Abstract
Building on the prior work of use of pentostatin in chronic lymphocytic leukemia (CLL), we initiated a trial of combined pentostatin (2 mg/m2), cyclophosphamide (600 mg/m2), and rituximab (375 mg/m2) for 65 symptomatic, previously untreated patients. Of 64 evaluable patients, 34 (53%) were high Rai risk, 71% were nonmutated for the immunoglobulin heavy-chain variable region gene, 34% were CD38+, and 34% were ZAP-70+. Thirty patients (52%) had one anomaly detected by fluorescence in situ (FISH) hybridization, and 21 (36%) had complex FISH defects. Thirty-eight patients (58%) had grade 3+ hematologic toxicity but minimal transfusion needs and no major infections. Responses occurred in 58 patients (91%), with 26 (41%) complete responses (CRs), 14 (22%) nodular partial responses (nodular PRs), and 18 (28%) partial responses (PRs). Many patients with a CR also lacked evidence of minimal residual disease by 2-color flow cytometry. Examination of prognostic factors demonstrated poor response in the 3 patients with del(17p). In contrast, we found this regimen was equally effective in young versus older (> 70 years) patients and in del(11q22.3) versus other favorable prognostic factors. Thus, this novel regimen of pentostatin, cyclophosphamide, and rituximab for previously untreated patients with CLL demonstrated significant clinical activity despite poor risk-based prognoses, achievement of minimal residual disease in some, and modest toxicity.
Introduction
Original therapeutic approaches for progressive chronic lymphocytic leukemia (CLL) involved the use of alkylating agents, chlorambucil, or cyclophosphamide as single agents or with prednisone. These drugs, when given in an adequate dose for a sufficient duration, frequently yield response rates over 70%, although complete responses (CRs) originally reported in the 5% to 10% range1-4 are more likely less than 5% CR rates using more rigorous criteria. More recently, the purine analogs fludarabine, pentostatin, and cladribine have all demonstrated activity in B-CLL. Although they are very similar in structure, there are subtle differences that likely play a role in both pharmacokinetics and clinical activity for these agents. Fludarabine therapy in CLL has generated objective responses in up to 60% of patients with alkylator-resistant CLL and approximately 80% of previously untreated patients.5,6 An intergroup, multi-institutional study comparing chlorambucil with fludarabine as initial treatment for B-cell CLL (B-CLL) found a higher response rate for fludarabine (63% versus 37%) as well as a higher disease-free survival (33 months for fludarabine versus 17 months for chlorambucil).7 Encouragingly, several reports have found that poly-therapies have substantial activity in both previously untreated and treated B-CLL.
The combination of fludarabine and cyclophosphamide have generated improved response rates including more CRs.8-13 More recently, the combination of pentostatin and cyclophosphamide14 and pentostatin and chlorambucil15 have generated excellent clinical response rates in pretreated B-CLL patients. Use of pentostatin and cyclophosphamide in heavily pretreated CLL patients generated a 74% response rate with 4 CRs in a pilot study involving 23 patients.14 Importantly, the partial response (PR) rate was 50% and a “best response” of 41% was found. This latter response category refers to the combination of pentostatin and cyclophosphamide generating the best clinical response when compared to their multiple prior therapies. The marrow suppression of this regimen appears to be minimal as evidenced by the few platelet transfusions given to this patient cohort. Finally, the addition of rituximab, the monoclonal antibody specific for CD20, to the combination of pentostatin or fludarabine used with cyclophosphamide has been shown to generate significantly more overall and complete clinical responses (CCRs) for either previously treated or untreated patients with CLL, respectively.16-18 Given the reported efficacy of chemoimmunotherapy (CIT) combinations in CLL and the promising activity and toxicity profile of pentostatin combinations, we designed a unique trial of pentostatin, cyclophosphamide, and rituximab for previously untreated patients with CLL. This report documents the very high response rates, achievement of minimal residual disease (MRD) status, and minimal marrow suppression for this cohort of CLL patients.
Patients, materials, and methods
Clinical trial sites and patient eligibility
This was a phase 2 clinical trial conducted at the Mayo Clinic, Rochester, MN, and The Ohio State University, Columbus. This was a clinical trial as well as correlative laboratory analyses designed to assess risk-stratification parameters and estimation of MRD. Patients were required to have a diagnosis of progressive CLL using the criteria of the National Cancer Institute (NCI) Working Group.19 Patients had to be previously untreated with normal renal and hepatic function, have an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 3, and no other neoplasms with the exception of squamous or basal cell skin cancers or carcinoma in situ of the cervix. The protocol was reviewed and approved by the Institutional Review Board at each participating institution. All patients were required to provide written informed consent prior to entry on this study, in accordance with the Declaration of Helsinki.
Study design and treatment plan
After giving written informed consent, eligible CLL patients received a regimen consisting of pentostatin (P; 2 mg/m2) on day 1, cyclophosphamide (C; 600 mg/m2), and rituximab (R; 375 mg/m2) given intravenously on day 1. This PCR regimen is given on a 21-day, 6-cycle schedule. However, the initial cycle of treatment uses thrice weekly rituximab as described by us earlier.20 In brief, this was rituximab at 100 mg/m2 on day 1 and 375 mg/m2 on days 3 and 5 of the first week only. Treatment was discontinued in cases of progressive disease (PD) or excessive toxicity. Prophylactic sulfamethoxazole-trimethoprim and acyclovir were given to all patients for 1 year starting on the first cycle of therapy with PCR. All patients were given filgrastim on day 3 of each treatment cycle for 10 consecutive days or until the total neutrophil count was more than 1.0 × 109/L (1000/μL) for 2 consecutive days.
Dose modifications for toxicity
The first cycle was administered at full dose regardless of preexisting cytopenias. Hematologic toxicity was graded according to NCI Working Group guidelines and nonhematologic toxicity was assessed according to the NCI Common Toxicity Criteria.21 Toxicity was graded using the Common Toxicity Criteria (version 2) of the NCI supplemented by the CLL Working Group grading scale for hematologic toxicity.22
Risk-stratification parameters
The following were done: CD38 phenotype, fluorescence in situ hybridization (FISH)–detectable defects, immunoglobulin heavy-chain variable region (IgVH) status, and ZAP-70 levels. These risk-stratification parameters were done on blood cells from all patients as they enrolled in the clinical trial and were carried out as in previously published methods.23-25
Criteria for response
Responses were graded according to NCI Working Group criteria with bone marrows collected after 2 months' completion of 6 cycles of treatment.17 All responding patients were then followed-up at 3-month intervals for 5 years or until documented progression. For patients with CR, nodular PR, or PR, we evaluated for CLL B cells using 2-color flow cytometry at the 2-month bone marrow collection time point.
Estimation of MRD
For patients with clinical responses, we also tested for MRD using 2-color flow cytometry. Peripheral blood mononuclear cells (PBMCs) were separated from heparinized venous blood by density gradient centrifugation. To remove adherent cells, PBMCs were suspended in RPMI 1640 supplemented with 10% fetal calf serum and incubated in plastic dishes at 37°C for 1 hour prior to collection of nonadherent cells. These isolated PBMCs were then used for flow cytometry analysis. The 2-color flow involved determination of the percentage of cells positive for CD5+/CD19+ cells. The PBMCs were stained by a 2-color (fluorescein isothiocyanate [FITC]/phycoerythrin [PE]) flow cytometric assay. A FITC- and PE-conjugated anti-CD19 or anti-CD5 (BD Biosciences, San Diego, CA) was used to determine the presence of CD5+/CD19+ B cells. Mouse IgG1 and IgG2 (BD Biosciences) were included as isotype controls. A flow cytometry negative status was defined as patients with less than or equal to 1% positive CD5+/CD19+ cells.
Statistical design and analysis
A single-stage, phase 2 study, with an interim analysis, of pentostatin, cyclophosphamide, and rituximab (PCR) was conducted in patients with previously untreated B-cell CLL to assess the clinical complete response (CCR) rate of the treatment. This trial was designed to test the null hypothesis that the true CCR rate in this patient population is at most 25%. The smallest CCR rate that would imply that this regimen warranted further study was 50%. Due to meeting interim criteria as early evidence of positive results, an additional 32 patients were accrued to the original 33 to more fully evaluate the risk factors and correlative analyses as well as to provide more precision in evaluation of the toxicity profile and response rate in these patients treated with PCR. The distribution of time to response and duration of response was estimated using the method of Kaplan-Meier.26 Time to response was evaluated overall as well as by patient groups. In addition, key patient characteristics were compared between the 2 participating sites to evaluate differences in the types of patients accrued at each institution. Differences were evaluated using either the Fisher exact test, Wilcoxon rank sum test, or Kruskal-Wallis test, depending on whether the continuous variables being compared between 2 groups or more than 2 groups. Overall, statistical significance was declared for P < .05.
Results
Patient demographics
Overall, 65 patients were enrolled at the Mayo Clinic and The Ohio State University between March 2002 and July 2005. One major treatment violation occurred because the patient had inadvertently received concomitant therapy during the first cycle of treatment; therefore, this patient is excluded from all clinical outcome analyses, including toxicity. The characteristics of the remaining 64 evaluable patients are presented in Table 1, by participating site. Most of the patients accrued to this trial exhibited high-risk factors at study entry. The median age of study participants was 63 years (range, 38-80 years), with 18 (28%) being 70 years or older. The majority of patients (34 of 64; 53%) in this study had stage 3 or 4 high-risk disease by Rai criteria at study entry.27 Molecular features including IgVH mutational analysis and interphase cytogenetics, as shown in Table 1, demonstrated that a high proportion of the CLL patients enrolled had high-risk disease representative of phase 3 studies reported by others.11,28 It is of interest that only one third of our patients were ZAP-70+. These findings do, however, point out that populations of progressive CLL may be quite heterogeneous even in terms of risk stratification as we have pointed out before.29
Patient characteristics by site
| Characteristic . | Site 1 . | Site 2 . | P* . |
|---|---|---|---|
| No. | 41 | 23 | |
| Rai stage | |||
| Low | 2 | 1 | |
| Intermediate | 13 | 14 | .049 |
| High | 26 | 8 | |
| 0 | 2 | 1 | |
| 1 | 4 | 7 | |
| 2 | 9 | 7 | .019 |
| 3 | 12 | 5 | |
| 4 | 14 | 3 | |
| Sex | |||
| Male | 32 | 17 | .76 |
| Female | 9 | 6 | |
| Age, y | |||
| Younger than 70 | 29 | 17 | |
| 70 and older | 12 | 6 | .99 |
| ECOG PS | |||
| 0 | 29 | 5 | |
| 1 | 11 | 13 | .001 |
| 2 | 1 | 4 | |
| 3 | 0 | 1 | |
| CD38+ B cells | |||
| 30% or less | 26 | 15 | .99 |
| Greater than 30% | 13 | 8 | |
| ZAP-70 B cells | |||
| 20% or less | 20 | 15 | .07 |
| Greater than 20% | 15 | 3 | |
| VH status | |||
| Mutated | 12 | 6 | .99 |
| Nonmutated | 29 | 15 | |
| FISH | |||
| Normal | 6 | 1 | |
| +12 | 8 | 3 | |
| 11q- | 9 | 4 | |
| 17p- | 0 | 3 | |
| 6q- | 1 | 0 | |
| 13q- | 10 | 10 | |
| 13q - x1 | 7 | 8 | |
| 13q - x2 | 3 | 2 | |
| Other | 2 | 1 | |
| No. of genetic defects | |||
| 0 | 6 | 1 | |
| 1 | 16 | 14 | .90 |
| 2+ | 14 | 7 | |
| Bone marrow pattern | |||
| Diffuse | 12 | 19 | < .001 |
| Not diffuse | 29 | 2 | |
| Characteristic . | Site 1 . | Site 2 . | P* . |
|---|---|---|---|
| No. | 41 | 23 | |
| Rai stage | |||
| Low | 2 | 1 | |
| Intermediate | 13 | 14 | .049 |
| High | 26 | 8 | |
| 0 | 2 | 1 | |
| 1 | 4 | 7 | |
| 2 | 9 | 7 | .019 |
| 3 | 12 | 5 | |
| 4 | 14 | 3 | |
| Sex | |||
| Male | 32 | 17 | .76 |
| Female | 9 | 6 | |
| Age, y | |||
| Younger than 70 | 29 | 17 | |
| 70 and older | 12 | 6 | .99 |
| ECOG PS | |||
| 0 | 29 | 5 | |
| 1 | 11 | 13 | .001 |
| 2 | 1 | 4 | |
| 3 | 0 | 1 | |
| CD38+ B cells | |||
| 30% or less | 26 | 15 | .99 |
| Greater than 30% | 13 | 8 | |
| ZAP-70 B cells | |||
| 20% or less | 20 | 15 | .07 |
| Greater than 20% | 15 | 3 | |
| VH status | |||
| Mutated | 12 | 6 | .99 |
| Nonmutated | 29 | 15 | |
| FISH | |||
| Normal | 6 | 1 | |
| +12 | 8 | 3 | |
| 11q- | 9 | 4 | |
| 17p- | 0 | 3 | |
| 6q- | 1 | 0 | |
| 13q- | 10 | 10 | |
| 13q - x1 | 7 | 8 | |
| 13q - x2 | 3 | 2 | |
| Other | 2 | 1 | |
| No. of genetic defects | |||
| 0 | 6 | 1 | |
| 1 | 16 | 14 | .90 |
| 2+ | 14 | 7 | |
| Bone marrow pattern | |||
| Diffuse | 12 | 19 | < .001 |
| Not diffuse | 29 | 2 | |
P values represent where significant differences were seen between the 2 participating sites.
Toxicity and tolerability
At the time of this report, all patients have completed active treatment. Some patients discontinued treatment early for the following reasons: disease progression (3 patients, or 4.7%), refusing further treatment (4 patients, or 6.3%), unacceptable toxicity (3 patients, or 4.7%), other medical problems (1 patient, or 1.6%), and death during treatment (2 patients, or 3.2%). While on treatment, 25 patients (39%) had the dose held or modified, and 14 of these patients had dose delays or modifications due to hematologic adverse events. Of these 14 patients, 9 patients (14%) had their dose held, 3 patients (4.7%) had a dose modification, and 2 patients (3.2%) had both a delay and a modification. All of these delays or modifications to dose levels occurred at cycle 4 or beyond. In detail, of the 11 patients who had a dose delay due to hematologic adverse events, 7 occurred on cycle 6, 2 on cycle 4, 2 on both cycles 4 and 6, and 1 on cycles 4 and 5.
The frequency and severity of the severe toxicities (grade 3 or greater) are shown in Table 2. Overall, 34 patients had a maximum of grade 3 or 4 hematologic adverse events and 28 patients had a maximum of grade 3+ nonhematologic adverse events (regardless of perceived attribution). In terms of a cycle-based analysis of tolerability, only 16.4% of treatment cycles had reported grade 3 or greater neutropenia and only 4.5% had reported grade 3 or greater thrombocytopenia. For all infections (with or without neutropenia, major or minor), only 1% of treatment cycles had grade 3 or greater events reported.
MC0183: Toxicity
| Toxicity . | Grade . | ||
|---|---|---|---|
| 3 . | 4 . | 5 . | |
| Secondary malignancy | 0 | 1 | 0 |
| Neutropenia | 12 | 14 | 0 |
| Leukopenia | 4 | 1 | 0 |
| Transfusion, pRBCs | 2 | 0 | 0 |
| Neutropenia, BMT | 1 | 0 | 0 |
| Platelets | 11 | 2 | 0 |
| Hemoglobin | 1 | 0 | 0 |
| Infection, ANC | 6 | 0 | 0 |
| Hyperuricemia | 1 | 0 | 0 |
| Ataxia | 1 | 0 | 0 |
| Neurosensory | 1 | 0 | 0 |
| Myalgia | 1 | 0 | 0 |
| Neuralgia | 1 | 0 | 0 |
| Pain | 1 | 0 | 0 |
| Dyspnea | 1 | 1 | 0 |
| Hypoxia | 1 | 1 | 1 |
| Autoimmune disorder | 1 | 0 | 0 |
| Syndromes | 1 | 0 | 0 |
| Hypotension | 2 | 0 | 1 |
| Sinus bradycardia | 0 | 1 | 0 |
| Ischemia/infarction | 0 | 1 | 0 |
| Fatigue | 1 | 0 | 0 |
| Fever, no ANC | 4 | 0 | 0 |
| Pruritus | 1 | 0 | 0 |
| Rash | 1 | 0 | 0 |
| Nausea | 6 | 0 | 0 |
| Vomiting | 4 | 0 | 0 |
| Toxicity . | Grade . | ||
|---|---|---|---|
| 3 . | 4 . | 5 . | |
| Secondary malignancy | 0 | 1 | 0 |
| Neutropenia | 12 | 14 | 0 |
| Leukopenia | 4 | 1 | 0 |
| Transfusion, pRBCs | 2 | 0 | 0 |
| Neutropenia, BMT | 1 | 0 | 0 |
| Platelets | 11 | 2 | 0 |
| Hemoglobin | 1 | 0 | 0 |
| Infection, ANC | 6 | 0 | 0 |
| Hyperuricemia | 1 | 0 | 0 |
| Ataxia | 1 | 0 | 0 |
| Neurosensory | 1 | 0 | 0 |
| Myalgia | 1 | 0 | 0 |
| Neuralgia | 1 | 0 | 0 |
| Pain | 1 | 0 | 0 |
| Dyspnea | 1 | 1 | 0 |
| Hypoxia | 1 | 1 | 1 |
| Autoimmune disorder | 1 | 0 | 0 |
| Syndromes | 1 | 0 | 0 |
| Hypotension | 2 | 0 | 1 |
| Sinus bradycardia | 0 | 1 | 0 |
| Ischemia/infarction | 0 | 1 | 0 |
| Fatigue | 1 | 0 | 0 |
| Fever, no ANC | 4 | 0 | 0 |
| Pruritus | 1 | 0 | 0 |
| Rash | 1 | 0 | 0 |
| Nausea | 6 | 0 | 0 |
| Vomiting | 4 | 0 | 0 |
These are side effects felt to be at least possibly related to treatment.
BMT indicates bone marrow transplantation; ANC, absolute neutrophil count.
Even if we include all cycles of evaluation (treatment and actively monitored observation), only 2% of cycles had grade 3 or greater infection of any type reported. Focusing only on those deemed at least possibly related to treatment, 22 patients had a maximum of grade 3+ nonhematologic toxicity and 33 had a grade 3+ hematologic toxicity. The most common severe toxicities were hematologic, where 26 patients (41%) had grade 3 or 4 neutropenia and 13 patients (21%) had grade 3 or 4 thrombocytopenia. Despite these toxicities, only 2 patients had a transfusion of packed red blood cells (pRBCs) that was felt to be attributable to treatment. One received transfusions on cycles 2, 3, and 4 but completed treatment. The other patient received transfusions on cycle 1 but also had many other unrelated nonhematologic severe events and discontinued treatment after that cycle. Three other patients required transfusions (2 pRBCs, 1 platelets) not considered related to treatment and all occurred on cycle 1 of therapy. The most common grade 3 or greater nonhematologic toxicities included nausea (n = 6), infection (n = 6), vomiting (n = 4), and fever without neutropenia (n = 4). The infections were related to upper respiratory tract sites. Two patients died during the study. One patient developed fulminate fever, hypoxia, and hypotension following the first cycle of therapy and died. No progression of disease was documented in this patient, cultures were negative, and death was felt to possibly be related to the study treatment. The other patient experienced grade 5 hypoxia and pneumonia that was possibly related to study treatment. Both patients had comorbid illnesses including chronic obstructive pulmonary disease and uncontrolled diabetes that may have contributed to the risk of adverse treatment outcome with treatment. Two patients had a possible autoimmune hemolytic anemia with one patient developing hemolysis on cycle 2 but completing therapy and achieving an nodular PR with the other patient developing hemolysis on cycle 1 but needing to be removed from treatment because of disease progression.
Response to therapy
Overall, 40 of all 64 patients (63%) had a CCR. Although the CCR rate was the primary end point of this trial, we also evaluated response rate by NCI Working Group 1996 criteria.19 Of the 64 patients, 26 (41%; 95% CI, 29%-54%) attained a CR, 14 (22%) a nodular PR, and 18 (28%) a PR. Thus, the overall response (CR plus nodular PR, and PR) with this treatment was 91% (95% CI; 81%-96%). Of the 58 patients who responded to treatment, the median time to first documentation of any response (ie, CCR or PR) was 33 days (range, 24-110 days). Interestingly, there was a significant difference in the time to response between patients at the 2 participating sites, indicating a potential differentiation in the patients at each of the sites (Figure 1). Specifically, the median time to response for site 1 patients was 29 days versus 75 days for site 2 patients (P < .001). In looking further at differences in the patients enrolled at each of the 2 sites, the only statistically significant differences were that site 2 patients had a worse pretreatment performance status (P = .001), higher pretreatment Rai stages (P = .019), and more diffuse bone marrow patterns pretreatment (P < .001; Table 1). Beyond site, the only other clinical factor significantly associated with time to response was whether or not patients had a diffuse bone marrow pattern at baseline; however, when both were included in a multivariable model, only site remained significant.
The time to NCI Working Group 96 response between patients enrolled at site 1 versus site 2.
The time to NCI Working Group 96 response between patients enrolled at site 1 versus site 2.
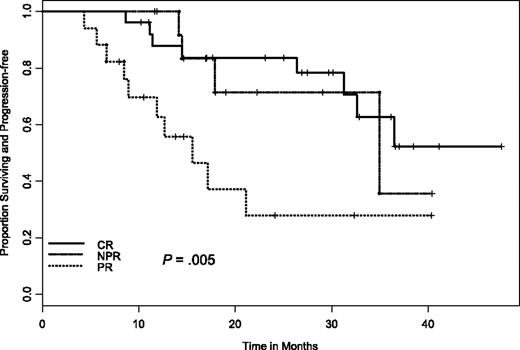
At the time of these analyses, only 9 patients have died and 24 have progressed. The median follow-up on living patients is 26 months (range, 4-48 months). For duration of response in those 58 patients who responded to therapy, the estimated median duration of response was 34 months (95% CI, 25.3 to not yet reached). A significant difference was observed in duration of response between those who did achieve a CR and those who did not (median, 35.6 versus 20.3 months, respectively; P = .038). Similarly, there was a significant difference in duration of response if we combined nodular PR and CR responders together versus PR responders (median, 35.6 versus 13.1 months, respectively; P = .002) as well as across each response group (CR versus nodular PR versus PR; P = .005; Figure 2). It is important to note that despite the significant difference in time to response between sites, there was no significant difference in duration of response or progression-free survival (PFS) by site (P = .42).
Time to progression in relation to NCI Working Group 96 response criteria. CR indicates complete remission; NPR, nodular partial remission; PR, partial remission.
Time to progression in relation to NCI Working Group 96 response criteria. CR indicates complete remission; NPR, nodular partial remission; PR, partial remission.
Examination of outcome for all 64 patients enrolled in this study demonstrates a median PFS of 32.6 months (95% CI, 21.1 to not yet reached). For all 64 patients, 28 patients had either died or progressed and were considered an event. Overall, 5 patients progressed or died within 6 months of study entry. These included 2 PR patients (1 of whom completed treatment but progressed right after), 2 patients who had, at best, stable disease, and the patient who refused treatment after the first cycle and subsequently progressed and died shortly thereafter. FISH diagnosis at baseline was available for all 5 patients, 3 of whom were del(17p13.1), one was del(13q12-14), and the other normal. No other known risk factors appeared to influence this rapid progression or death in these patients.
Assessment of MRD following treatment
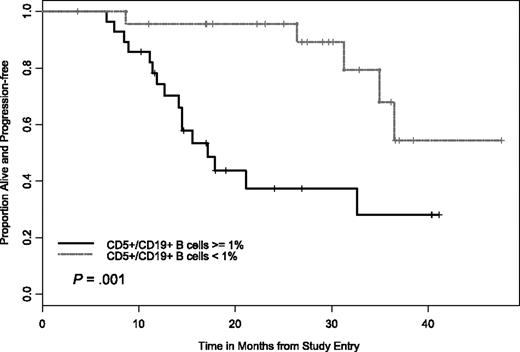
Given the results of previous studies demonstrating that elimination of MRD (as measured by 2-color flow cytometry) correlates with extended PFS, we examined this as part of our trial. These data were available on 52 patients, where 24 had fewer than 1% CD5+/CD19+ B cells with 19 of these patients having a CR and 5 a nodular PR. Examination of outcome among patients with CR and nodular PR for PFS by flow cytometry status (negative versus positive, ie, ≤ 1% CD5+/CD19+ versus ≥ 1% CD5+/CD19+) demonstrated improvement in outcome for patients who attained flow cytometry negativity (Figure 3). More patients with a CR exhibited this “elimination” of MRD than did patients with a nodular PR (23 versus 14, P = .009) In addition, those patients who achieved an “elimination” of MRD had a PFS advantage over those who did not as shown in Figure 3 (HR = 0.22, P = .003).
PFS by CD5+/CD19+ levels. Difference in the 2 curves was significant at P < .001.
PFS by CD5+/CD19+ levels. Difference in the 2 curves was significant at P < .001.
Correlative prognostic factors for predicting treatment outcome
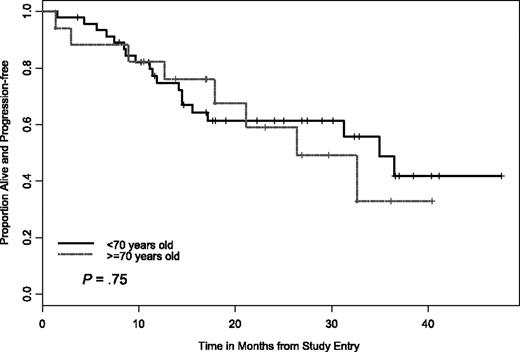
In addition to the clinical baseline characteristics, biologic correlative markers that can indicate risk status for CLL progression or treatment response were also evaluated relative to treatment response and PFS. We were also interested in determining if PCR therapy can be equally effective for older patients (≥ 70 years) with CLL. Analysis with statistical comparison including CR, nodular PR, attainment of flow cytometry-negative status, and PFS were done for the following groups; IgVH mutational status (≤ 98% versus ≥ 98% from germline sequence) CD38+ versus CD38− patients (≤ 30% as cutoff for negative), ZAP-70+ versus ZAP-70− patients (≤ 20% as cutoff for negative), and comparison of high-risk versus low-risk FISH groups. For example, we considered del(17p13.1), del(11q22.3), trisomy (+) 12 patients or complex FISH defects (ie, more than one FISH defect) as high-risk versus normal FISH or del(13q12-14) FISH as low risk. Importantly, no high-risk factor (ie, age, FISH, mutational status, CD38+, ZAP-70+) precluded attaining a CR or nodular PR. In addition, our analysis found that PCR treatment was equally effective at inducing durable responses in patients with del(11q22.3) and patients 70 years of age and older versus patients 70 years of age and younger (Figures 4–5).
PFS by del(11q22.3) versus other FISH cohorts. There was no significant difference in PFS between the 2 CLL cohorts.
PFS by del(11q22.3) versus other FISH cohorts. There was no significant difference in PFS between the 2 CLL cohorts.
PFS by age cutoff of 70 years. There was no significant difference in PFS between the 2 age cohorts.
PFS by age cutoff of 70 years. There was no significant difference in PFS between the 2 age cohorts.
Discussion
In this multicenter phase 2 study, we describe a novel combination-based therapy of pentostatin, cyclophosphamide, and rituximab (PCR) for previously untreated patients with CLL in which we demonstrate that 63% of patients attain either a CR or nodular PR to therapy. This high level of response was not accompanied by excessive marrow toxicity or serious infections. Similar to other combination regimens such as fludarabine, cyclophosphamide, and rituximab (FCR),17 treatment with PCR resulted in a high frequency of flow cytometry–negative bone marrows at completion of therapy that was significantly associated with extended PFS. To our knowledge, the PCR regimen differentiates itself from fludarabine-based combinations such as FCR in that subset analysis of young (< 70 years of age) versus older (≥ 70 years of age) patients had similar benefit from treatment. Specifically, CLL patients over the age of 70 did not carry excessive morbidity and adverse outcome as compared to younger patients. These results contrast with patients over the age of 70 treated with FCR who had a worse outcome. Finally, in our study, we were able to demonstrate that unlike fludarabine or fludarabine-rituximab–containing regimens, patients with high-risk del(11q22.3) interphase cytogenetic abnormalities appear to have a similar PFS as do those patients without high-risk interphase cytogenetics. Thus, the PCR-based treatment represents a potential advance for CLL treatment that now requires additional testing in randomized trials against alternative standard combination therapies.
The favorable outcome of patients receiving chemoimmunotherapy in this study is, in part, reflective of the lack of significant or unacceptable hematologic toxicity. Despite the majority of patients included in this trial with advanced stage, the degree of marrow suppression resulted in little or no need for red cell or platelet transfusions. Only 14 patients required delays or dose modifications for treatment based on hematologic toxicities. In addition, with the exception of 1 patient who may have died from bacterial sepsis within 1 cycle of therapy, we detected only 5 patients with documented infections that were felt to be at least possibly related to treatment. The dose of pentostatin was chosen at 2 mg/m2 because of 2 factors: (1) earlier work showed this dose in combination with chlorambucil can be associated with significant neutropenia13 and (2) we know this dose of pentostatin can completely inhibit adenosine deaminase function for 10 to 14 days (M.R.G., personal communication, 2006). Although aggressive prophylaxis with antibiotics, antiviral therapy, and growth factor support were used, our regimen was both feasible and well tolerated in all subsets of patients with CLL including those over the age of 70. The importance of this distinction from other combination therapies such as FCR is important for several reasons. In the FCR treatment reported by Keating et al,17 patients over the age of 70 did not appear to gain the dramatic benefit seen from that regimen in younger patients. CLL in the community represents a disease of the elderly with a median age at diagnosis of 71 years, making aggressive combinations such as FCR less applicable to the general population of individuals with this disease. In contrast, our data preliminarily support that PCR might be effective in this subset of patients. A final important contrast that is very relevant for older patients with CLL is the ease of therapy. In our estimation, PCR treatment requires only 8 physician office visits over a 4-month period. In contrast, FCR requires 18 physician visits over 6 months. Although our data are preliminary, they support further exploration of the PCR regimen in older patients with CLL.
An important finding of our work is confirmation of previous studies demonstrating that attainment of flow cytometry–negative bone marrow status at completion of therapy correlates with a significantly longer PFS.17 Patients in our trial with a CR had a higher frequency of flow cytometry–negative bone marrows as compared to those with nodular PR. Although clinical practice often has not included posttreatment bone marrow assessment due to presence of remaining externally measurable disease, newer therapies such as PCR and FCR can result in a majority of patients attaining flow cytometry–negative disease. Use of this easily obtained diagnostic test provides information for patients relative to their expected remission duration and might also identify a subset that would benefit from consideration of additional cytoreduction in well-defined clinical trials. The CLL Research Consortium is currently performing such a trial with cytoreductive alemtuzumab for patients having only flow cytometry–evident disease following cytoreductive therapy. Flavopiridol is another therapy that is being pursued in this setting within the CLL Research Consortium.
Unlike other trials that reported only clinical data, our study includes a comprehensive battery of pretreatment prognostic factors that have been identified in previous work to be important for predicting disease progression from time of diagnosis. Consistent with prior reports,30-33 CLL patients with del(17p13.1) did not fare well in our study. Thus, our small subset (n = 3) of patients with del(17p13.1) failed to gain meaningful response and had a shorter PFS and overall survival as compared to the other patients enrolled in this trial. In contrast to regimens using fludarabine or fludarabine and rituximab,28,34 patients with del(11q22.3) performed similarly with respect to both response and PFS. Our data suggest that PCR may overcome the adverse treatment outcome observed by others in patients with del(11q22.3). In addition, our data support that alternative therapies likely will be required for patients with del(17p13.1) disease.
Pursuit of alternative regimens for patients with newly diagnosed CLL outside of FCR might be criticized based on promising results reported by Keating and colleagues.17 In 224 patients, an overall response rate was found to be 95%, with a 70% complete remission rate with only 10 of 204 not responding. Although our CR rate with PCR is lower than that observed with FCR, a greater percentage of the FCR patients were Rai stage 0 to 2 and younger than 70 years of age compared with those treated in our study. For each of these disease subsets, a higher CR would be expected. Additionally, the FCR study was performed at a single institution. It is of interest that the intergroup study of concurrent versus sequential rituximab with fludarabine had response rates not dissimilar from our PCR study (ie, 90% overall response rates with a CR rate of 47% for concurrent rituximab).35 Our own study demonstrates the value of including more than one center because we observed differences in time to response between the 2 participating sites. This aspect further emphasizes the utility of evaluating these agents across multiple sites to obtain results more widely applicable to the CLL research community. As noted in Table 1 and reviewed in “Results,” there were some distinct clinical and biologic differences in the B-cell clone for each site despite the common eligibility criteria. Trials conducted through a multi-institution group can help make the results of these trials more applicable to the community because they reflect practices at several institutions.
In summary, the PCR regimen for previously untreated relatively high-risk CLL patients outlined in this report was very well tolerated and yielded high response rates including flow cytometry negativity in a subset of patients. In this study we also provide validation of flow cytometry–negative status at the completion of therapy as significantly associated with extended PFS as compared with the patients assessed at this same time with evidence of residual disease. In this multicenter study, we demonstrated that many prognostic factors used to predict early CLL progression had no impact on prognosis for treatment outcome. Finally, our data demonstrate that PCR was beneficial to virtually all subsets of symptomatic patients with CLL including those with del(11q22.3) and older individuals except those possessing del(17p13.1). Based on these data, we propose that future comparative studies with this novel regimen versus FR or FCR are warranted.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: N.E.K. designed and performed research, contributed vital reagents and analytical tools, collected and analyzed data, and wrote the paper; S.M.G. analyzed data and wrote the paper; T.G.C. designed and performed the research; T.D.S. designed and performed research and wrote the paper; C.S.Z. designed and performed the research; D.F.J. collected and analyzed data and wrote the paper; R.T. performed research and collected data; N.D.B. performed research and collected data; G.W.D. designed the research and collected and analyzed data; T.S.L. designed and performed research; N.A.H. performed research and collected data; L.S. designed and performed research; M.R.G. designed and performed research; and J.C.B. designed and performed research, collected and analyzed data, and wrote the paper.
Acknowledgment
We thank Ms Susan McLean for her administrative support.
This work was supported in part by a grant from the National Institutes of Health/National Cancer Institute CA 95241 and by philanthropic support from Mr E. Spencer and Mr R. Donner.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal