In elegant murine experiments, Taylor and colleagues have demonstrated that preformed antibody represents the primary engraftment barrier in allosensitized hosts, and have identified interventions to target this biology for potential clinical benefit.
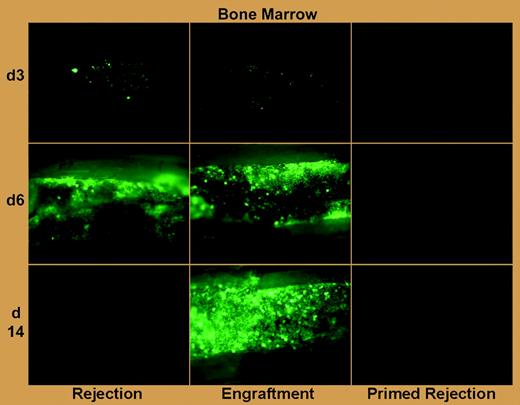
Graft rejection after allogeneic hematopoietic cell transplantation (HCT), which is an increasing threat in the age of low-intensity host conditioning and HCT across greater genetic barriers, is particularly problematic in patients with aplastic anemia and hemoglobinopathy who require blood transfusions.1 Although an association of host antibody production with clinical graft rejection was made 3 decades ago,2 rejection in the allosensitized host has been primarily attributed to primed T lymphocytes, and interventions to counteract rejection in this setting have focused on intensification of T-cell–depleting preparative chemotherapy.3 The work of Taylor and colleagues in this issue of Blood confirms a role for T-cell immunity in allosensitized rejection, and, more important, makes the landmark observation using green fluorescent protein (GFP)–labeled grafts and bioluminescence imaging that preformed antibody can swiftly mediate rejection within hours of transplantation (see figure).
Antibody-mediated rejection of donor BM in primed mice is far more rapid than T-cell–mediated rejection in naive mice. See the complete figure in the article beginning on page 1307.
Antibody-mediated rejection of donor BM in primed mice is far more rapid than T-cell–mediated rejection in naive mice. See the complete figure in the article beginning on page 1307.
This demonstration of preformed antibody–mediated allograft rejection represents a rather complete characterization. Allosensitized rejection, which was induced by pretransplantation donor spleen cell infusion, clearly involved humoral immunity and appeared to involve antibody-dependent cell-mediated cytotoxicity (ADCC) because genetically B-cell– and FcR-deficient hosts were relatively protected. Furthermore, in a manner analogous to the clinical obstacle, preformed antibodies of cross-reactive or broadly reactive specificities enabled prompt rejection of third-party allografts. These findings are complementary to recent findings of humoral-based HCT rejection presented by Xu et al4 using murine hosts allosensitized by donor skin grafts.
Perhaps most significantly, Taylor and colleagues found that antibody neutralization through high-dose immunoglobulin therapy, when combined with other graft-enhancing methods such as megadose stem cell infusion and host T-cell depletion, facilitated engraftment in allosensitized hosts. This experimental result is consistent with clinical findings in solid organ transplantation, where removal of preformed antibody through plasmapheresis and intravenous immunoglobulin therapy served as effective therapy for refractory humoral rejection.5 Implementation of clinical trials to translate this knowledge, as the authors propose, is warranted and quite feasible. Care should be taken in potential transplantation candidates to prevent allosensitization during transfusion, perhaps through rituximab therapy, which blocks antibody production to neoantigen.6 And, as Taylor and colleagues propose, in light of the extremely rapid onset of rejection due to preformed antibody, attempts to either purge or block the ADCC mechanism of alloantibody must be performed prior to transplantation.
The author declares no conflicting financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal