finger E3s,50 Lee et al28 have shown that A20 ZnF domains bind to a novel region on Ub, the Asp58 patch, to selectively recruit Ub-loaded E2s, suggesting that ZnF domain E3s are a distinct family from RING-finger E3s.
Receptor trafficking events such as receptor internalization, delivery to the endosome, and endosomal sorting can be regulated by the covalent modification of activated receptors with Ub, as well as by the activation of Rab5 family proteins.20,24 Furthermore, a number of E3 ligases (eg, Cbl, Grail, and Itch) have been shown to play critical roles in the regulation of immune responses.20,49 Although deletion or mutation of the ZnF domain led to a more diffuse distribution of RabGEF1 within BMCMCs (Figure 2C), these mutations did not affect the ability of the NT and ZnF RabGEF1 mutants to restore FcϵRI aggregation-induced mast cell activation in −/− BMCMCs to +/+ levels (Figure 5). This indicates that the E3 ligase activity of RabGEF1 is not required for the regulation of FcϵRI internalization and mast cell functional activation.
In support of our findings in the mast cell system, Huang et al showed recently that ubiquitination of the epidermal growth factor receptor regulates receptor degradation, but not internalization, in PAE cells.51 Perhaps RabGEF1's ZnF domain plays an important role in the regulation of endocytic trafficking events other than receptor internalization.
Although the yeast ortholog of RabGEF1, Vps9p, binds to Ub via its CUE domain, all RabGEF1 mutants we examined, including CT2 (which lacks the proposed CUE domain) could bind Ub (Figure 5B). Our findings thus are in agreement with very recent reports showing that RabGEF1 binds Ub through 2 distinct domains, the ZnF domain and an inverted UIM (IUIM) immediately downstream of the ZnF.26–28 It remains to be determined whether this Ub binding is important for the cellular function of RabGEF1. However, the retention of Ub-binding domains in RabGEF1 suggests that Ub binding may be important for some aspects of RabGEF1's function.27 Indeed, 2 groups have independently shown that RabGEF1 is ubiquitinated in HeLa cells,26,27 and it is tempting to speculate that RabGEF1 may undergo coupled monoubiquitination, in a manner similar to other endocytic Ub-binding proteins (eg, eps15 and Hrs).20,52 In support of this hypothesis, we consistently observed a larger form of RabGEF1 in anti-HA or anti-RabGEF1 Western blots (Figures 2B and 7A), suggesting that RabGEF1 and its mutants undergo covalent modification in mast cells, and that the lower levels of modification observed with the ZnF mutant may reflect the decreased ability of this mutant to bind Ub.
Rabaptin-5 interaction
The Rab5 effector Rabaptin-5 is a known binding partner of RabGEF1.16–18 We noted decreased Rabaptin-5 protein, but not mRNA, levels in RabGEF1−/− BMCMCs (Figure 6). We showed that Rabaptin-5 binding is dependent on the coiled coil domain of RabGEF1 (Figure 7A), in agreement with a prior report.26 Furthermore, the decreased Rabaptin-5 levels we observed in CT2-expressing −/− BMCMCs were comparable to those in −/− BMCMCs, indicating that RabGEF1 binding is required to maintain wild-type basal levels of Rabaptin-5 protein. Of interest, even though the D313A mutant can bind Rabaptin-5, expression of D313A in −/− BMCMCs resulted in Rabaptin-5 levels that were intermediate between those in +/+ and −/− cells, suggesting that additional factors, which may be regulated by Rab5, are involved in maintaining Rabaptin-5 protein levels in mast cells.
The CT2 mutant allowed us to study which RabGEF1 functions are dependent on Rabaptin-5 binding. We found that Rabaptin-5 binding is not required for RabGEF1-mediated regulation of FcϵRI internalization within cells (Figure 7C), even though Rabaptin-5 binding has previously been shown to increase the GEF activity of RabGEF1 in vitro in a cell-free system.18 The localization of GFP-tagged CT2 was more diffuse than WT RabGEF1 in BMCMCs (Figure 2C) and we observed poor colocalization of CT2 with EEA1 and Rab5 in fibroblasts (although CT2-expressing −/− fibroblasts had enlarged EEA1-positive endosomes), suggesting that Rabaptin-5 binding may regulate, at least in part, the efficient endosomal localization of RabGEF1.
In addition to RabGEF1, Rabaptin-5 contains independent binding sites for Rab4, Rab5, γ-adaptins, and GGAs.53–55 Therefore, it will be important to determine whether the decreased Rabaptin-5 levels have additional effects on −/− cells independent of RabGEF1. For example, we found that lower basal surface receptor expression of FcϵRI and β1 integrin correlated with the ability of RabGEF1 to bind/maintain high endogenous levels of Rabaptin-5. Each of these receptors is thought to be recycled back to the cell surface after internalization,39,56 and one of the proposed functions of Rabaptin-5 is to link Rab5 endosomes with Rab4 recycling endosomes. It is tempting to speculate that low Rabaptin-5 levels may diminish the ability to recycle certain receptors back to the surface and may result in lower basal surface receptor expression.
However, the ability of the CT2 mutant to correct other −/− phenotypes without binding to Rabaptin-5 and/or restoring basal levels of Rabaptin-5 or FcϵRI surface expression indicates that decreased levels of cellular Rabaptin-5 and/or FcϵRI surface expression are not responsible for the increased IgE + Ag–induced functional activation observed in −/− BMCMCs.
Conclusions
Our studies demonstrate that RabGEF1 regulates basal FcϵRI surface expression, FcϵRI internalization, endosome fusion, and IgE + Ag–induced mediator release in mast cells. Moreover, the Vps9 domain of RabGEF1 plays a critical role in the regulation of endocytosis and functional activation in mast cells upon FcϵRI-dependent stimulation. The E3 ligase–encoding ZnF domain and the Rabaptin-5–binding coiled coil domain of RabGEF1 do not detectably influence the aspects of FcϵRI-dependent mast cell activation investigated herein. However, it is likely that these domains are important for the regulation of other cellular processes, as exemplified by the role of the coiled coil domain in the regulation of Rabaptin-5 protein levels and basal levels of surface receptor expression of FcϵRI and β1 integrin, receptors that play important roles in mast cell functional activation and adhesion.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants AI23990, AI070813, CA72074, and HL67674 (S.J.G.) and GM08205 (M.A.B.); a Florida International University Foundation grant (M.A.B.); a Natural Sciences and Engineering Research Council of Canada (NSERC) Fellowship (J.K.); and a Stanford MSTP grant no. 5-732-GM07365 (E.J.R.).
We thank members of the Galli laboratory and Dr Guangpu Li (University of Oklahoma, OK) for helpful discussions. We thank M. Liebersbach for animal husbandry.
Authorship
Contribution: J.K., E.J.R., C.-C.C., M.A.B., and S.J.G. designed research; J.K., E.J.R., C.-C.C., and M.A.B. performed research; J.K., E.J.R., M.A.B., M.T., S.-Y.T., and S.J.G. analyzed data; and J.K., E.J.R., and S.J.G. wrote the paper. J.K. and E.J.R. contributed equally to this work.
Conflict-of-interest disclosure: S.J.G., M.T., and S.-Y.T. have 2 US Patents (5/965/707 and 6/500/942) regarding this research (RIN2, a novel inhibitor of Ras-mediated signalling) and have filed a US Patent Application #11/158/655 (in vivo models for RabGEF-1–dependent signalling and functions).
Correspondence: Stephen J. Galli, Department of Pathology, Stanford University School of Medicine, Stanford, CA 94305-5324; e-mail: sgalli@stanford.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal