In this issue of Blood, Giles and colleagues report the remarkable clinical activity of MK-0457, an aurora kinase inhibitor, in 3 Philadelphia chromosome–positive leukemia patients harboring the highly resistant T315I mutation.
To counteract the problem of imatinib resistance due to BCR-ABL gene mutations in Philadelphia chromosome–positive (Ph+) leukemias, several new drugs have been tested in preclinical assays, and 3 of them are currently in clinical development: the dual-specificity Src/Abl inhibitors dasatinib (BMS354825) and SKI606, and the imatinib derivative nilotinib (AMN-107). Nevertheless, the T315I mutant has remained an Achilles heel for at least 2 of these second-generation inhibitors (ie, nilotinib and dasatinib).1,2 Structural analyses predict that the T315I eliminates a crucial hydrogen bond required for high-affinity binding of imatinib, dasatinib, and nilotinib, and alters adversely the topology of the ATP-binding pocket.3
In this issue of Blood, Giles and colleagues document for the first time the efficacy of a tyrosine kinase inhibitor in T315I-positive patients—2 patients with chronic myeloid leukemia (CML) in accelerated phase and a patient with Ph+ acute lymphoblastic leukemia (ALL), resistant both to imatinib and to dasatinib or nilotinib, who received MK-0457 within a phase 1/2 study. All 3 patients achieved clinical responses to doses that were not associated with adverse events.
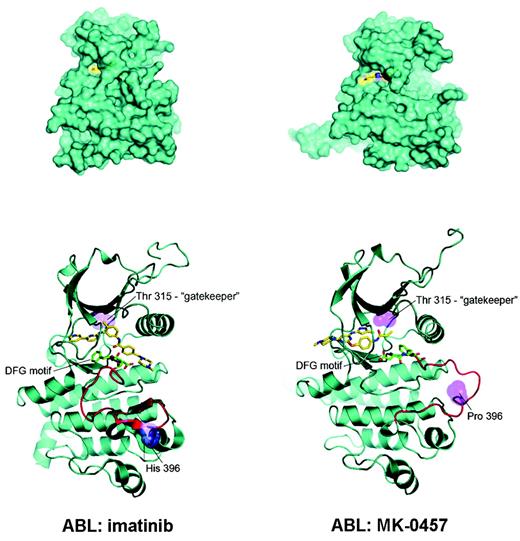
The use of this aurora kinase inhibitor in the treatment of Ph+ leukemias found its rationale in the results of a recent screening4 of existing chemical compounds for their ability to bind and inhibit drug-resistant mutant variants of Bcr-Abl, including the T315I. The study revealed that MK-0457 binds T315I Bcr-Abl with very high affinity (Kd = 5 nM). Subsequent cocrystal studies showed that MK-0457 binds Bcr-Abl in a mode that accommodates the substitution of the bulkier isoleucine for threonine at residue 315 (see figure), and for this reason effectively inhibits the kinase activity of both wild-type and T315I Bcr-Abl in vitro at micromolar concentrations.5 Despite the small number of T315I-positive patients treated with MK-0457 and the very short follow-up, the clinical observations of Giles and colleagues seem to fully validate this preclinical evidence.
Possible explanation for MK-0457 activity in T315I Bcr-Abl–positive CML and Ph+ ALL. (A) MK-0457 has been shown to be less deeply buried in the Abl kinase domain (right) with respect to imatinib (left). As a consequence, (B) a close encounter with threonine 315 is avoided, explaining the ability of MK-0457 to accommodate easily the substitution of threonine with isoleucine. Modified, with permission, from Young et al5 .
Possible explanation for MK-0457 activity in T315I Bcr-Abl–positive CML and Ph+ ALL. (A) MK-0457 has been shown to be less deeply buried in the Abl kinase domain (right) with respect to imatinib (left). As a consequence, (B) a close encounter with threonine 315 is avoided, explaining the ability of MK-0457 to accommodate easily the substitution of threonine with isoleucine. Modified, with permission, from Young et al5 .
If, on one hand, results of crystallographic and in vitro studies made it reasonable to assess this compound in Ph+ leukemia patients, the inhibition of aurora kinases by MK-0457 raises the question whether these targets may also harbor some pathogenetic role in CML and/or Ph+ ALL. Aurora kinases regulate diverse cell-cycle events, ranging from the entry into mitosis, centrosome function, mitotic spindle formation, and chromosome orientation to segregation. Aberrant activity of these kinases has been reported in many human tumors, but it is yet unclear whether, in T315I Bcr-Abl–positive CML and Ph+ ALL, there is a selective deregulation of aurora kinases and if auroras may be a suitable secondary target for inhibition. Whatever the actual mechanism of action of MK-0457 is, the observations of Giles et al may set the stage for a breakthrough in the management of patients with the T315I mutation, who are at present without an effective treatment, if we exclude allogeneic stem cell transplantation.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal