Abstract
The EEN (extra eleven nineteen) gene, located on chromosome 19p13, was cloned as a fusion with MLL from a patient with acute myeloid leukemia (AML) with translocation t(11;19)(q23;p13). In this study, we characterized the genomic structure of the EEN gene, including its 5′ regulatory region and transcription start site (TSS). We found that Sp1 could bind to the guanine-cytosine (GC)–stretch of the EEN promoter and was critical for the normal EEN expression, whereas the leukemia-associated fusion protein AML1-ETO could aberrantly transactivate the EEN gene through an AML1 binding site. Of note, overexpressed EEN showed oncogenic properties, such as transforming potential in NIH3T3 cells, stimulating cell proliferation, and increasing the activity of transcriptional factor AP-1. Retroviral transduction of EEN increased self-renewal and proliferation of murine hematopoietic progenitor cells. Moreover, Kasumi-1 and HL60-cell growth was inhibited with down-regulation of EEN by RNAi. These findings demonstrate that EEN might be a common target in 2 major types of AML associated with MLL or AML1 translocations, and overexpression of EEN may play an essential role in leukemogenesis.
Introduction
The t(8;21) is one of the most frequent chromosomal translocations associated with human acute myeloid leukemia (AML),1,2 accounting for approximately 40%-80% of M2 AML and 12%-20% of all de novo AML cases. The translocation juxtaposes the AML1 gene on chromosome 21 to the ETO gene on chromosome 8, resulting in AML1-ETO fusion, which has been demonstrated to possess transcriptional activity. AML1 controls a number of hematopoietic genes by binding to the core enhancer sequence TGT/CGGT,3 whereas AML1-ETO fusion protein functions mainly as a dominant-negative repressor on AML1 target genes. Moreover, AML1-ETO also targets genes not controlled by AML1, such as Bcl2,4 TIS11b,5 and G-CSF,6 indicating the unique gain-of-functions of AML1-ETO. However, how these AML1-ETO target genes are related to the etiology of leukemia remains unclear.
The MLL (myeloid-lymphoid leukemia) gene, located on 11q23, is involved in another type of recurrent cytogenetic abnormality in hematologic malignancies, especially in infant acute leukemia and therapy-related leukemia.7-9 To date, 87 chromosomal regions were reported to be translocated to 11q23, and 51 MLL fusion partner genes have been cloned.10 Among these events, MLL is broken in an 8.3-kb breakpoint cluster region (BCR) and fused in-frame with its corresponding partners, which generates aberrant fusion protein responsible for leukemogenesis.11
The EEN (extra eleven nineteen) gene is a fusion partner of MLL, identified on chromosome 19p13.12 EEN belongs to the Src homology 3 (SH3) domain–containing protein family.13 So far, the physiologic function of EEN has not been well established. EEN was previously reported to play a role in endocytosis and signal transduction. It was the binding partner of endocytic proteins, such as synaptojanin, dynamin, and amphiphysin, which were implicated in synaptic vesicle trafficking.14-16 EEN was also a binding partner of the G protein–coupled β1-adrenergic receptor.17 And EEN could interact with voltage-gated Ca2+ channels in synaptic vesicle endocytosis.18 EEN could bind BPGAP1 and was involved in the activation of EGF receptor endocytosis and ERK1/2 signaling.19 Through its binding partner EBP (EEN binding protein), EEN was engaged in Ras signaling and cellular transformation.20 However, the functions of EEN in the hematopoietic cells and in oncogenesis are poorly understood. And it is particularly interesting to note that EEN is the only member in the family expressed in hematopoietic cells.12
To explore how the expression of the EEN gene is regulated, we tried to identify the regulatory elements of EEN. Interestingly, we found that AML1-ETO fusion protein could aberrantly regulate EEN transcription. Moreover, overexpression of EEN turned out to be oncogenic and down-regulation of EEN attenuated cell growth. Our findings indicate that EEN might be a common target in leukemogenesis associated with AML1-ETO or MLL fusion proteins.
Materials and methods
Genomic library screening and DNA sequencing
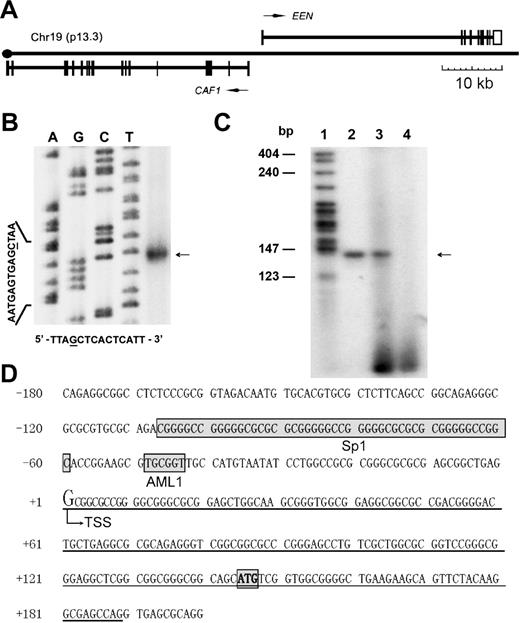
The human genomic DNA EMBL λ phage library (Clontech, Palo Alto, CA) was screened using an EEN cDNA probe and 1 positive clone, EEN5, corresponding to the sequence of the EEN gene from intron 1 to exon 10 without exon 1, was obtained. To get the entire EEN gene, we screened the human BAC library from the California Institute of Technology (CITB) (Research Genetics, Huntsville, AL) using EEN intron 2 as the probe and obtained 1 positive BAC clone, BAC335C9, which covered the rest of the EEN genomic sequence. EEN5 and BAC335C9 were subjected to DNA sequencing using shotgun strategy with BigDye Terminator Cycle Sequencing Kits (Applied Biosystems, Foster City, CA). With a coverage of 10-fold, sequences were assembled and finished with the Phred/Phrap/Consed package (University of Washington; www.phrap.org) (Figure 1A).21
Genomic structure and transcription start site (TSS) of the EEN gene. (A) Schematic diagram of the structure of the contig-containing EEN gene and head-to-head–located CAF1 gene. (B) Identification of the TSS of the EEN gene by RNase protection assay. The sequencing result of M13mp18 is shown as the length reference (left 4 lanes). The corresponding M13mp18 sequence is shown at the bottom. The protected RNA band is indicated by the arrow. (C) Further verification of the EEN TSS by primer extension assay. A specific reverse transcription primer EEN-exten was end-labeled with γ-32P by T4 polynucleotide kinase, annealed to 20 μg total RNA from HL60 cells, and extended by reverse transcriptase. The extension product (lane 3) was analyzed on 7M urea, 8% polyacrylamide gel along with a 32P-labeled 140 bp PCR product (lane 2) and MspI-digested pBR322 (lane 1). Lane 4 was loaded with blank control without RNA in the reverse transcription reaction. (D) The genomic sequence containing the TSS of the EEN gene. The first exon is underlined and the start codon is bolded and boxed. TSS is used as the reference point (+1, with an enlarged G) for numbering the EEN promoter sequence. The positions of the SP1 and AML1 binding sites are also boxed.
Genomic structure and transcription start site (TSS) of the EEN gene. (A) Schematic diagram of the structure of the contig-containing EEN gene and head-to-head–located CAF1 gene. (B) Identification of the TSS of the EEN gene by RNase protection assay. The sequencing result of M13mp18 is shown as the length reference (left 4 lanes). The corresponding M13mp18 sequence is shown at the bottom. The protected RNA band is indicated by the arrow. (C) Further verification of the EEN TSS by primer extension assay. A specific reverse transcription primer EEN-exten was end-labeled with γ-32P by T4 polynucleotide kinase, annealed to 20 μg total RNA from HL60 cells, and extended by reverse transcriptase. The extension product (lane 3) was analyzed on 7M urea, 8% polyacrylamide gel along with a 32P-labeled 140 bp PCR product (lane 2) and MspI-digested pBR322 (lane 1). Lane 4 was loaded with blank control without RNA in the reverse transcription reaction. (D) The genomic sequence containing the TSS of the EEN gene. The first exon is underlined and the start codon is bolded and boxed. TSS is used as the reference point (+1, with an enlarged G) for numbering the EEN promoter sequence. The positions of the SP1 and AML1 binding sites are also boxed.
Plasmids
The region −309 to +83 of the EEN promoter was inserted into pBlueScript II SK(+) (plasmid pBS-EENP) to produce antisense RNA probe for an RNase protection assay (RPA). Different fragments of the EEN promoter, corresponding to the regions from −59, −155, −248, −336, −439, −530, or −621 to +67, were inserted into pGL3-Basic plasmid and named pGB-EENP1 to pGB-EENP7, respectively. To produce mutant EEN promoter reporter plasmids, the Sp1 binding site (−107 to −60) and the AML1 binding site (−49 to −44) in pGB-EENP7 were mutated into AAGCTT using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) and named pGB-EENP-ΔSp1 and pGB-EENP-ΔAML1, respectively. A full-length Sp1 coding sequence from IMAGE clone 5 928 633 was inserted into pGEX-4T3 (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom; for use in electrophoretic mobility shift assay [EMSA]) and pSG5M (Stratagene). Full-length EEN was inserted into pEGFP-C1 (Clontech; plasmid pEGFP-EEN) and MIGR1. For EMSA, reporter luciferase assay, and chromatin immunoprecipitation, Flag-tagged AML1-ETO, AML1b, and AML1-ETO with the DNA binding domain mutation (L148D)22 were subcloned into pSG5M. All constructs were confirmed by DNA sequencing.
RNase protection and primer extension assays
RNase protection assay (RPA) was performed with RPA Starter Package (Pharmingen, San Diego, CA). Antisense RNA probe was transcribed using T7 RNA polymerase from linearized pBS-EENP. The probe was incubated with total RNA from HL60 cells and the RNase protection fragment was separated in polyacrylamide gel electrophoresis (PAGE). The sequencing result of M13mp18 with the T7 Sequencing Kit (Amersham Pharmacia Biotech) was taken as the length reference of the fragment. The primer used in the sequencing reaction was gtaaaacgacggccagt. Primer extension assay was carried out according to the Primer Extension System (Promega, Madison, WI) instructions. The specific reverse transcription primer (EEN-exten: 5′-atgctgccgcccgccgccgag), a 140-bp polymerase chain reaction (PCR) fragment, and DNA marker MspI-digested pBR322 were labeled with γ-32P ATP. The reverse transcription product was run on an 8% denaturing polyacrylamide gel, then fixed and autoradiographed.
Cell culture, transfection, and electroporation
HL60, K562, and U937 cells were cultured in RPMI-1640 (< 1 × 106/mL). NIH3T3 and COS7 cells were cultured in Dulbecco modified Eagle medium (DMEM). The media were supplemented with 10% fetal calf serum (FCS; Gibco BRL, Gaithersburg, MD), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. All cell cultures were incubated at 37°C in humidified air with 5% CO2. Transfections of NIH3T3 and COS7 cells were carried out with SuperFect (Qiagen, Hilden, Germany) according to the manufacturer's instructions. Transfection of HL60, K562, and U937 cells was performed by electroporation using the Gene Pulser Electroprotocol (Bio-Rad Laboratories, Hercules, CA). The stable cell lines were obtained after cells were cultured in the medium plus 1 mg/mL G418 (Promega) for about 20 days, and were maintained in 0.2 mg/mL G418. For all studies, pooled populations of G418-resistant cells were used to eliminate clone variations.
RT-PCR and Western blot
Total RNA was isolated using Trizol LS Reagent (Invitrogen, Carlsbad, CA) and treated with DNase I to remove contaminating genomic DNA. RNA (1 μg) was converted to random-primed cDNA using the SuperScript first-strand synthesis system (Invitrogen) according to the manufacturer's instructions. Genomic DNA and cDNA were subjected to PCR analysis. The primers for EEN amplification are: forward 5′-cagctggtcagtgagaaggtc, reverse 5′-gccggcatccagcaatgcgtc. The primers for AML1-ETO amplification are: forward 5′-gttgtgatgcgtatccccgtag, reverse 5′-ctggttcttggagctccttgag. The primers for CAF1 amplification are: forward 5′-aacgacaagttggcatttcc, reverse 5′-cataggcaccttccctttga. The primers for Sp1 amplification are: forward 5′-tgcagcagaattgagtcacc, reverse 5′-cacaacatactgcccaccag. The primers for G3PDH amplification are: forward 5′-cctgcaccaccaactgctta, and reverse 5′-gcctgcttcaccaccttctt. Quantitative real-time reverse transcription (RT)–PCR was conducted using an iCycler iQ real-time PCR detection system (Bio-Rad) with SYBR green I fluorescent dye (Applied Biosystems). The RNA level of EEN and/or CAF1 was analyzed in the following samples: Kasumi-1, an AML cell line harboring t(8; 21); HL60, an AML cell line with promyelocytic features; U937, a monocytic cell line; NB4, an AML-M3 cell line with t(15; 17); Jurkat, a T-ALL cell line; Namalwa and Raji, human Burkitt lymphoma cell lines; and primary samples from AML (including AML-M2 subtypes with or without t(8; 21) and resultant AML1-ETO and AML-M3 subtype) and chronic myeloid leukemia (CML). AML and CML each included 8 patient samples.
Stably transfected U937, NIH3T3, HL60, and 293T cells were lysed in SDS sample buffer. After separation on standard 10% SDS-PAGE gel, proteins were blotted to nitrocellulose. The antibodies against GFP, ETO (C-20), EEN (S-20), and Sp1(SC-59) were provided by Santa Cruz Biotechnology (Santa Cruz, CA). Bands were quantified using the volume tools function in Quantity One software (Bio-Rad).
Luciferase assay
Cells were transfected by luciferase reporter vectors alone or cotransfected by mammalian expression vectors. Parent empty expression vectors were cotransfected as a control. The pRL-TK (Promega) vector constitutively expressing Renilla luciferase was also cotransfected as internal control. After incubation for 36 hours, cells were harvested and subjected to the luciferase assay using the Dual-Luciferase Reporter assay system (Promega) on a Lumat LB 9507 luminometer (EG&G Berthold, Bad Wildbad, Germany). The relative luciferase units (RLUs) were calculated according to the firefly luciferase activities normalized by the activities of Renilla luciferase. Each set of experiments was repeated at least 3 times.
EMSA
GST fusion protein Sp1 was expressed and purified from host strain BL21 and used in the EMSA experiments with a DIG Gel Shift Kit (Roche Diagnostic, Mannheim, Germany). Flag-tagged protein AML1-ETO, AML1b, and AML1-ETO (L148D) were produced using the TNT Quick Coupled Transcription/Translation System (Promega) and verified by Western blot with anti–FLAG M2 antibody (Sigma, St Louis, MO). Two complementary oligonucleotides (forward, 5′-ccggaagcgtgcggttgccatgtaatat; reverse, 5′-atattacatggcaaccgcacgcttccgg) containing the AML1 binding site were annealed and labeled with γ-32P as the probe for EMSA. Kasumi-1 nuclear extracts were prepared as previously described.23 Supershift analysis was accomplished by first incubating nuclear extracts with 2 μg ETO antibody (C-20) for 20 minutes at room temperature, then adding labeled probe or 100-fold molar excess of unlabeled competitor, and incubating for 30 minutes longer on ice.
Chromatin immunoprecipitation and PCR detection
Chromatin immunoprecipitation (ChIP) was performed following the described protocol24 using anti–FLAG M2 antibody. A quantity of 10 μg pSG5M, pSG5M-AML1b, pSG5M-AML1-ETO, or pSG5M-AML1-ETO (L148D) was electroporated into the HL60 cells. ChIP DNA was detected using standard PCR with primer pairs located in the promoter region (forward, 5′-gccggcaccggaagcgtgcggttg; reverse, 5′-tctgcgcgcctcagcagtccccgtcg) or in the last EEN exon (forward, 5′-gccgcccctggaccagccgagctgc; reverse, 5′-cacgggtgagtcactgcggcagg).
Colony growth in soft agar and tumor growth in nude mice
Six-well plates were prepared with 0.7% agar solution in DMEM with 10% FCS containing 1 mg/mL G418 sulfate, and 2 × 103 stably transfected NIH3T3 cells were suspended in 0.35% agar solution in growth medium. Colonies larger than 0.1 mm in diameter were scored 21 days after being plated. For tumor formation in nude mice, 1 × 106 stably transfected NIH3T3 cells in 200 μL phosphate-buffered saline (PBS) was injected into the flank of 4-week-old athymic, Balb-c/nu/nu mice. The mice were inspected weekly for the development of tumors for up to 8 weeks.
Myeloid transformation assays
Transduction of murine bone marrow (BM) cells and retrovirus preparation were performed as previously described,25 with minor modifications. Briefly, retrovirus was prepared by transient transfection of 293T packaging cells with MIGR1 constructs. And the retroviral titers were estimated through the amount of infected NIH3T3 cells by flow cytometry assay. Six-week-old Balb/c mice were treated intravenously with 5-flurouracil (150 mg/kg), and BM cells were harvested from femurs and tibias 4 days later and maintained in Iscove modified Dulbecco medium (IMDM) supplemented with l-glutamine, penicillin/streptomycin, 15% FCS, 20 ng/mL stem-cell factor (SCF), and 10 ng/mL each of IL-3 and IL-6. In the following 2 days, BM cells were infected twice by spinoculation at 1100g for 2 hours at 32°C. For methylcellulose colony assays, infected BM cells were plated in 0.9% methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada) in the presence of SCF, IL-3, IL-6, and granulocyte macrophage–colony-stimulating factor (GM-CSF). After 7 to 10 days, colonies were counted, pooled, and replated (104 suspended single cells).
EEN RNA interference using recombinant feline immunodeficiency virus (FIV)
Two RNAi candidate target sequences designed by the Dharmacon siDESIGN Center were cloned into pFIV-H1/U6-copGFP and named FIV-R1(TGACCAACCAGATCGATGA) and FIV-R2(AGATCGATGAGAACTGGTA). FIV-R1 or FIV-R2 (3 μg) was transfected into the 293T cells with 0.5 μg mammalian expression vector PCIneo-EEN in each well of the 6-well plate. Western blot was carried out to determine the RNAi efficiency after 48 hours. The FIV-based recombinant virus was packaged using Lentivector Expression Systems (SBI, Mountain View, CA). Kasumi-1 and HL60 cells were infected twice by spinoculation at 1100g for 2 hours and cultured in 1640 media containing 10% FBS. After 2 days, GFP+ cells were sorted by Moflow and stable populations expressing the siRNA were selected serially in the next 3 weeks (∼98% to 99% GFP+ purity; DakoCytomation, Glostrup, Denmark). Experiments were performed with sorted GFP+ cells named Kasumi-FIV, Kasumi-R1, HL60-FIV, and HL60-R1.
Analysis of cell cycle
Cells were harvested, washed, and fixed overnight in 70% cold ethanol. After being washed with PBS, cells were incubated in PBS supplemented with 50 μg/mL RNase A. Cell-cycle distribution was then analyzed by staining the cells with 50 μg/mL propidium iodine (PI; Santa Cruz Biotechnology) and evaluated in an EPICS XL flow cytometer (Beckman Coulter, Hialeah, FL). Gating was used to remove debris and doublets before collection. Data were quantitated using MultiCycle software (Phoenix Flow Systems, San Diego, CA).
Results
Determination of the genomic structure and the transcription start site of the EEN gene
With the shotgun strategy, we obtained the complete genomic DNA sequence of the EEN gene (Figure 1A). Total random shotgun sequences produced one contig of 96 kb. Interestingly, there were 2 genes in this region, EEN and the CAF1 gene (GenBank accession no. U20 979),26 that are located head-to-head in close proximity (Figure 1A). The complete genomic DNA sequence of EEN from the TSS to the end of the polyadenylation signal was about 40 kb (GenBank accession no. AF190 465). Of note, the expression pattern of CAF1 was quite different from that of EEN (Figure 2A-B), probably due to the presence of an insulator-like element between these 2 genes (data not shown).
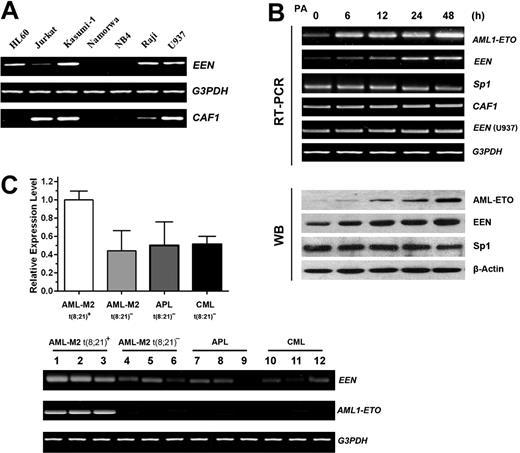
High expression levels of the EEN gene in leukemic cells with AML1-ETO. (A) RT-PCR analyses of EEN and CAF1 mRNA in leukemic cell lines. Myeloid lineages: Kasumi-1, HL60, U937, and NB4; lymphoid lineages: Jurkat, Namalwa, and Raji. (B) RT-PCR (top 6 rows) and Western blot analyses (bottom 4 rows) of AML1-ETO, EEN, Sp1, and CAF1 in the Ponasterone A (PA)–inducible, AML1-ETO–expressing U937 cell line. Cells were treated with 5 μM Ponasterone A to induce AML1-ETO expression. A 2-fold increase of EEN expression was observed 48 hours after PA treatment, in contrast to Sp1, which showed no obvious change in expression level. (C) RT-PCR analysis (bottom) and quantitative real-time RT-PCR analysis (top) of EEN in leukemic patient samples with or without AML1-ETO. Each leukemia subtype included 8 cases. The EEN-expression level in M2 t(8;21)+ was significantly higher than the other 3 subtypes without t(8;21) (P < .01), whereas no difference was detected between patients negative for t(8;21) (P > .1). Each column represents the mean ± SD in 8 cases. AML indicates acute myeloid leukemia; APL, acute promyelocytic leukemia; CML, chronic myeloid leukemia.
High expression levels of the EEN gene in leukemic cells with AML1-ETO. (A) RT-PCR analyses of EEN and CAF1 mRNA in leukemic cell lines. Myeloid lineages: Kasumi-1, HL60, U937, and NB4; lymphoid lineages: Jurkat, Namalwa, and Raji. (B) RT-PCR (top 6 rows) and Western blot analyses (bottom 4 rows) of AML1-ETO, EEN, Sp1, and CAF1 in the Ponasterone A (PA)–inducible, AML1-ETO–expressing U937 cell line. Cells were treated with 5 μM Ponasterone A to induce AML1-ETO expression. A 2-fold increase of EEN expression was observed 48 hours after PA treatment, in contrast to Sp1, which showed no obvious change in expression level. (C) RT-PCR analysis (bottom) and quantitative real-time RT-PCR analysis (top) of EEN in leukemic patient samples with or without AML1-ETO. Each leukemia subtype included 8 cases. The EEN-expression level in M2 t(8;21)+ was significantly higher than the other 3 subtypes without t(8;21) (P < .01), whereas no difference was detected between patients negative for t(8;21) (P > .1). Each column represents the mean ± SD in 8 cases. AML indicates acute myeloid leukemia; APL, acute promyelocytic leukemia; CML, chronic myeloid leukemia.
To determine the position of the TSS, we performed an RPA and revealed a major protected dsRNA band of 189 bp (Figure 1B). A major TSS 144 bp upstream of the ATG was identified. Furthermore, a primer extension assay was carried out to identify the TSS precisely and the primer used was complementary to the DNA sequence upstream of EEN's ATG. The reaction yielded a single 140-bp product, which was consistent with the RPA result (Figure 1C). TSS was used as the reference point (+1) for numbering the EEN promoter sequence (Figure 1D).
Cells expressing AML1-ETO had elevated levels of EEN
Since EEN is the only endophilin family member expressed in hematopoietic cells, we were particularly interested in the role of EEN in hematopoiesis. We first studied the expression of EEN in myeloid and lymphoid lineages, including Kasumi-1, HL60, U937, NB4, Jurkat, Namalwa, and Raji. Kasumi-1 cells with AML1-ETO fusion protein27,28 had significantly higher levels of EEN transcription than other cell lines (Figure 2A). To see if the up-regulation of the EEN gene was associated with AML1-ETO, we tested the expression level of the EEN gene in the Ponasterone A (PA)–inducible, AML1-ETO–expressing U937 cell line.29 When AML1-ETO mRNA and protein level were increased,30 EEN mRNA and protein also increased accordingly, whereas Sp1 level did not increase after PA administration (Figure 2B). To further exclude the possibility that the up-regulation of EEN was caused by PA induction, we also tested the expression of EEN in parent U937 cells treated by PA, but EEN level was unchanged (Figure 2B). We also measured the EEN level in leukemic cells from AML-M2 patients with t(8; 21) and AML1-ETO, AML-M2 without t(8; 21), AML-M3, and patients with CML without AML1-ETO fusion using semiquantitative RT-PCR and quantitative real-time RT-PCR. As expected, higher levels of EEN transcripts were observed in primary M2 leukemia cells expressing AML1-ETO as compared with those without AML1-ETO (Figure 2C). These results indicate that AML1-ETO fusion protein may have a regulatory role in EEN expression.
The EEN gene promoter contains an AML1 binding site
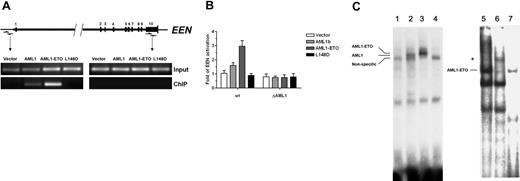
Next, we examined the EEN promoter sequence and identified an AML1 binding site (TGCGGT),3 from −49 to −44 (Figure 1D). We then performed ChIP assay to determine whether AML1b, the major isoform of AML1 protein,31 and AML1-ETO proteins could bind to this EEN promoter region. Anti-FLAG ChIP was performed in Flag-AML1-ETO–, Flag-AML1b–, or Flag-AML1-ETO (L148D)–expressing HL60 cells and control HL60 cells transfected with the pSG5M plasmid. Results showed that both AML1-ETO and AML1b could bind to the promoter sequence containing the AML1 binding site, and the binding was specific and strictly localized to the AML1 consensus site (Figure 3A). EMSA also confirmed that this binding was specific, as no binding was detected by the L148D mutant (Figure 3C). Furthermore, using supershift EMSA, we also demonstrated the specific binding of AML1-ETO to the EEN promoter with the nuclear extracts prepared from Kasumi-1 cells (Figure 3C).
Induced expression of EEN by AML1-ETO. (A) AML1-ETO targets the promoter region, as revealed by chromatin immunoprecipitation (ChIP) assay. The primers for the promoter region and the last exon (as control) are indicated by arrows. The numbers above the bar indicate the exon numbers. pSG5M, pSG5M-AML1b, pSG5M-AML1-ETO, and pSG5M-AML1-ETO (L148D) (10 μg each) were electroporated into the HL60 cells and immunoprecipitated by anti–FLAG M2 antibody. Input indicates input chromatin; ChIP, immunoprecipited chromatin. (B) AML1-ETO activates the wild-type (wt) promoter of the EEN gene. The EEN promoter–luciferase reporter gene construct (pGB-EENP7) was cotransfected with the expression vectors of AML1b, AML1-ETO, or mutant AML1-ETO (L148D). AML1b slightly increased the promoter activity, but the mutant AML1-ETO (L148D) did not. Mutant EEN promoter without the AML1 binding site (ΔAML1) was not activated by AML1-ETO or AML1b. The average firefly luciferase / Renilla luciferase activity on pGL3-EENP7 of the vector control was assigned a value of 1. Results are expressed as the means of 3 independent experiments plus or minus standard deviations. (C) AML1-ETO binds the wild-type (wt) promoter of the EEN gene. Flag-tagged recombinant proteins were prepared by in vitro translation. AML1b, AML1-ETO, and AML1-ETO (L148D) (lanes 2-4) were incubated with γ-32P–labeled double-strand probe and run on a 4.5% native polyacrylamide gel. A retarded band was seen in AML1-ETO and AML1b, but not in AML1-ETO (L148D). Lane 1 was a negative control without template in in vitro translation. For the EMSA supershift assay the antibody against ETO was added to nuclear extracts from Kasumi-1 cells before incubation with radiolabeled probe. A supershift band is indicated by the asterisk (lane 6). Lane 7 was obtained with 100-fold molar excess of unlabeled competing oligonucleotides.
Induced expression of EEN by AML1-ETO. (A) AML1-ETO targets the promoter region, as revealed by chromatin immunoprecipitation (ChIP) assay. The primers for the promoter region and the last exon (as control) are indicated by arrows. The numbers above the bar indicate the exon numbers. pSG5M, pSG5M-AML1b, pSG5M-AML1-ETO, and pSG5M-AML1-ETO (L148D) (10 μg each) were electroporated into the HL60 cells and immunoprecipitated by anti–FLAG M2 antibody. Input indicates input chromatin; ChIP, immunoprecipited chromatin. (B) AML1-ETO activates the wild-type (wt) promoter of the EEN gene. The EEN promoter–luciferase reporter gene construct (pGB-EENP7) was cotransfected with the expression vectors of AML1b, AML1-ETO, or mutant AML1-ETO (L148D). AML1b slightly increased the promoter activity, but the mutant AML1-ETO (L148D) did not. Mutant EEN promoter without the AML1 binding site (ΔAML1) was not activated by AML1-ETO or AML1b. The average firefly luciferase / Renilla luciferase activity on pGL3-EENP7 of the vector control was assigned a value of 1. Results are expressed as the means of 3 independent experiments plus or minus standard deviations. (C) AML1-ETO binds the wild-type (wt) promoter of the EEN gene. Flag-tagged recombinant proteins were prepared by in vitro translation. AML1b, AML1-ETO, and AML1-ETO (L148D) (lanes 2-4) were incubated with γ-32P–labeled double-strand probe and run on a 4.5% native polyacrylamide gel. A retarded band was seen in AML1-ETO and AML1b, but not in AML1-ETO (L148D). Lane 1 was a negative control without template in in vitro translation. For the EMSA supershift assay the antibody against ETO was added to nuclear extracts from Kasumi-1 cells before incubation with radiolabeled probe. A supershift band is indicated by the asterisk (lane 6). Lane 7 was obtained with 100-fold molar excess of unlabeled competing oligonucleotides.
AML1-ETO activates the EEN promoter
Having demonstrated the sequence-specific binding of AML1-ETO to the EEN promoter, the next question was whether AML1-ETO fusion protein could regulate the transcriptional activity of the EEN promoter. Therefore, we tested AML1-ETO and AML1b on the EEN promoter by transient transfection assays. We cotransfected the EEN promoter–luciferase reporter gene construct (pGB-EENP7) with the expression vectors of AML1b, AML1-ETO, or AML1-ETO (L148D). We also made the mutant plasmid (pGB-EENP7-ΔAML1) with the mutated AML1 binding site (TGCGGT was changed to AAGCTT). The wild-type EEN promoter was activated by the AML1-ETO protein but not by AML1-ETO (L148D), whereas the activity of the EEN promoter with the mutant AML1 binding site showed no obvious response to both proteins (Figure 3B). Moreover, the transcriptional activity of AML1b was much lower than AML1-ETO.
AML1 binding site was insufficient for the full activity of EEN promoter
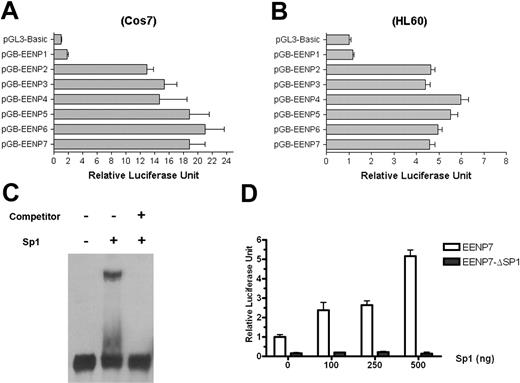
To understand the role of the AML1 binding site in the regulation of EEN expression and to search for other essential transcription factors, the promoter sequence of EEN was subject to investigation of other elements that could be critical for the full activity of the EEN promoter. Different fragments of the EEN promoter EENP1 to EENP7, corresponding to the regions from −59, −155, −248, −336, −439, −530, and −621 to +67, were amplified by PCR and subcloned into the pGL3-basic plasmid upstream of the firefly luciferase reporter gene. As shown in Figure 4A, when transfected into Cos7 cells, EENP2 (−155 to +67) exhibited significant luciferase activity. EENP2 showed 7-fold activation compared with EENP1 (−59 to +67) and 13-fold compared with the empty pGL3-basic plasmid. The other 5 fragments (EENP3 to EENP7) showed slightly increased activity compared with EENP2. In this assay system an EENP7 reporter construct with a mutation of the AML1 binding site displayed no significant reduction of promoter activity (data not shown), indicating that binding motifs other than the AML1 site may play a major role in transcriptional regulation of the EEN gene. Similar results were obtained from HL60 cells (Figure 4B). Because EENP2 alone was sufficient to drive high expression of EEN, we reasoned that an indispensable cis-element to the transactivation of EEN should lie in the fragment from −155 bp to −59 bp.
Transcriptional regulation of the EEN gene by Sp1. (A) Deletion analysis of the activity of the EEN promoter in the Cos7 cell line. A significant difference is observed between EENP1 and other EEN promoter constructs. (B) Deletion analysis of the activity of the EEN promoter in the HL60 cell line shows similar results as in Cos7 cells. (C) EMSA showing the binding of Sp1 to the GC-stretch of EEN promoter with a DIG-labeled probe spanning the −107/−60 site. (D) Transcriptional activation of the EEN promoter by Sp1 in a dose-dependent manner. Each column represents the mean ± SD in 3 independent experiments.
Transcriptional regulation of the EEN gene by Sp1. (A) Deletion analysis of the activity of the EEN promoter in the Cos7 cell line. A significant difference is observed between EENP1 and other EEN promoter constructs. (B) Deletion analysis of the activity of the EEN promoter in the HL60 cell line shows similar results as in Cos7 cells. (C) EMSA showing the binding of Sp1 to the GC-stretch of EEN promoter with a DIG-labeled probe spanning the −107/−60 site. (D) Transcriptional activation of the EEN promoter by Sp1 in a dose-dependent manner. Each column represents the mean ± SD in 3 independent experiments.
Identification and functional analysis of an Sp1 binding motif in the EEN promoter
To search for candidates that may be involved in the EEN activation through the fragment from −155 bp to −59 bp, we analyzed this GC-rich fragment, which lacks the classic TATA-box and CAAT-box, characteristic of housekeeping gene promoters. Moreover, the ubiquitous expression pattern also indicated that the EEN gene might be a housekeeping gene.12 Transcription initiation of housekeeping genes was often controlled by an Sp1 transcription factor,32,33 which binds the GC box.34 The GC-stretch does not contain a classic GC-box in the −107/−60 site; however, EMSA with a digoxin (DIG)–labeled probe spanning the −107/−60 site demonstrated that in the presence of Sp1, there was a retarded migration of the probe (Figure 4C). Moreover, the mutation of the putative Sp1 binding site −107/−60 (EENP7-ΔSp1) significantly decreased the promoter activity (Figure 4D). These results suggested that the −107/−60 region might be a functional binding site for SP1.
Characterization of oncogenic properties of EEN
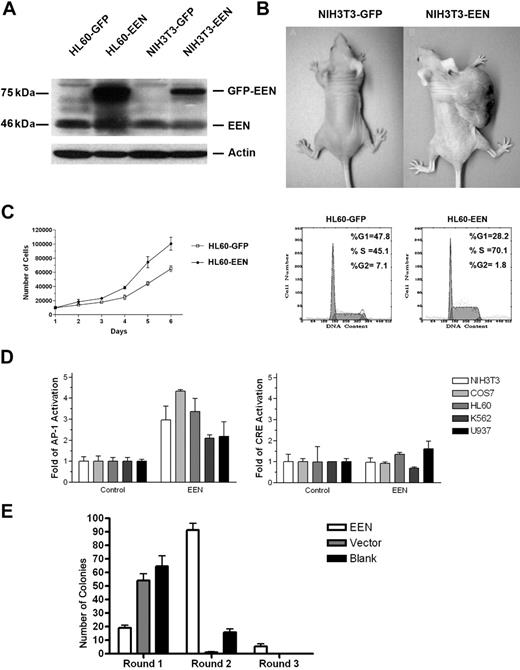
To address the possible contribution of up-regulated EEN to leukemogenesis, EEN was transfected into NIH3T3 cells, a classic model system commonly used to test the transforming potential of leukemic proteins and the cellular behaviors.35 pEGFP-EEN–transfected cells (NIH3T3-EEN) were compared with control cells transfected with pEGFP-C1 (NIH3T3-GFP). Western blot showed a 2.3-fold increase in EEN protein in transfected cells (Figure 5A). Colony assay showed 109.0% ± 35.4 per 1000 cells in pEGFP-EEN–transfected cells as compared with none in control (Table 1) To determine the in vivo oncogenicity of EEN, NIH3T3 cells were injected subcutaneously into the flanks of nude mice. As shown in Figure 5B for a typical animal experiment, all 6 mice injected with NIH3T3-EEN cells developed tumors after a latency period of 2 weeks and the tumors grew to more than 1 cm in diameter by 6 weeks. Mice injected with NIH3T3-GFP cells did not develop tumors (Table 1). These results indicate that overexpression of EEN was oncogenic.
Oncogenic properties of cells with EEN overexpression. (A) Western blot analysis showing overexpression of EEN in stably transfected NIH3T3 and HL60 cells (2.3-fold in NIH3T3-EEN and 4-fold in HL60-EEN compared with the endogenous level). (B) Tumor formation in nude mice injected with NIH3T3-EEN. (C) Cell proliferation and cell-cycle distribution of HL60-GFP and HL60-EEN cells. The result of 1 representative experiment is shown. (D) Activation of AP-1 (left panel), but not CRE (right panel), by EEN. (E) Colonies generated per 104 transduced BM cells were determined in first-, second-, and third-round cultures for MigR1-EEN construct, MigR1 empty vector, and nontransfected BM cells. GFP+ colonies were counted at the end of each round. Bars represent the mean plus or minus SD for 3 experiments.
Oncogenic properties of cells with EEN overexpression. (A) Western blot analysis showing overexpression of EEN in stably transfected NIH3T3 and HL60 cells (2.3-fold in NIH3T3-EEN and 4-fold in HL60-EEN compared with the endogenous level). (B) Tumor formation in nude mice injected with NIH3T3-EEN. (C) Cell proliferation and cell-cycle distribution of HL60-GFP and HL60-EEN cells. The result of 1 representative experiment is shown. (D) Activation of AP-1 (left panel), but not CRE (right panel), by EEN. (E) Colonies generated per 104 transduced BM cells were determined in first-, second-, and third-round cultures for MigR1-EEN construct, MigR1 empty vector, and nontransfected BM cells. GFP+ colonies were counted at the end of each round. Bars represent the mean plus or minus SD for 3 experiments.
Summary of the transforming properties of EEN
| Cell line . | Growth in soft agar, no. per 1000 cells* . | Tumor formation in nude mice, no. positive / no. of injection sites† . |
|---|---|---|
| NIH3T3-GFP | 0.0 ± 0.0 | 0/5 |
| NIH3T3-EEN | 109.0 ± 35.4 | 6/6 |
| Cell line . | Growth in soft agar, no. per 1000 cells* . | Tumor formation in nude mice, no. positive / no. of injection sites† . |
|---|---|---|
| NIH3T3-GFP | 0.0 ± 0.0 | 0/5 |
| NIH3T3-EEN | 109.0 ± 35.4 | 6/6 |
*The values for soft agar colonies are means ± standard deviations of 4 separate experiments.
†Tumors (+) were at least 1 cm in diameter after 6 weeks.
To explore the possible functions of EEN on hematopoietic cells, HL60 cells were transfected with pEGFP-EEN (HL60-EEN). The GFP-EEN fusion protein was also examined by Western blot, which showed an approximately 4-fold increase of GFP-EEN compared with the endogenous level (Figure 5A). Notably, the HL60-EEN cells grew much faster than control cells (Figure 5C). After 6 days, the difference in cell number between HL60-EEN and HL60-GFP was statistically significant (P < .05). The increased proliferation of HL60-EEN cells was also confirmed by the cell-cycle distribution (Figure 5C), the percentage of HL60-EEN cells in the proliferation phases (S + G2/M) of the cell cycle (70.1%) being higher than that of HL60-GFP (45.1%).
Activation of AP-1 by EEN
It has been well established that AP-1 family transcriptional factors are important targets of leukemia-associated fusion proteins. Moreover, the transforming activity of AML1-ETO protein was reported to be correlated with its ability to up-regulate AP-1.35 Overexpression of EEN did transactivate AP-1 in the NIH3T3 and HL60 cell lines, as well as in COS7, K562, and U937 cells (Figure 5D). We also tested the effect of EEN on the cAMP responsive element (CRE) as control. As shown in Figure 5D, EEN had no obvious effect on CRE, indicating EEN had selected effect on AP-1.
Myeloid transformation assays and transplantation
A methylcellulose serial replating assay36 was used to assess the effects of EEN on the growth properties of murine myeloid progenitors in vitro (Figure 5E). The MIGR1 was employed to transduce freshly harvested BM cells from mice treated with 5-fluorouracil. A portion (104) of the initial lineage-depleted cells was plated into methylcellulose. After 8 to 10 days, the GFP+ clones were counted and 104 cells from the first round were replated. On the first round, the number of GFP+ clones infected by MIGR1-EEN was much less than the number of those infected by MIGR1, probably due to the lower titer of MIGR1-EEN virus. In the second round plating, however, MIGR1-EEN GFP+ clones outnumbered MIGR1 GFP+ clones. And in the third round, only the MIGR1-EEN GFP+ clones could be observed (Figure 5E).
EEN RNA interference using FIV
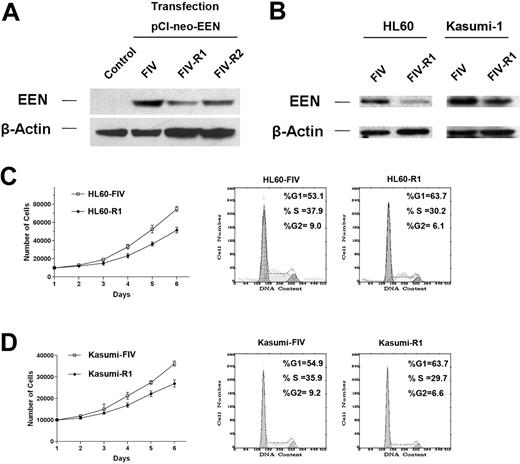
In the plasmid pFIV-H1/U6-copGFP, the sense and antisense strands were transcribed separately from 2 independent promoters, U6 and H1, to form the siRNA duplex. Cotransfection of EEN- and siRNA-expression constructs (3:1) FIV-R1 or FIV-R2 in 293T cells demonstrated that FIV-R1 (25.2%) was much more efficient than FIV-R2 (39.6%) and was chosen for RNAi (Figure 6A). EEN decreased to 28.3% in HL60 and 51.7% in Kasumi-1 cells (Figure 6B). Cell-cycle analysis of HL60 showed that the EEN siRNA inhibited cell growth. In the proliferation phases (S + G2/M), the percentage of HL60-R1 cells (36.3%) was lower than that of HL60-FIV cells (46.9%). Also, for Kasumi-1 cells, in the proliferation phases (S + G2/M), the percentage of Kasumi-R1 cells (36.3%) was lower than that of Kasumi-FIV (45.1%).
EEN RNAi in the hematopoietic cells. Western blot analysis showing RNA interference in 293T cells (A) and HL60 and Kasumi-1 cells (B). (C) The cell proliferation curves and cell-cycle distribution of HL60 cells with FIV-R1 as compared with the control (FIV). Note that the difference in the proliferation between the 2 cell populations becomes significant on day 6 (P = .030). These results were repeated at least 3 times and a representative one is shown. (D) The cell-proliferation curves and cell-cycle distribution of Kasumi-1 with FIV-R1 as compared to the control (FIV). Note the difference in cell proliferation was significant on day 6 (P = .045).
EEN RNAi in the hematopoietic cells. Western blot analysis showing RNA interference in 293T cells (A) and HL60 and Kasumi-1 cells (B). (C) The cell proliferation curves and cell-cycle distribution of HL60 cells with FIV-R1 as compared with the control (FIV). Note that the difference in the proliferation between the 2 cell populations becomes significant on day 6 (P = .030). These results were repeated at least 3 times and a representative one is shown. (D) The cell-proliferation curves and cell-cycle distribution of Kasumi-1 with FIV-R1 as compared to the control (FIV). Note the difference in cell proliferation was significant on day 6 (P = .045).
Discussion
Our previous work demonstrated that MLL-EEN fusion protein was oncogenic.13 The fusion protein is localized in the nucleus, and could also bring the wild-type EEN protein into the nucleus. EEN was reported to bind with Abi16 and Abi in turn binds with ENL (eleven nineteen leukemia). Both Abi and ENL were MLL fusion partners.37 It was hypothesized that most of the MLL fusion partners interact to form a complex.38 Therefore, it was possible that EEN might be localized in the nucleus in some MLL translocation-associated leukemias.
Most of the studies of the endophilin family proteins have been focused on their functions in endocytosis in neuronal cells. It was reported that EEN is localized in the nucleus and exhibited nucleocytoplasmic shuttling in human leukemic cell lines HL60 and U937,39 in contrast to the cytoplasmic localization in neurons and osteoclasts. The nuclear EEN in U937 was associated with the cytokinesis and fluctuated with cyclin A and cyclin B, suggesting its possible role in the cell cycle and mitosis.39 We demonstrated that both the middle coiled-coil domain and the C-terminal SH3 domain of EEN had transactivation property.13 Although the mechanism remains unclear, the overexpressed nuclear EEN is likely to harbor some aberrant transactivation properties, which may contribute to leukemogenesis.
In the SH3 domain–containing endophilin family, EEN is the only member expressed in hematopoietic cells.12 However, both the cellular functions of EEN in hematopoietic-cell development and leukemogenesis were poorly understood. We screened several common hematopoietic cells of different AML subtypes. Interestingly, we found that EEN was highly expressed in Kasumi-1. Furthermore, we demonstrated that this high level of EEN expression was associated with cells carrying the AML1-ETO fusion gene, such as the PA-inducible, AML1-ETO–expressing U937 cell line and primary patient samples with AML1-ETO, but not with other common AML subtypes (AML-M2 without AML1-ETO, AML-M3) and CML. Meanwhile, we also found that the expression of the CAF1 gene, located head-to-head in the same genomic locus as EEN, was not affected by AML1-ETO due to an insulator element between the CAF1 and EEN (data not shown).
To study the regulation of EEN expression, we first determined the genomic structure and the TSS of the EEN gene. Analysis of its 5′-flanking and noncoding region revealed that the promoter −155/+67 contains neither TATA nor CCAAT boxes. The GC-stretch from −107 to −60 has an Sp1-binding site critical for EEN expression. Sp1 is the founding member of a growing family that can bind to the GC boxes.34 We demonstrated here that Sp1 could bind to the GC-stretch of the EEN promoter and transactivate EEN. The mutation in the Sp1 binding site greatly impaired the activity of the EEN promoter. Cotransfection of the EEN promoter–driven luciferase reporter with the Sp1 expression vector dramatically increased the expression of luciferase gene, suggesting a critical role of Sp1 in EEN expression. However, we did not find that the Sp1 level was increased in the PA-treated, AML1-ETO–expressing U937 cell line. This excluded the possibility that the up-regulation of EEN in AML1-ETO–containing cells was caused by elevated Sp1. The AML1 binding site might be unnecessary for the basal activity of the promoter since the activity of mutant EEN promoter with the deletion of this site showed no significant difference compared with the wild type. Of note, this site could be targeted by AML1-ETO in the AML-M2 subtype with t(8;21) and thus may represent a mechanism of aberrant transcriptional regulation.
As an aberrant transcriptional factor, AML1-ETO exerts inhibitory effects on the target genes of AML1, a well-known transcriptional activator in hematopoiesis.2 In certain cases, it was also reported that AML1-ETO was involved in transcriptional activation. The transactivation by AML1-ETO seemed to be through diverse mechanisms. For example, an AML1 binding site, but not AML1b, could directly activate the promoter of Bcl2 by AML1-ETO.4 AML1-ETO and AML1 work synergistically to transactivate the M-CSFR promoter40 ; G-CSFR was activated by AML1-ETO through the up-regulation of CEBPϵ.6 The genes could also be activated by blocking the repression by PLZF,41 and the expression of c-jun could be induced by AML1-ETO in an indirect, JNK-dependent manner.42 UBP43,43 TIS11b,5 and TRK44 may also be up-regulated by AML1-ETO, but the mechanism is not clear. The mechanism of EEN transactivation by AML1-ETO may be similar to that of Bcl2. Our data further support the idea that AML1-ETO has unique gain-of-functions other than its function as the dominant-negative repressor of AML1.
AML1-ETO has been shown to induce growth arrest in AML cells.45 However, AML1-ETO is shown to block myeloid and erythroid differentiation. It is reasonable to speculate that AML1-ETO is capable of modulating a large number of genes involved in the regulation of cell proliferation, differentiation, and apoptosis. With regard to the growth control, it is possible that more genes are regulated in favor of growth arrest either directly or indirectly by AML1-ETO, such as CX4346 and the JNK signaling pathway.42 Therefore, the overall result of the gene-expression network on the effect of AML1-ETO could be translated into a phenotype of decreased cell proliferation. It is nevertheless important to point out that in the clinical course of leukemogenesis, AML1-ETO does not work alone but may cooperate with other genetic events. For example, activation of the c-KIT pathway may stimulate cell proliferation, and hence surmount the growth arrest effect of AML1-ETO. Previous work also showed that c-KIT could activate RAS signaling,47 whereas EEN was also involved in the regulatory network of RAS pathway. The fact that EEN renders NIH3T3 cells tumorigenic also gave support to this, since EEN may well cooperate with the existing aberrant pathways or networks, such as that of c-MYC, in NIH3T3 cells. In short, interaction of AML1-ETO with other molecular abnormalities may reconcile the observations at the individual gene level toward a comprehensive insight into the leukemogenesis of t(8; 21)-related AML.
We also demonstrated the oncogenic properties of overexpressed EEN. First, AML1-ETO had the ability to transform NIH3T3 cells.35 Coincidently, EEN was also capable of transforming NIH3T3 and enhancing the self-renewal capacity and proliferative potential of clonogenic hematopoietic progenitors in vitro. Second, the growth of the t(8;21) myeloid leukemia cell line could be inhibited by antisense oligonucleotides complementary to the AML1-ETO fusion mRNA48 or cleavage of AML1-ETO RNA with specific ribozymes,49 indicating that AML1-ETO was important for the continuous growth of these cells. Here, we also found that EEN could enhance the proliferation of HL60 cells, whereas EEN RNAi could slow down the cell growth significantly. Third, AML1-ETO was shown to up-regulate AP-1,35 whereas overexpression of EEN also transactivated AP-1 expression. It can be speculated that up-regulated EEN may be one of the key points for AML1-ETO leukemogenesis. Whether EEN is indispensable to the leukemogenesis of AML1-ETO should be further investigated.
Taken together, the aberrant EEN localization associated with MLL translocation, as well as the abnormal EEN up-regulation associated with t(8;21) translocation, manifest different mechanisms involved in leukemogenic potential of EEN. It can be concluded that EEN might be the common target of 2 major types of acute myeloid leukemia associated with AML1-ETO fusion and MLL fusion.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: Q.-H.H., Q.-H.Z., B.-L.L., Z.C., and S.-J.C. designed the research plan and analyzed data. X.-W.Z., Y.-Y.W., and H.-Y.L. assisted with experiments. B.C., H.X., H.L., and L.-H.M. performed experiments and analyzed data. L.-H.M. and H.L. wrote the paper, and Z.C. and S.-J.C. revised the paper.
L.-H.M., H.L., and H.X. contributed equally to this work.
Acknowledgments
We acknowledge Dr G.-Q. Chen for his constructive discussion on this work; Prof M. Lubbert for providing the PA-inducible, AML1-ETO–expressing U937 cells; Prof S.W. Hiebert for providing the construct containing AML1-ETO (L148D); Drs J.-S. Yan and H. Kang for technical support; and Drs Y. Fang, J.-X. Liu, and T.-X. Liu for critical reading. We thank all members of the Shanghai Institute of Hematology for their continuous support and encouragement.
This work was supported in part by grants from the Chinese National High Tech Program 863, the Chinese National Key Program for Basic Research (973), the National Natural Science Foundation of China (NSFC), the Shanghai Commission for Science and Technology, Leading Academic Discipline of the Shanghai Commission for Education (Y0201), and the Samuel Waxman Cancer Research Foundation. L.-H.M. is partly supported by NSFC (no. 303 00 139); the Doctoral Constructive Fund of the School of Medicine, Shanghai Jiao Tong University; and the Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP, no. 200 502 66 017). H.L. is supported by the K.C. Wong Education Foundation (Hong Kong) and the China Postdoctoral Science Foundation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal