In this issue of Blood, Min and colleagues report that RANKL is a potent activator of endothelial nitric oxide synthase (eNOS) and induces vascular hyperpermeability and angiogenesis in an eNOS-NO–dependent manner.
Receptor activator of nuclear factor (NF)–κB ligand (RANKL, also known as ODF, osteoclast differentiation factor; OPGL, osteoprotegerin ligand; and TRANCE, TNF-related activation-induced cytokine) is a key regulator of bone homeostasis, lymphocyte development, lymph node organogenesis, and mammary gland formation.1 RANK is a signaling receptor of RANKL—which is counterbalanced by a decoy receptor OPG. In addition to the well-studied effects on the skeletal and immune systems, the OPG/RANKL/RANK system appears to mediate vascular pathology such as atherosclerosis, calcification, and plaque destabilization. However, its physiologic role and downstream signaling pathways in the vascular system are not known.
Nitric oxide (NO) is a multifunctional gaseous molecule that plays important roles in blood vessel physiology and pathology.2 NO mediates endothelial-cell migration and proliferation and recruitment of endothelial precursor and perivascular cells, and thus, facilitates angiogenesis and vessel maturation. NO regulates vessel tone and blood flow via relaxation of vascular smooth muscle cells. NO also inhibits platelet aggregation and leukocyte adhesion, and mediates vascular hyperpermeability in pathologic settings. Endothelial NO synthase (eNOS) predominantly mediates these vascular functions of NO.
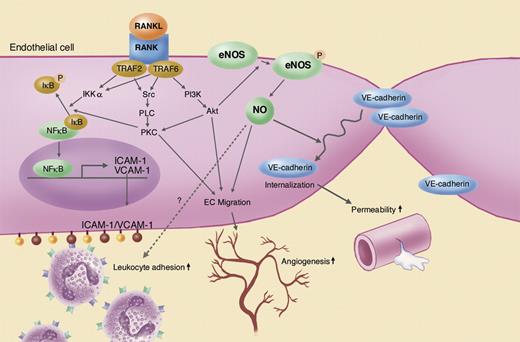
Kwon and colleagues previously reported that RANKL activates vascular endothelial cells and induces adhesion molecule expression, endothelial tube formation, and angiogenesis in vivo (Kim et al3 and Min et al4 ). Now Min and colleagues show that for the first time RANKL increases vascular permeability and that various RANKL-induced vascular events—leukocyte extravasation, increased permeability, and angiogenesis—are mediated by eNOS (see figure). Exogenously applied RANKL phosphorylates Ser1177 in eNOS via the tumor necrosis factor receptor–associated factor 6 (TRAF6) and PI3K-Akt pathway. eNOS-NO mediates RANKL-induced endothelial-cell migration and tube formation as well as angiogenesis in a Matrigel assay. NO also induces VE-cadherin relocation from endothelial-cell membrane to cytosol and increases leakage of Evans blue dye in the skin and FITC-dextran in the retina. These findings shed new light on the role of RANKL in the vascular system, and reveal the central role of eNOS as a mediator of RANKL's function in the vasculature.
Vascular effects of RANKL and its downstream signaling pathways in endothelial cells. Illustration by Marie Dauenheimer.
Vascular effects of RANKL and its downstream signaling pathways in endothelial cells. Illustration by Marie Dauenheimer.
This exciting study also raises many unanswered questions. For example, what is the role of endogenous RANKL in the vasculature under physiologic or pathologic conditions? What are the spatial and temporal regulations of endogenous RANKL/OPG/RANK during angiogenesis and/or inflammation? Can the causal role of endogenous RANKL on eNOS and vasculature be unraveled by blocking its signaling with OPG, OPG-like proteins, soluble RANK, or RANKL neutralizing antibodies?
It is noteworthy that the effects of RANKL are not exclusively eNOS-NO dependent. For example, a NOS inhibitor or eNOS deletion significantly diminishes, but does not completely abolish, the effects of RANKL. Similarly, dominant-negative TRAF2 expression or a Src inhibitor blocks RANKL-induced endothelial-cell migration but does not affect eNOS activity. As originally discovered, RANKL activates NF-κB via activation of IKKα. In turn, NF-κB mediates up-regulation of cell-adhesion molecules such as ICAM-1 and VCAM-1 induced by RANKL.4 NF-κB is also a potent inducer of inducible NOS (iNOS). iNOS may be involved in and/or interfere with RANKL functions in vivo. Thus, NF-κB may also affect RANKL-induced angiogenesis and vascular hyperpermeability. Finally, the reduction in RANKL-induced leukocyte extravasation in eNOS−/− mice is counterintuitive. NO is known to down-regulate cell-adhesion molecules including ICAM-1 and VCAM-1. One would expect to see increased leukocyte adhesion in eNOS−/− mice rather than a decrease. Further studies to resolve this apparent discrepancy are needed. In the meantime, the report by Min and colleagues opens new connections between the fields of vascular biology and inflammation.
The authors declare no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal