Abstract
Chronic lymphocytic leukemia (CLL) is the most prevalent form of leukemia in adults in western countries. A genome scan of CLL-prone families revealed a lod score of one in band 13q22.1. To investigate this finding, we selected 6 CLL families consisting of 63 individuals (CLL affected, n = 19; unaffected, n = 44) for fine mapping of a 23-megabase region in 13q14.2-q22.2. Interphase fluorescence in situ hybridization (FISH) revealed 13q14 deletion in 85% (11/13) of CLL patients. Four CLL families shared a 3.68-Mb minimal region in 13q21.33-q22.2. Two asymptomatic siblings who shared the 13q21.33-q22.2 at-risk haplotype exhibited CD5+ monoclonal B-cell lymphocytosis (MBL) on flow cytometry. One of these individuals also had a 13q14 deletion by FISH. These 2 individuals with MBL shared the at-risk haplotype with their CLL-affected relatives, providing further evidence of the relationship between CLL and MBL, as well as of the biologic significance of this novel region. Using direct DNA sequencing analysis, we screened 13 genes for mutations, but no frameshift or nonsense mutations were detected. Our studies revealed that 11 of the 13 genes in the candidate region were expressed in immune tissues, supporting their functional relevance in investigations of familial CLL. In conclusion, we identified a novel candidate region that may predispose to familial CLL.
Introduction
B-cell chronic lymphocytic leukemia (CLL) is a monoclonal disorder characterized by a progressive accumulation of mature-appearing, but functionally incompetent, lymphocytes. It is the most common form of leukemia among adults in western countries. Data from the Surveillance, Epidemiology and End Results (SEER) Program estimate 9730 new cases of CLL in the United States for 2005 (http://seer.cancer.gov/csr/1975_2000/).1 Clinical diagnosis requires an absolute lymphocytosis higher than 5 × 109 mature-appearing lymphocytes/μL in the peripheral blood. Flow cytometry confirms the diagnosis by showing the presence of light chain–restricted B lymphocytes expressing CD5, CD19, dim CD20, CD23 antigens, and an absent or dim FMC-7 and CD79b staining.
Abnormalities of chromosome band 13q14 in CLL DNA were first reported by Fitchett et al in 1987.2 Frequent deletion of 13q14 has suggested that loss of this region may be involved in malignant transformation to CLL and has prompted efforts to define a minimal deleted region (MDR) predisposing to CLL. In 1993, several investigators3-5 reported an MDR in 13q14.3 in CLL tumor DNA (Figure 1), suggesting the presence of a putative tumor suppressor gene at this region. Molecular studies revealed a 1.6-megabase (Mb) deleted region bordered by the retinoblastoma gene (RB) and D13S25. Then, RB was excluded as a CLL candidate gene. Kitamura et al6 constructed a 650-kilobase (kb) contig derived from BAC clones surrounding the putative locus between D13S319 and D13S25 and identified potential CLL candidate genes in this region. Subsequently, molecular studies failed to identify pathologic changes related to CLL. Corcoran et al7 defined a 130-kb commonly deleted region overlapping the MDR defined by markers D13S272 and 6E3.2 (D13S319).8,9 Bullrich et al8 completely sequenced a 790-kb region spanning the CLL MDR, but failed to identify mutations in leukemia cases. Deletion 13q14 is the most frequent chromosomal aberration (∼ 55%) detected in sporadic CLL and is associated with the longest median treatment-free interval.10 Deletions of chromosome band 13q14 have also been found in non-Hodgkin lymphoma,11 acute lymphocytic leukemia,11 and mantle-cell lymphoma.12
Calin et al13 identified 2 microRNAs (miR15a and miR16-1) within a minimally deleted region of 13q14 and found reduced expression of miR15a and miR16-1 in 68% (41/60) of CLL samples analyzed in comparison with normal CD5+ B cells. The majority of these cases exhibits loss of heterozygosity (LOH) by microsatellite analysis.13 Mechanisms other than LOH (ie, promoter hypermethylation) were not identified as a causal factor for reduced miR expression in CLL samples. In 2005, Calin et al14 reported a nonsense polymorphism in ARLTS1, a gene with homology to the ADP-ribosylation factor family. The frequency of this polymorphism (Trp149Stop) was similar in controls and patients with sporadic CLL. Both of these studies were inconclusive in establishing a causal link between these genes and the pathogenesis of CLL.
There is accumulating evidence that a subset of CLL is due to genetic susceptibility.15-17 More than 80 families with aggregation of CLL have been reported.18 Population studies have shown a 7-fold increased risk of CLL and a 2-fold increased risk for lymphoproliferative diseases among first-degree relatives of patients with CLL.16 Goldin et al19 performed a whole genome scan of 94 individuals (38 affected individuals) from 18 families containing at least 2 living members with CLL. Lod scores of 1.0 or greater were found for loci on chromosomes 1, 3, 6, 12, 13, and 17. None of these loci achieved statistical significance19 ; however, 4 of these loci coincide with, or are adjacent to, regions of recurrent chromosomal abnormalities in CLL (6q, 13q, 12q, and 17p). Linkage results suggested a region of interest in band 13q22.1 (marker D13S156) among a subgroup of CLL families.19 To investigate this region for a CLL susceptibility gene, we selected 6 CLL-prone families for fine mapping of the 13q14.2-q22.2 region to determine if a shared minimal candidate region (MCR) may be defined.
Patients, materials, and methods
This study was approved by the National Cancer Institute (NCI) institutional review board, and informed consent was obtained from all subjects in this report. Families with 2 or more cases of CLL have been enrolled in the NCI Familial B-CLL Registry since 1967. All available affected individuals and their first-degree relatives were evaluated, and biospecimens were collected at the NIH clinical center or on field trips. CLL diagnostic criteria were documented by available history, medical records, physical examination, clinical laboratory studies, and pathologic review of slides. Clinical data on families 1 to 4 were previously reported.20 Among these 6 families, there are 3 deceased individuals (1-2001, 5-1008, and 6-4004) with a history of CLL confirmed by pathology reports that did not have DNA available for genotyping and thus were not included in the analysis.
Genotyping
DNA was extracted from peripheral blood cells according to standard procedures. Genotyping was performed with fluorescent-labeled microsatellite markers from Applied Biosystems ([ABI], Foster City, CA). Seven markers were used in the initial assay to genotype the region from 13q14.2 to the telomeric recombinant marker D13S1306 at 13q22.2 identified by the whole genome scan.19 The order of the markers was as follows: centromere-D13S165-D13S273-D13S319-D13S1269-D13S1320-D13S1296-D13S156-D13S1306-telomere. Based on initial genotyping data, 6 additional microsatellite markers situated between D13S1296 and D13S156 (D13S279, D13S288, D13S1324, D13S1291, D13S1318, D13S166) and 3 between D13S156 and D13S1306 (D13S792, D13S162, D13S782) were typed in the 6 families to identify the centromeric and telomeric recombinant borders of the MCR. Positions of markers were confirmed with University of California, Santa Cruz (UCSC) BLAT21 and the latest genome assembly. Polymerase chain reaction (PCR) was performed per the manufacturer's direction using ABI Prism True Allele Premix. Alleles were separated on an ABI 3100 genetic analyzer and analyzed with ABI GeneMapper software 3.0.
Sequencing
DNA was extracted from peripheral blood leukocytes according to standard procedures. Bidirectional dye-terminator sequencing was used for mutation analysis as previously described.22 PCR conditions and primer sequences are available upon request. Exon-intron boundaries were determined by BLAT alignment with the assembled genomic sequence (NCBI Build 34; National Center for Biotechnology Information [NCBI], Bethesda, MD).
Tissue-expression profile
Tissue expression of 13 candidate genes from the minimal candidate region in 13q21.33-q22.2 was evaluated using the Human Immune cDNA panel (Clontech, Mountain View, CA). Primers were designed to amplify the 3′ UTR regions of the candidate genes. Titanium Taq (Clontech) was used for PCR amplification of cDNA from the Human Immune panel (Clontech). Primers that failed to amplify with the human control cDNA were tested for PCR efficiency using Centre d'Etude du Polymorphisme Humaine (CEPH) genomic DNA. PCR products were analyzed with a 2% agarose gel.
Functional studies
Total RNA was extracted from frozen lymphocytes using the RNeasy MinElute Cleanup Kit (Qiagen, Valencia, CA). First-strand cDNA was synthesized using the cDNA Archive Kit (ABI). A sporadic CLL leukemic clone and normal B cells were isolated by flow cytometry for the purpose of studying differential expression of the candidate genes in 13q21.33-q22.2. Ten candidate genes showing expression in peripheral leukocytes and bone marrow were selected for gene expression analysis using real-time PCR and the TaqMan gene expression assay (ABI). PCR primers and Taqman probes for the 10 candidate genes (target genes) and β-actin (ACTB, calibrator gene) were designed by Applied Biosystems (ABI). The relative standard curve quantitation method was used to measure the expression level of the 10 target genes relative to ACTB gene-expression level.23 A standard curve using fibroblast cDNA was generated for the 10 target genes and the ACTB calibrator gene. cDNA archived from total RNA was diluted 1/10 for the CLL leukemic clone and 1/4 for the B-cell control and aliquoted in triplicate for real-time PCR quantitation of the 10 target genes and the ACTB calibrator gene. Mean mRNA expression levels of each target gene were normalized to the ACTB calibrator gene expression level measured by real-time PCR. The normalized target gene expression value(s) of the CLL leukemic clone was divided by the normalized value(s) of their respective target gene(s) from the B-cell control to derive a fold-difference change in target gene expression. Loc387937 and Chr13_441 were excluded from gene expression measurement as they were not expressed in the immune tissue assay. Loc387934 did not have unique coding sequence (as determined by NCBI BLAST24 ) necessary for design of Taqman probe/primers so it was not measured for gene expression.
Flow cytometry
Flow cytometry was performed on affected family members to confirm their CLL diagnosis. Whole-blood lysis and flow cytometric immunophenotyping were performed as previously described.25-31 Flow cytometric immunophenotyping was performed by 2- and 3-color immunofluorescent staining using a combination of directly conjugated reagents. Blood samples from patients with known sporadic CLL were used as positive controls for affected members of the families. Samples from blood-bank donors and unaffected spouses were used as controls for unaffected members. CellQuest (Becton Dickinson Biosciences [BDB], San Jose, CA) was used to acquire and analyze the flow cytometric immunophenotyping data. FACSComp (BDB) and QuickCal (Flow Standards, San Juan, PR) microbead standards were used to validate instrument performance and linearity. All monoclonal antibodies were obtained from BDB except anti-CD22 phycoerythrin (PE), which was obtained from Caltag (Burlingame, CA).
Cytogenetics
Buffy coats from fresh, heparinized peripheral blood were cultured in duplicate with each of the following mitogens: phytohemagglutinin (PHA), phorbol 12-myristate 13-acetate (PMA), pokeweed mitogen (PWM), and E coli lipopolysaccharide (LPS). After 72 (PHA) or 96 (PMA, PWM, LPS) hours in a humidified 5% CO2 incubator at 37°C, cells were harvested and fixed in 3:1 methanol–glacial acetic acid. Routine G-banded karyotype analysis and interphase fluorescence in situ hybridization (FISH) with a panel of 6 probes were performed according to standard protocols. The D13S319 and D13S25 probes (Vysis, Downers Grove, IL) were used to detect deletions in band 13q14. Minimums of 200 interphase nuclei were scored for hybridization signals for each probe. Based upon our results in healthy controls, losses and gains were interpreted as negative if they occurred in 4% or fewer of the nuclei; however, none of the positive results were equivocal as all were present in 12% or more of the nuclei.
Results
Clinical description of CLL families
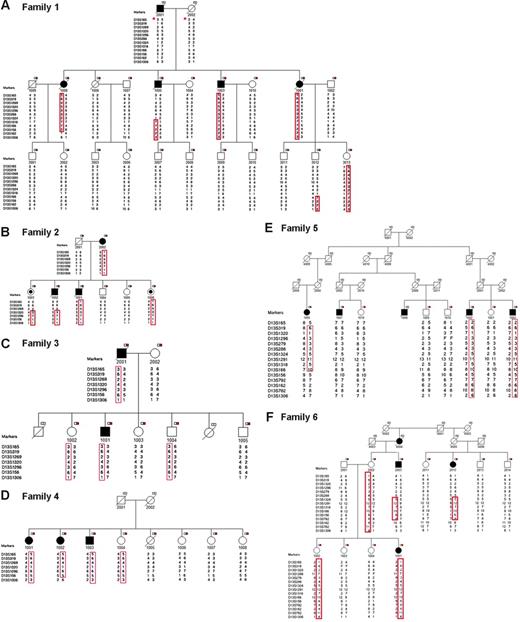
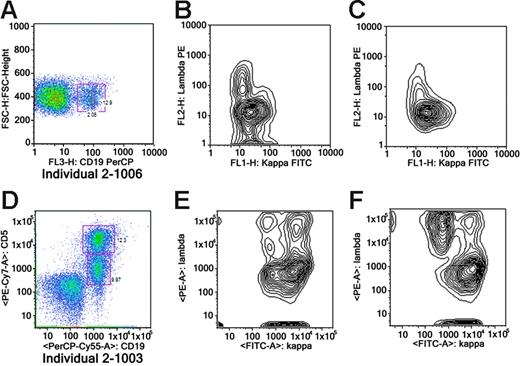
Six CLL-prone families were identified for fine mapping. Families were selected to maximize the number of available affected individuals and their unaffected first-degree relatives for molecular studies. Families 1, 2, 4, 5, and 6 had 3 or more affected members. Family 3 had 2 affected individuals and 5 unaffected first-degree relatives. Family 6 included 3 affected individuals (2 affected half-first cousins [6-2003 and 6-2012] and uncle-niece [6-2003 and 6-1001]). A total of 63 individuals (affected, n = 19; unaffected, n = 44) were available for genotyping (Figure 2). There were 19 family members affected with CLL with median age at diagnosis of 52 years (range, 38 to 76 years), and a slight preponderance of males (11 males, 8 females) (Table 1). The peripheral white blood cell (WBC) count of CLL cases ranged from 20.9 × 109/L to 186.8 × 109/L. Rai stage in CLL cases was as follows: stage 0, 47% (9/19); stage 1, 16% (3/19); stage 2, 16% (3/19); stage 3, 5% (1/19); and stage 4, 16% (3/19). Flow cytometry confirmed the diagnosis of CLL in all 14 cases tested (Table 1). Flow cytometry analysis of peripheral blood from members of family 2 revealed monoclonal B-cell lymphocytosis (MBL) in 2 asymptomatic siblings (2-1003 and 2-1006) (Figure 3) and no abnormalities in 2 other asymptomatic siblings (2-1004 and 2-1005). FISH analysis revealed 13q14 deletions in 85% (11/13) of CLL-affected individuals studied. An unaffected sibling (2-1006) in family 2 who had MBL was also found to have the 13q14 deletion by FISH, while a sibling (2-1005) who did not share the at-risk haplotype was found to be normal by FISH.
Pedigrees and haplotype analysis of 6 families in the study. A black square indicates individual affected with CLL; a black circle, individual with MBL (monoclonal B-cell lymphocytosis); a white-and-red box, individual with DNA available for analysis; *, reconstructed haplotype; and F, failed genotype.
Pedigrees and haplotype analysis of 6 families in the study. A black square indicates individual affected with CLL; a black circle, individual with MBL (monoclonal B-cell lymphocytosis); a white-and-red box, individual with DNA available for analysis; *, reconstructed haplotype; and F, failed genotype.
Clinical and cytogenetic features of CLL families
| Individual . | Sex . | Age at diagnosis, y . | Diagnosis . | WBC count, × 109/L . | Stage . | Survival status and age, y . | Flow cytometry . | Cytogenetic interphase FISH . |
|---|---|---|---|---|---|---|---|---|
| 1-1001 | F | 47 | CLL | 112 | Rai 3 | Dead, 76 | CD5+ CLL | del 13q14 |
| 1-1003 | M | 52 | CLL | 59.6 | Rai 1 | Dead, 66 | np | np |
| 1-1005 | M | 47 | CLL | 144.6 | Rai 1 | Dead, 61 | np | np |
| 1-1008 | F | 58 | CLL | 32.2 | Rai 0 | Dead, 86 | np | no del 13q14 |
| 2-2002 | F | 69 | CLL | 17.8 | Rai 0 | Living, 80 | CD5+ CLL | del 13q14 |
| 2-1001 | M | 45 | CLL | 186.8 | Rai 4 | Dead, 49 | CD5+ CLL | del 13q14 |
| 2-1002 | M | 50 | CLL | 251 | Rai 2 | Dead, 54 | CD5+ CLL | del 13q14 |
| 2-1003 | F | 53 | Asymptomatic | 6.41 | NA | Living, 60 | CD5+ MBL | np |
| 2-1006 | F | 39 | Asymptomatic | 6.16 | NA | Living, 45 | CD5+ MBL | del 13q14 |
| 3-2001 | M | 76 | CLL | 12.6 | Rai 0 | Dead, 82 | CD5+ CLL | np |
| 3-1001 | M | 49 | CLL | 68.0 | Rai 0 | Living, 59 | CD5+ CLL | del 13q14 |
| 4-1001 | F | 47 | CLL | 98.4 | Rai 0 | Living, 82 | CD5+ CLL | np |
| 4-1002 | F | 70 | CLL | 24.6 | Rai 0 | Living, 81 | CD5+ CLL | np |
| 4-1003 | M | 68 | CLL | 52.1 | Rai 2 | Living, 76 | CD5+ CLL | np |
| 5-1002 | M | 62 | CLL | 63 | Rai 4 | Dead, 71 | CD5+ CLL | no del 13q14 |
| 5-1003 | M | 47 | CLL | 64 | Rai 0 | Living, 61 | CD5+ CLL | del 13q14 |
| 5-1005 | F | 58 | CLL | 56.7 | Rai 0 | Living, 69 | CD5+ CLL | del 13q14 |
| 5-1007 | M | 73 | CLL | 27.1 | Rai 4 | Living, 81 | CD5+ CLL | del 13q14 |
| 6-2003 | M | 59 | CLL | 20.9 | Rai 0 | Living, 64 | np | del 13q14 |
| 6-2012 | F | 51 | CLL | 35.9 | Rai 2 | Living, 55 | CD5+ CLL | del 13q14 |
| 6-1001 | F | 38 | CLL | 26.1 | Rai 0 | Living, 42 | np | del 13q14 |
| Individual . | Sex . | Age at diagnosis, y . | Diagnosis . | WBC count, × 109/L . | Stage . | Survival status and age, y . | Flow cytometry . | Cytogenetic interphase FISH . |
|---|---|---|---|---|---|---|---|---|
| 1-1001 | F | 47 | CLL | 112 | Rai 3 | Dead, 76 | CD5+ CLL | del 13q14 |
| 1-1003 | M | 52 | CLL | 59.6 | Rai 1 | Dead, 66 | np | np |
| 1-1005 | M | 47 | CLL | 144.6 | Rai 1 | Dead, 61 | np | np |
| 1-1008 | F | 58 | CLL | 32.2 | Rai 0 | Dead, 86 | np | no del 13q14 |
| 2-2002 | F | 69 | CLL | 17.8 | Rai 0 | Living, 80 | CD5+ CLL | del 13q14 |
| 2-1001 | M | 45 | CLL | 186.8 | Rai 4 | Dead, 49 | CD5+ CLL | del 13q14 |
| 2-1002 | M | 50 | CLL | 251 | Rai 2 | Dead, 54 | CD5+ CLL | del 13q14 |
| 2-1003 | F | 53 | Asymptomatic | 6.41 | NA | Living, 60 | CD5+ MBL | np |
| 2-1006 | F | 39 | Asymptomatic | 6.16 | NA | Living, 45 | CD5+ MBL | del 13q14 |
| 3-2001 | M | 76 | CLL | 12.6 | Rai 0 | Dead, 82 | CD5+ CLL | np |
| 3-1001 | M | 49 | CLL | 68.0 | Rai 0 | Living, 59 | CD5+ CLL | del 13q14 |
| 4-1001 | F | 47 | CLL | 98.4 | Rai 0 | Living, 82 | CD5+ CLL | np |
| 4-1002 | F | 70 | CLL | 24.6 | Rai 0 | Living, 81 | CD5+ CLL | np |
| 4-1003 | M | 68 | CLL | 52.1 | Rai 2 | Living, 76 | CD5+ CLL | np |
| 5-1002 | M | 62 | CLL | 63 | Rai 4 | Dead, 71 | CD5+ CLL | no del 13q14 |
| 5-1003 | M | 47 | CLL | 64 | Rai 0 | Living, 61 | CD5+ CLL | del 13q14 |
| 5-1005 | F | 58 | CLL | 56.7 | Rai 0 | Living, 69 | CD5+ CLL | del 13q14 |
| 5-1007 | M | 73 | CLL | 27.1 | Rai 4 | Living, 81 | CD5+ CLL | del 13q14 |
| 6-2003 | M | 59 | CLL | 20.9 | Rai 0 | Living, 64 | np | del 13q14 |
| 6-2012 | F | 51 | CLL | 35.9 | Rai 2 | Living, 55 | CD5+ CLL | del 13q14 |
| 6-1001 | F | 38 | CLL | 26.1 | Rai 0 | Living, 42 | np | del 13q14 |
CLL indicates chronic lymphocytic leukemia; np, not performed; NA, not applicable; and MBL, monoclonal B-cell lymphocytosis.
Flow cytometry of 2 unaffected individuals with MBL. Panels A-C show data on 2-1003; panels D-F, on 2-1006. (A) Analysis of whole blood stained with CD19 PerCP. The plot shows forward scatter versus CD19-isolating B cells. (B) Overall kappa-positive clone. (C) A subgate showing that the CD5 population is monoclonal (kappa light chain restricted). (D) The analysis of StemSep-enriched B cells (StemSep, Vancouver, BC, Canada). The plot shows CD19PerCP Cy5.5 versus CD5 PE Cy7. The top box shows a bright CD5 population, and the bottom box shows a dim CD5 population. (E) A subgate showing that the brighter CD5 population shown in panel D is monoclonal (kappa light chain restricted). (F) A subgate showing that the lower dim CD5 population present in panel D is polyclonal.
Flow cytometry of 2 unaffected individuals with MBL. Panels A-C show data on 2-1003; panels D-F, on 2-1006. (A) Analysis of whole blood stained with CD19 PerCP. The plot shows forward scatter versus CD19-isolating B cells. (B) Overall kappa-positive clone. (C) A subgate showing that the CD5 population is monoclonal (kappa light chain restricted). (D) The analysis of StemSep-enriched B cells (StemSep, Vancouver, BC, Canada). The plot shows CD19PerCP Cy5.5 versus CD5 PE Cy7. The top box shows a bright CD5 population, and the bottom box shows a dim CD5 population. (E) A subgate showing that the brighter CD5 population shown in panel D is monoclonal (kappa light chain restricted). (F) A subgate showing that the lower dim CD5 population present in panel D is polyclonal.
Mapping
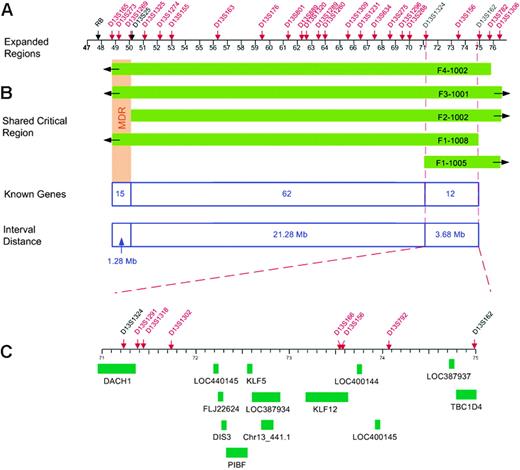
Fine mapping of the 13q region allowed us to investigate whether the CLL susceptibility locus overlaps with the MDR and identify critical recombinants that define the MCR (MCR). Six polymorphic microsatellite markers between D13S165 and D13S1306 were selected for initial genotyping. Nine additional markers were typed to define the MCR. An integrated genetic and physical map of the region was constructed using information derived from public and private databases UCSC Genome Bioinformatics32 ; NCBI BLAST; Ensembl Genome Browser33 ; and Celera, Rockville, MD) (Figure 1). Haplotype analysis showed the individuals with CLL in families 1 to 4 shared a region from marker D13S165 to D13S1306 (Figure 2). Four individuals shared a smaller region due to recombination events at these markers: 1-1005 (D13S1324), 1-1008 (D13S162), 2-1002 (D13S1269), and 4-1002 (D13S782) (Figure 4). Two critical recombinants (1-1005, 1-1008) set the centromeric boundary at D13S1324 and telomeric boundary at D13S162, narrowing the CLL candidate region to a 3.68-Mb in 13q21.33-q22.2. Families 1 to 4 shared this critical region in all CLL-affected members. Family 5 did not share the minimal region at 13q21.33-q22.2 among all their CLL-affected members and was excluded from mutation analysis. Family 6 had a low probability of sharing this minimal candidate region as individuals 6-2003 and 6-1012 would both require double recombination events within a 3.68-Mb region (Figure 2F). The 6 families had the following backgrounds and ancestry: family 1 (Greek), family 2 (English/German/Scandinavian), family 3 (Irish/German/French), family 4 (German/Hungarian), family 5 (Eastern European/Jewish), and family 6 (Irish/German/Native American). The 4 families who shared the haplotype were of diverse ethnic backgrounds. We found no correlation between the families who shared the at-risk haplotype at 13q21.33-q22.2 and their ethnic origin.
Physical map of the CLL candidate region. (A) Locations of polymorphic markers, shown in red. A physical map of the region was constructed using information derived from public and private databases (UCSC Genome Bioinformatics32 ; NCBI; Ensembl Genome Browser33 ; and Celera). (B) Regions shared by the families (shown in green) with critical recombinants identifying the new minimal 3.68-Mb candidate region. (C) Location of the 13 genes within the new CLL candidate region.
Physical map of the CLL candidate region. (A) Locations of polymorphic markers, shown in red. A physical map of the region was constructed using information derived from public and private databases (UCSC Genome Bioinformatics32 ; NCBI; Ensembl Genome Browser33 ; and Celera). (B) Regions shared by the families (shown in green) with critical recombinants identifying the new minimal 3.68-Mb candidate region. (C) Location of the 13 genes within the new CLL candidate region.
Sequence analysis
The 3.68-Mb critical region at 13q21.33-q22.2 contained 12 reference genes and one predicted gene in the UCSC Genome Bioinformatics database (Figure 4). There were no reference microRNAs in the Sanger miRBase34 for this candidate region. These 13 genes were screened for mutations using bidirectional sequencing and a panel of germ-line DNA from affected and unaffected individuals from families 1 to 4 and 6. Eighty-five polymorphisms were identified; 56 were located in exons and 29 were intronic (Table 2). Twenty-nine of 56 exonic polymorphisms were located in the 5′ and 3′ untranslated regions. Within the coding region, 15 nucleotide substitutions resulted in conservative amino acid changes, one codon triplication resulted in the insertion of 2 glycines in DACH1, and 11 substitutions resulted in synonymous changes (Table 2).
Polymorphism summary
| Gene exon/intron . | Position, bp . | Nucleotide change . | Amino acid change . |
|---|---|---|---|
| DIS3 (KIAA1008)NM_014953 | |||
| Exon 5 | c.794G>A | G to A | S265N |
| Intron 5 | IVS + 81T>C | T to C | Noncoding |
| Exon 6 | c.977C>G | C to G | T326R |
| Exon 14 | c.1743A>G | A to G | T581T |
| Intron 16 | IVS16 + 106C > T | C to T | Noncoding |
| Chr13_441 | |||
| Intron 2 | IVS2−18T>C | T to C | Noncoding |
| Exon 3 | c.198G>T | G to T | T66T |
| FLJ22624NM_024808 | |||
| Exon 1 | c.115C>T | C to T | Noncoding |
| Intron 3 | IVS3−69G>A | G to A | Noncoding |
| Intron 3 | IVS3−31A>G | A to G | Noncoding |
| Exon 12 | c.4G>A | G to A | Noncoding |
| KLF5NM_001730 | |||
| Intron 1 | IVS1 + 168C>A | C to A | Noncoding |
| Intron 2 | IVS2 + 56_ + 57dupGT | Duplication GT | Noncoding |
| Intron 2 | IVS2 + 56_ + 57dupGTGT | Duplication GTGT | Noncoding |
| Loc387934 XM_370729 | |||
| Alt Exon 1 | c.294G>A | G to A | A98A |
| Exon 1 | c.438A>G | A to G | A146A |
| Loc440145 XM_495961 | |||
| Exon 3 | c.300A>T | A to T | S100S |
| Loc400145 XM_375031 | |||
| Exon 1 | c.85T>C | T to C | Noncoding |
| Exon 2 | c.156C>T | C to T | H52H |
| Exon 2 | c.245G>A | G to A | R82H |
| Exon 2 | c.403G>A | G to A | G135S |
| DACHINM_080759 | |||
| Exon 1 | c.374C>T | C to T | Noncoding |
| Exon 1 | c.369T>C | T to C | Noncoding |
| Exon 1 | c.308C>T | C to T | Noncoding |
| Exon 1 | c.304C>T | C to T | Noncoding |
| Exon 1 | c.299C>T | C to T | Noncoding |
| Exon 1 | c.298C>A | C to A | Noncoding |
| Exon 1 | c.298C>T | C to T | Noncoding |
| Exon 1 | c.298delC | Deletion C | Noncoding |
| Exon 1 | c.118C>T | C to T | Noncoding |
| Exon 1 | c.293C>T | C to T | Noncoding |
| Exon 1 | c.292C>T | C to T | Noncoding |
| Exon 1 | c.270_−305del36 | Deletion 36 | Noncoding |
| Exon 1 | c.201_−309del109 | Deletion 109 | Noncoding |
| Exon 1 | c.190_−309del120 | Deletion 120 | Noncoding |
| Exon 1 | c242_243dupCGGCGG | Duplication CGGCGG | G81_S82insGG |
| Intron 4 | IVS4 + 14insT | Insertion T | Noncoding |
| TBC1D4NM_014832 | |||
| Exon 1 | c.84C>G | C to G | P28R |
| Exon 1 | c.302C>T | C to T | A101V |
| Exon 1 | c.350T>C | T to C | F117S |
| Exon 1 | c.363C>T | C to T | H121H |
| Intron 1 | IVSI + 8G>A | G to A | Noncoding |
| Intron 1 | IVSI + 18del61 | Deletion 61 | Noncoding |
| Intron 1 | IVSI + 84A>G | A to G | Noncoding |
| Exon 2 | c.606C>T | C to T | F202F |
| Alt intron 1 | Alt IVS1 + 75insG | Ins G | Noncoding |
| Alt intron 4 | Alt IVS4−92T>C | T to C | Noncoding |
| Alt intron 4 | Alt IVS4−107A>G | A to G | Noncoding |
| Exon 7 | c.1611T>G | T to G | S537S |
| Intron 7 | IVS7 + 10insT | Insertion T | Noncoding |
| Intron 9 | IVS9 + 139C>A | C to A | Noncoding |
| Intron 10 | IVS10−59T>G | T to G | Noncoding |
| Intron 13 | IVS13−3C>T | C to T | Noncoding |
| Exon 14 | c.2455A>G | A to G | 1819V |
| Intron 14 | IVS14−60C>T | C to T | Noncoding |
| Intron 15 | IVS15 + 63_ + 64delTT | Deletion TT | Noncoding |
| Exon 16 | c.2904C>T | C to T | L968L |
| Exon 19 | c.3443C>T | C to T | T1148M |
| Exon 20 | c.3620A>G | A to G | N1207S |
| Exon 20 | c.3628C>G | C to G | L1210V |
| Intron 20 | IVS20−19dupT | Duplication T | Noncoding |
| Exon 21 | c.3827C>T | C to T | A1276V |
| Exon 21 | c.980G>A | G to A | Noncoding |
| Exon 21 | c.1599T>C | T to C | Noncoding |
| PIBF1(C13orf24)NM_006346 | |||
| Intron 3 | IVS3 + 19A>T | A to T | Noncoding |
| Intron 3 | IVS3 + 78T>G | T to G | Noncoding |
| Exon 4 | c.499A>G | A to G | 1167V |
| Intron 4 | IVS4 + 61T>C | T to C | Noncoding |
| Intron 8 | IVS8 + 23C>T | C to T | Noncoding |
| Intron 10 | IVS10-68_−71delGTAA | Deletion GTAA | Noncoding |
| Intron 12 | IVS11 + 41delT | Deletion T | Noncoding |
| Exon 15 | c.1888A>G | A to G | 1628V |
| KLF12NM_007249 | |||
| Exon 1 | c.127_−128delTG | Deletion TG | Noncoding |
| Intron 2 | IVS2−3T>C | T to C | Noncoding |
| Exon 4 | c.272C>T | C to T | T91M |
| Exon 6 | c.840C>A | C to A | G280G |
| Loc387937 XM_373569 | |||
| Exon 4 | c.141C>T | C to T | Noncoding |
| Loc400144 XM_378421 | |||
| Exon 1 | c.260C>T | C to T | Noncoding |
| Exon 2 | c.412A>G | A to G | Noncoding |
| Exon 2 | c.535A>G | A to G | Noncoding |
| Exon 2 | c.597A>T | A to T | Noncoding |
| Exon 2 | c.868(TG) 14-20 | TG dinucleotide repeat 14-20 | Noncoding |
| Exon 2 | c.1712A>G | A to G | Noncoding |
| Exon 2 | c.2097C>T | C to T | Noncoding |
| Exon 2 | c.2442T>G | T to G | Noncoding |
| Gene exon/intron . | Position, bp . | Nucleotide change . | Amino acid change . |
|---|---|---|---|
| DIS3 (KIAA1008)NM_014953 | |||
| Exon 5 | c.794G>A | G to A | S265N |
| Intron 5 | IVS + 81T>C | T to C | Noncoding |
| Exon 6 | c.977C>G | C to G | T326R |
| Exon 14 | c.1743A>G | A to G | T581T |
| Intron 16 | IVS16 + 106C > T | C to T | Noncoding |
| Chr13_441 | |||
| Intron 2 | IVS2−18T>C | T to C | Noncoding |
| Exon 3 | c.198G>T | G to T | T66T |
| FLJ22624NM_024808 | |||
| Exon 1 | c.115C>T | C to T | Noncoding |
| Intron 3 | IVS3−69G>A | G to A | Noncoding |
| Intron 3 | IVS3−31A>G | A to G | Noncoding |
| Exon 12 | c.4G>A | G to A | Noncoding |
| KLF5NM_001730 | |||
| Intron 1 | IVS1 + 168C>A | C to A | Noncoding |
| Intron 2 | IVS2 + 56_ + 57dupGT | Duplication GT | Noncoding |
| Intron 2 | IVS2 + 56_ + 57dupGTGT | Duplication GTGT | Noncoding |
| Loc387934 XM_370729 | |||
| Alt Exon 1 | c.294G>A | G to A | A98A |
| Exon 1 | c.438A>G | A to G | A146A |
| Loc440145 XM_495961 | |||
| Exon 3 | c.300A>T | A to T | S100S |
| Loc400145 XM_375031 | |||
| Exon 1 | c.85T>C | T to C | Noncoding |
| Exon 2 | c.156C>T | C to T | H52H |
| Exon 2 | c.245G>A | G to A | R82H |
| Exon 2 | c.403G>A | G to A | G135S |
| DACHINM_080759 | |||
| Exon 1 | c.374C>T | C to T | Noncoding |
| Exon 1 | c.369T>C | T to C | Noncoding |
| Exon 1 | c.308C>T | C to T | Noncoding |
| Exon 1 | c.304C>T | C to T | Noncoding |
| Exon 1 | c.299C>T | C to T | Noncoding |
| Exon 1 | c.298C>A | C to A | Noncoding |
| Exon 1 | c.298C>T | C to T | Noncoding |
| Exon 1 | c.298delC | Deletion C | Noncoding |
| Exon 1 | c.118C>T | C to T | Noncoding |
| Exon 1 | c.293C>T | C to T | Noncoding |
| Exon 1 | c.292C>T | C to T | Noncoding |
| Exon 1 | c.270_−305del36 | Deletion 36 | Noncoding |
| Exon 1 | c.201_−309del109 | Deletion 109 | Noncoding |
| Exon 1 | c.190_−309del120 | Deletion 120 | Noncoding |
| Exon 1 | c242_243dupCGGCGG | Duplication CGGCGG | G81_S82insGG |
| Intron 4 | IVS4 + 14insT | Insertion T | Noncoding |
| TBC1D4NM_014832 | |||
| Exon 1 | c.84C>G | C to G | P28R |
| Exon 1 | c.302C>T | C to T | A101V |
| Exon 1 | c.350T>C | T to C | F117S |
| Exon 1 | c.363C>T | C to T | H121H |
| Intron 1 | IVSI + 8G>A | G to A | Noncoding |
| Intron 1 | IVSI + 18del61 | Deletion 61 | Noncoding |
| Intron 1 | IVSI + 84A>G | A to G | Noncoding |
| Exon 2 | c.606C>T | C to T | F202F |
| Alt intron 1 | Alt IVS1 + 75insG | Ins G | Noncoding |
| Alt intron 4 | Alt IVS4−92T>C | T to C | Noncoding |
| Alt intron 4 | Alt IVS4−107A>G | A to G | Noncoding |
| Exon 7 | c.1611T>G | T to G | S537S |
| Intron 7 | IVS7 + 10insT | Insertion T | Noncoding |
| Intron 9 | IVS9 + 139C>A | C to A | Noncoding |
| Intron 10 | IVS10−59T>G | T to G | Noncoding |
| Intron 13 | IVS13−3C>T | C to T | Noncoding |
| Exon 14 | c.2455A>G | A to G | 1819V |
| Intron 14 | IVS14−60C>T | C to T | Noncoding |
| Intron 15 | IVS15 + 63_ + 64delTT | Deletion TT | Noncoding |
| Exon 16 | c.2904C>T | C to T | L968L |
| Exon 19 | c.3443C>T | C to T | T1148M |
| Exon 20 | c.3620A>G | A to G | N1207S |
| Exon 20 | c.3628C>G | C to G | L1210V |
| Intron 20 | IVS20−19dupT | Duplication T | Noncoding |
| Exon 21 | c.3827C>T | C to T | A1276V |
| Exon 21 | c.980G>A | G to A | Noncoding |
| Exon 21 | c.1599T>C | T to C | Noncoding |
| PIBF1(C13orf24)NM_006346 | |||
| Intron 3 | IVS3 + 19A>T | A to T | Noncoding |
| Intron 3 | IVS3 + 78T>G | T to G | Noncoding |
| Exon 4 | c.499A>G | A to G | 1167V |
| Intron 4 | IVS4 + 61T>C | T to C | Noncoding |
| Intron 8 | IVS8 + 23C>T | C to T | Noncoding |
| Intron 10 | IVS10-68_−71delGTAA | Deletion GTAA | Noncoding |
| Intron 12 | IVS11 + 41delT | Deletion T | Noncoding |
| Exon 15 | c.1888A>G | A to G | 1628V |
| KLF12NM_007249 | |||
| Exon 1 | c.127_−128delTG | Deletion TG | Noncoding |
| Intron 2 | IVS2−3T>C | T to C | Noncoding |
| Exon 4 | c.272C>T | C to T | T91M |
| Exon 6 | c.840C>A | C to A | G280G |
| Loc387937 XM_373569 | |||
| Exon 4 | c.141C>T | C to T | Noncoding |
| Loc400144 XM_378421 | |||
| Exon 1 | c.260C>T | C to T | Noncoding |
| Exon 2 | c.412A>G | A to G | Noncoding |
| Exon 2 | c.535A>G | A to G | Noncoding |
| Exon 2 | c.597A>T | A to T | Noncoding |
| Exon 2 | c.868(TG) 14-20 | TG dinucleotide repeat 14-20 | Noncoding |
| Exon 2 | c.1712A>G | A to G | Noncoding |
| Exon 2 | c.2097C>T | C to T | Noncoding |
| Exon 2 | c.2442T>G | T to G | Noncoding |
Small insertions/deletions were found within deep intronic regions of DACH1, KLF5, KLF12, TBC1D4, and PIBF1 (also known as C13orf24, chromosome 13 open-reading frame 24), (Table 2). One polymorphism resulted in the insertion of 2 glycines between codons 81 and 82 of DACH1. This amino acid change appears to be nondeleterious, did not cosegregate with disease, and was detected among all CEPH controls. A single nucleotide polymorphism (snp) located in the −3 position of the KLF12 intron 2 splice acceptor was detected in 4 affected individuals and 1 unaffected individual from 3 of 5 families. This polymorphism was previously reported by Rozenblum et al.35
Five intronic polymorphisms and 2 conservative amino acid changes (I167V, I628V) detected in PIBF1 did not cosegregate with disease. A deep intronic polymorphism (IVS10−68_−71delGTAA) was detected in PIBF1 that cosegregated with 3 affected relatives (2-2002, 2-1001, 2-1002), and 2 unaffected relatives (2-1003, 2-1006) in family 2 who shared the affected haplotype in band 13q21.33-q22.2 (Figure 2B). This polymorphism was not detected in the other 4 families screened. PIBF1 IVS10−68_−71delGTAA polymorphism was detected at a frequency of 0.38 among 100 unrelated CEPH controls, indicating that this is a common polymorphism among Europeans. Three deep intronic polymorphisms were detected in KLF5 (Table 2). Two of these polymorphisms were located within intron 2 at a dinucleotide GT repeat and were present in both affected and unaffected individuals in 4 of 5 families. Both affected and unaffected individuals in families 3 and 4 were heterozygous for the KLF5 IVS2+56_+57dupGT or the KLF5 IVS2+56_+57dupGTGT polymorphisms. However, these polymorphisms in intron 2 of KLF5 did not cosegregate with disease. There were 13 intronic polymorphisms found in TBC1D4. These polymorphisms were detected in both affected and unaffected individuals among CLL families or solely in unaffected individuals who did not carry the affected haplotype at 13q21.33-q22.2. No additional polymorphisms were found that cosegregated with affected individuals among the remaining families analyzed. No frameshift or nonsense mutations were detected in the coding region of the 13 genes screened.
Functional studies
We examined the 13 genes in our candidate region for expression in a cDNA human immune panel that included peripheral leukocytes, spleen, bone marrow, liver, and thymus. Of the 13 genes identified in the candidate region, 11 were expressed in immune tissues, specifically leukocytes and bone marrow (Table 3). This finding supports that the genes analyzed in our study may have functional relevance in familial CLL.
Tissue-expression profile of CLL candidate genes at 13q21.33-q22.2
| Candidate gene . | Spleen . | Lymph node . | Thymus . | Tonsil . | Leukocyte . | Bone marrow . | Liver . | Positive control . |
|---|---|---|---|---|---|---|---|---|
| loc440145 | + | + | + | + | + | + | + | + |
| TBC1D4 | + | + | + | + | + | + | + | + |
| PIBF1 | + | + | + | + | + | + | + | + |
| KLF5 | + | + | + | + | + | + | + | + |
| DIS3 | + | + | + | + | + | + | + | + |
| DACH1 | + | + | + | + | + | + | + | + |
| KLF12 | + | + | + | + | + | + | + | + |
| FLJ22624 | + | + | + | + | + | + | + | + |
| loc400144 | + | + | + | + | + | + | − | + |
| loc400145 | + | + | − | − | + | + | + | + |
| loc387934 | + | + | + | + | + | + | + | + |
| loc387937 | − | − | − | − | − | − | − | + |
| CHR13_441 | − | − | − | − | − | − | − | + |
| Candidate gene . | Spleen . | Lymph node . | Thymus . | Tonsil . | Leukocyte . | Bone marrow . | Liver . | Positive control . |
|---|---|---|---|---|---|---|---|---|
| loc440145 | + | + | + | + | + | + | + | + |
| TBC1D4 | + | + | + | + | + | + | + | + |
| PIBF1 | + | + | + | + | + | + | + | + |
| KLF5 | + | + | + | + | + | + | + | + |
| DIS3 | + | + | + | + | + | + | + | + |
| DACH1 | + | + | + | + | + | + | + | + |
| KLF12 | + | + | + | + | + | + | + | + |
| FLJ22624 | + | + | + | + | + | + | + | + |
| loc400144 | + | + | + | + | + | + | − | + |
| loc400145 | + | + | − | − | + | + | + | + |
| loc387934 | + | + | + | + | + | + | + | + |
| loc387937 | − | − | − | − | − | − | − | + |
| CHR13_441 | − | − | − | − | − | − | − | + |
+ indicates positive expression; −, negative expression.
The gene expression level of 10 candidate genes in the 13q21.33-q22.2 region was evaluated in a sporadic CLL leukemic clone compared with normal B cells. The CLL leukemic clone showed a decreased level of expression among 7 candidate genes compared with the normal B cells. The gene expression level of the CLL leukemic clone ranged from a decrease of −2.2-fold (FLJ22624) to −12.9-fold (TBC1D4) compared with normal B cells (Table 4). Two genes (DACH1 and loc400145) were not expressed in CLL cells or B cells. Loc400144 was not expressed in the CLL clone but showed low expression in the normal B cells.
Candidate gene expression comparison between sporadic CLL and B cells
| Candidate gene expression . | CLL gene expression activity ratio to B cells, fold change . |
|---|---|
| loc440145/β-actin | −2.8 |
| TBC1D4/β-actin | −12.9 |
| PIBF1/β-actin | −2.7 |
| KLF5/β-actin | −5.3 |
| DIS3/β-actin | −2.8 |
| DACH1/β-actin | Not expressed in lymphocytes |
| KLF12/β-actin | −3.0 |
| FLJ22624/β-actin | −2.2 |
| loc400144/β-actin | Not expressed |
| Loc400145/β-actin | Not expressed in lymphocytes |
| Candidate gene expression . | CLL gene expression activity ratio to B cells, fold change . |
|---|---|
| loc440145/β-actin | −2.8 |
| TBC1D4/β-actin | −12.9 |
| PIBF1/β-actin | −2.7 |
| KLF5/β-actin | −5.3 |
| DIS3/β-actin | −2.8 |
| DACH1/β-actin | Not expressed in lymphocytes |
| KLF12/β-actin | −3.0 |
| FLJ22624/β-actin | −2.2 |
| loc400144/β-actin | Not expressed |
| Loc400145/β-actin | Not expressed in lymphocytes |
Discussion
In this study, we present 6 families in which multiple members were diagnosed with CLL in successive generations, suggesting that inherited genes contribute to a subset of CLL.16 First-degree relatives of patients with CLL have an increased risk of developing CLL and other lymphoproliferative diseases, suggesting a shared genetic susceptibility in these families.16 Therefore, studies of familial CLL are particularly important in discovering CLL susceptibility genes.
Our study provides further evidence that familial CLL has unique clinical and molecular genetic features. In screening asymptomatic individuals using flow cytometry, we identified 2 individuals with CD5+ MBL who carried the at-risk haplotype at 13q21.33-q22.2 (Figure 3). These 2 individuals with MBL shared the at-risk haplotype with their CLL-affected relatives, providing further evidence for a relation between CLL and MBL. This finding is consistent with reports that MBL occurs in about 14% to 18% of asymptomatic relatives of familial CLL cases18,36 and supports the hypothesis that CD5+ MBL may be a precursor of CLL or a surrogate marker for carrier status. However, it remains to be determined through prospective studies if MBL is a predictor of eventual development of CLL among asymptomatic members of CLL families. The ability to detect MBL with flow cytometry should facilitate gene mapping studies in high-risk families.
Our study revealed that 85% of patients with familial CLL had deletions in 13q14. The frequency among familial cases is higher than reported in sporadic CLL (55%).10 In family 2, all affected individuals with CLL had deletions in 13q14 and shared a haplotype at 13q21.33-q22.2, suggesting that inherited factors may predispose to CLL. One asymptomatic individual who carried the at-risk haplotype at 13q21.33-q22.2 and exhibited CD5+ MBL on flow cytometry also had a 13q14 deletion by FISH. This is one of the first reported cases of an asymptomatic individual with 13q14 deletion. Deletions in 13q14 may represent a very early genomic change in a cascade of genetic events that predispose to developing CLL and/or MBL. Consistent with this is the finding that 13q14 deletion is the most frequent chromosomal aberration detected in sporadic CLL and that sporadic cases with 13q14 deletions as the sole abnormality have the longest estimated survival.10 However, the clinical significance of 13q14 deletion in an asymptomatic relative in the setting of familial CLL is unknown. It remains to be determined through prospective studies if the presence of a 13q14 deletion is a risk factor for development of CLL among asymptomatic members of CLL families.
This study identified a new candidate region 13q21.33-q22.2 that may predispose to familial CLL and MBL. Somatic deletions of 13q21-q22 have been observed in several tumors including sporadic37 and hereditary breast cancer,38 prostate cancer,39 and hepatoblastoma,40 but to our knowledge have not been reported in CLL. Using haplotype analysis, we identified a shared 3.68-Mb region at 13q21.33-q22.2 among 4 CLL families. Our studies revealed that 11 of the 13 genes identified in this 3.68-Mb candidate region were expressed in immune tissues, specifically leukocytes and bone marrow. This finding supports that the 11 genes in the candidate region have functional relevance in investigations of CLL. Using direct sequencing analysis, we screened 13 genes for germ-line mutations, and 85 polymorphisms were identified. In family 2, we found one deep intronic polymorphism in PIBF1 that cosegregated with the haplotype shared by 3 affected members. However, no conserved splice site or coding mutations were identified in this gene. PIBF1 is a lymphocyte-secreted immunomodulatory molecule41 that is expressed in spleen35 and overexpressed in breast tumor cell lines.42 PIBF1 is a microtubule-associated protein that localizes to centrosomes,42 where abnormalities are frequently observed in cancer cells, although their role in tumorigenesis is unknown.43
Of the 12 reference genes at 13q21.33-q22.2, 5 candidate genes have putative function related to cell growth that makes them potentially important. DIS3 is the human homolog of mitotic control protein Dis3 of Saccharomyces pombe,44 and DACH1 inhibits transforming factor-β (TGF-β)–induced apoptosis.45 KLF5 is found to be deleted and down-regulated in prostate cancer46 and reintroduction of KLF5 in breast tumor cell lines with 13q21 deletion inhibited cell growth in vitro.47 KLF12 represses expression of activator protein-2 alpha (AP-2α).48 Decreased expression of AP-2α is associated with disease progression and metastases in breast cancer.49 DIS3 and KLF12 are expressed in peripheral leukocytes.35 Our gene expression investigations of a CLL clone showed a 2.2- to 12.9-fold decreased level of expression among 7 candidate genes including PIFB1 and KLF5. In addition, there was complete loss of expression of one gene (loc400144) compared with the normal B cells. It is unlikely that the decrease or loss of expression of genes in this region may be due to microRNAs since we found no reference microRNAs in this candidate region. Further gene expression studies of CLL clones will be conducted to confirm our findings. Future cytogenetic studies to determine whether large chromosomal deletions in 13q21.33-q22.2 are responsible for the decrease and/or loss of gene expression in this region are highly relevant since somatic deletions have been observed in tumors in this region.37-40
We identified CpG islands in 7 of 13 genes near the promoter region of these respective genes. However, our literature search did not reveal evidence of maternal or paternal imprinting effects on chromosome 13 based on the reported normal phenotype of individuals who exhibit uniparental disomy of chromosome 13.50-52 Therefore, inherited methylation changes are unlikely to be relevant to the pathogenesis of CLL in these families. Our future studies will investigate the role of methylation in regulating gene expression in our new candidate region.
Two genome-wide linkage studies of CLL families have pointed to several potential candidate regions.19,53 Recently, Sellick et al53 conducted a genome-wide linkage analysis of 115 CLL families and found a nonparametric linkage score of 3.14 at locus 11p11 and HLOD scores higher than 1.15 at 4 loci (5q22-q23, 6p22, 10q25, and 14q32) consistent with genetic heterogeneity among these families.53 To date, a CLL susceptibility gene has not been identified using direct sequencing by our group and other investigators. Epigenetic mechanisms, microRNAs, and multiple genes may be involved in transcriptional silencing of gene(s) underlying CLL leukemogenesis. Several factors make the mapping and identification of CLL susceptibility genes quite challenging. First, the late onset of the disease makes it difficult to collect DNA from families with multigeneration-affected individuals. We will increase the power of linkage studies through consortial approaches that pool data and samples on CLL families to uncover CLL susceptibility genes. Second, familial CLL is a genetically heterogeneous disease with variable penetrance and a probable high phenocopy rate. In conclusion, we identified a novel candidate region that may predispose to familial CLL and MBL. Future cytogenetic and gene expression studies are planned to clarify the biological significance of this novel region.
Authorship
Contribution: D.N., M.-H.W., and J.R.T. designed the research; L.F., J.F.F., L.R.G., and N.C. collected and analyzed the patient data; D.N., D.C.A., O.T., and F.A. performed research; D.N., D.C.A., G.E.M., and J.R.T. analyzed data; D.N. and J.R.T. wrote the paper; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jorge R. Toro, Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, 6120 Executive Blvd, Executive Plaza South, Rm 7012,Rockville, MD 20892-7231; e-mail: toroj@mail.nih.gov.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Contribution: D.N., M.-H.W., and J.R.T. designed the research; L.F., J.F.F., L.R.G., and N.C. collected and analyzed the patient data; D.N., D.C.A., O.T., and F.A. performed research; D.N., D.C.A., G.E.M., and J.R.T. analyzed data; D.N. and J.R.T. wrote the paper; and all authors read and approved the final version of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal