Abstract
New blood vessel formation requires the coordination of endothelial cell division and the morphogenetic movements of vessel expansion, but it is not known how this integration occurs. Here, we show that endothelial cells regulate division orientation during the earliest stages of blood vessel formation, in response to morphogenetic cues. In embryonic stem (ES) cell–derived vessels that do not experience flow, the plane of endothelial cytokinesis was oriented perpendicular to the vessel long axis. We also demonstrated regulated cleavage orientation in vivo, in flow-exposed forming retinal vessels. Daughter nuclei moved away from the cleavage plane after division, suggesting that regulation of endothelial division orientation effectively extends vessel length in these developing vascular beds. A gain-of-function mutation in VEGF signaling increased randomization of endothelial division orientation, and this effect was rescued by a transgene, indicating that regulation of division orientation is a novel mechanism whereby VEGF signaling affects vessel morphogenesis. Thus, our findings show that endothelial cell division and morphogenesis are integrated in developing vessels by flow-independent mechanisms that involve VEGF signaling, and this cross talk is likely to be critical to proper vessel morphogenesis.
Introduction
Blood vessels form and expand in both development and disease, via processes that include vasculogenesis, angiogenesis, and intussusception (reviewed in Risau,1 Eichmann et al,2 Coultas et al3 ). Sprouting angiogenesis is the coordinated migration of groups of endothelial cells from vessels and their subsequent fusion to form new interconnections. In this way, simple vascular tubes are ramified and extended to form a primitive vascular plexus. This vessel plexus forms at numerous sites in the embryo, including the yolk sac, the head mesenchyme, and surrounding the neural tube. The primitive vascular plexus is then remodeled under the influence of blood flow and interactions with mural cells. Thus, the initial pattern of vessels serves as a template for remodeling that leads to a mature vasculature.
During formation of the primitive vascular plexus, several cellular processes must be regulated and integrated. Specifically, endothelial cells respond to some morphogenetic cues by sprouting, while actively dividing to expand the pool of endothelial cells. One level of integration occurs via the signaling pathways that promote angiogenesis, because many affect both endothelial cell division and morphogenesis. The VEGF signaling pathway is an example of this mode of integration, because it regulates both cell division and branching morphogenesis (reviewed in Rousseau et al,4 Kliche and Waltenberger,5 Ferrara et al,6 Nagy and Senger,7 and Shibuya and Claesson-Welsh8 ). VEGF-A (VEGF) binds 2 high-affinity receptors on endothelial cells, flk-1 (VEGFR-2) and flt-1 (VEGFR-1), and perturbation of VEGF signaling by genetic deletion of either receptor affects both endothelial cell division and morphogenesis.9-12 Several lines of evidence, however, suggest that different regulatory nodes in the VEGF signaling pathway influence endothelial cell division and morphogenesis. VEGF signaling through flk-1 promotes endothelial cell division through the Raf/MEK/ERK pathway, whereas endothelial cell migration is stimulated through p38 Map kinase and adaptor proteins such as Shb and Nck that lead to regulation of the actin cytoskeleton.13-16 In addition, the overall level of available VEGF is thought to regulate the rate of endothelial cell division, whereas VEGF presentation, perhaps through the formation of a gradient, is thought to regulate sprouting angiogenesis and the formation of filopodia.11,12,17,18 Moreover, other pathways selectively affect endothelial cell division or morphogenesis. For example, signaling through p27/kip1 regulates the endothelial cell cycle,19,20 whereas the netrin-UNC and semaphorin-plexin pathways regulate endothelial branching morphogenesis and guidance.21-23 Thus, it is likely that endothelial cell division and morphogenesis are integrated at multiple levels during angiogenesis, but little is known about how this is achieved.
The orientation of the cleavage plane of cell division, which positions the daughter cells relative to other embryonic axes, is regulated in numerous developing tissues (reviewed in Ahringer,24 Cowan and Hyman,25 and Strutt26 ). The basis for this regulation is the interaction of the mitotic spindle with molecules differentially localized on the inner side of the plasma membrane (the cortex) (reviewed in Pellettieri and Seydoux27 ). The cortical cues include the PAR proteins first identified in Caenorhabditis elegans, atypical protein kinases, and molecules, such as LGN, NuMA, and Inscuteable, that appear to link microtubules to the cortex.28-30 The spatial regulation of these cues is complex and not completely understood, but the actin cytoskeleton and its interactions with the cortex are required for proper positioning of the cues and in turn the mitotic spindle.31 The actin cytoskeleton in turn is acted on by numerous inputs, among them growth factor signaling.
Complex structures such as epithelial sheets and tubes can exhibit a polarity of division orientation (reviewed in Metzger and Krasnow,32 Nelson,33 and Davies34 ). Madin-Darby canine kidney (MDCK) cells form tubes in culture in response to hepatocyte growth factor (HGF)/scatter factor signaling, and this signaling can reorient MDCK cell divisions so that daughter cells leave the epithelial sheet.35,36 Kidney tubules expand via cell divisions that are oriented to extend the length of the tubule.37 Epidermal cleavages oriented parallel to the long axis of the sheet resulted in daughter cells that formed a new layer during embryonic skin development, and loss of several pathways, including those involving β1 integrins, lead to increased randomization of divisions and aberrant stratification.29 Oriented cell divisions are also associated with flower bud formation, elongation of the avian primitive streak, shaping of the neural plate in avians and mouse, and extension of the zebra fish body axis at gastrulation.38-42
Endothelial cells orient their actin cytoskeleton and microtubule network in response to shear stress, such as that produced by blood flow in vivo (reviewed in Davies,43 McCue et al,44 and Orr et al45 ). However, despite the fact that endothelial cells actively divide while undergoing morphogenesis, the orientation of endothelial cell cleavages during normal and perturbed angiogenesis has not been investigated. We asked whether the orientation of endothelial division was regulated in a flow-independent model of dynamic angiogenesis in culture and in retinal vessels in vivo. Here, we show that endothelial cell cleavage is normally oriented perpendicular to the long axis of the vessel, which can promote vessel lengthening, a hallmark of these expanding vascular plexuses. Moreover, we show that orientation is randomized by a mutation that disrupts VEGF signaling and leads to vessel dysmorphogenesis, and it is rescued by genetic rescue of the mutation. These data indicate that endothelial cell division orientation is regulated by flow-independent morphogenetic cues, and that endothelial cell division is normally oriented by a process that involves VEGF signaling. Our findings also suggest that the integration of proliferative and morphogenetic processes is critical to proper vessel morphogenesis.
Materials and methods
Cell culture and in vitro differentiation
Embryonic stem (ES) cell lines used consisted of wild-type (WT; +/+) and flt-1−/− ES cells containing a transgene consisting of enhanced green fluorescent protein (eGFP) fused to histone 2B (H2B) under the transcriptional control of the platelet endothelial cell adhesion molecule (PECAM) promoter/intron enhancer element (Tg PECAM-H2B-GFP)12 (and this report), as well as WT ES cells containing a PECAM-eGFP transgene (Tg PECAM-GFP).12 Additional lines were WT, flt-1−/−, and 2 sflt1 rescue lines, flt-1−/−;Tg PECAM-sflt-1#3312 and flt-1−/−;Tg PECAM-sflt-1#26 (N.C.K., G.Z., J.B.K., Allan Nanney, Kimberly G. Kallianos, Amanda Schimizzi, Cam Patterson, and V.L.B.; “Soluble flt-1 (VEGFR-1) is the predominant flt-1 isoform regulating vessel branching,” manuscript submitted) that did not contain a GFP transgene. All ES cell cultures were maintained and differentiated in vitro as described previously.46,47 Embryoid bodies were plated onto slide flasks (Nunc, Rochester, NY) at day 3 of differentiation and cultured at 37°C in 5% CO2 until day 7 to 8, when time lapse imaging was performed or they were fixed and stained.
DNA constructs and electroporation
The SalI/NotI H2B-eGFP fragment was cut from pBOS-H2BGFP (BD Pharmingen, San Diego, CA) and cloned into the PECAM promoter-enhancer vector12 for electroporation into WT ES cells (designated PECAM-H2B-GFP). The same fragment was also cloned into PECAM-Hygro12 for electroporation into flt-1−/− ES cells and designated PECAM-H2B-GFP-Hygro. DNA was electroporated into ES cells as described previously.12 Briefly, 20 μg linearized PECAM-H2B-GFP or PECAM-H2B-GFP-Hygro DNAs were electroporated into 2 × 107 ES cells using a BioRad GenePulser II electroporator (250 V/300 μF; BioRad, Hercules, CA). WT ES cell selection was in 200 μg/mL G418 (Gibco, Carlsbad, CA), and selection of flt-1−/− ES cells was in 200 μg/mL hygromycin B (Roche Diagnostics, Indianapolis, IN). After 12 to 14 days, drug-resistant ES colonies were picked, expanded, and analyzed by in vitro differentiation and fluorescence imaging. We initially analyzed 5 WT transgenic lines (designated as WT;Tg PECAM-H2B-GFP) and 4 flt-1−/− transgenic lines (designated as flt-1−/−;Tg PECAM-H2B-GFP) that expressed the transgene in vessels and saw no differences except by genotype, so single WT and mutant lines were used for time-lapse imaging. Generation of the sflt-1 rescue lines has been previously described.12
Time-lapse imaging
Slide flasks containing day 7 to 8 in vitro–differentiated ES cell cultures were sealed, then placed on a heated stage on a Nikon TE300 inverted microscope (Melville, NY) with a Perkin Elmer spinning disk confocal head (Shelton, CT). Confocal images were acquired at 1-minute intervals using Metamorph software (version 6.0; Universal Imaging, Downingtown, PA) and a Hamamatsu Orca CCD camera (McHenry, IL) with 20×/0.45 NA or 10×/0.25 NA objectives as described.47
Antibody staining
Following time-lapse imaging, ES cell cultures were rinsed with phosphate-buffered saline (PBS) and fixed for 5 minutes in ice-cold methanol-acetone (50:50). Fixed cultures were reacted with rat anti–mouse PECAM at 1:1000 (MEC 13.3; BD Pharmingen, San Diego, CA) and donkey anti–rat immunoglobulin G (IgG; H + L) conjugated to FITC or TRITC at 1:200 (Jackson Immunoresearch, West Grove, PA) as described previously.46,48 In some cases PECAM-stained cultures were labeled with phosphohistone H3 as described,11 using rabbit antiphosphohistone at 1:500 (Upstate Biotechnology, Charlottesville, VA) and donkey anti–rabbit immunoglobulin G (IgG; H + L) conjugated to TRITC at 1:200 (Jackson Immunoresearch). All cultures were viewed and photographed with an Olympus IX-50 inverted microscope (Melville, NY) outfitted with epifluorescence, or a Zeiss 510 confocal microscope (Jena, Germany). PECAM-stained images were aligned with the last frame of each movie using Photoshop version 7.0 (Adobe Systems, San Jose, CA).
Rat retina dissection and staining
Rat pups (P3-5) were weighed and anesthetized by intraperitoneal injection of ketamine (20 mg/kg) and xylazine (6 mg/kg). Paraformaldehyde (PFA) was directly perfused (0.5 mL of 0.5% PFA) into the right ventricle, after which the pups were humanely killed by intracardiac injection of pentobarbital (80 mg/kg). Both eyes were enucleated, and whole eyes were fixed in 2% PFA for 2 hours before being washed in PBS. The retinas were dissected using a modification of a described method.49,50
The flat-mounted retinas were incubated in ice-cold ethanol for 30 minutes, then permeabilized with 1% Triton X-100 in PBS for 30 minutes. The retinas were reacted with isolectin GS-I B4 conjugated to Alexa Fluor 488 at 1:100 (Invitrogen, Carlsbad, CA) overnight at 4°C. The samples were washed once with PBS, then blocked in 1% Triton X-100 and 5% goat serum in PBS for 1 hour. Antibodies and reaction conditions used were as follows: rabbit polyclonal antiphosphohistone H3 at 1:500 (Upstate Biotechnology), overnight at 4°C, donkey anti–rabbit immunoglobulin G (IgG; H + L) conjugated to TRITC at 1:100 (Jackson Immunoresearch), 3 hours at 37°C. All incubations were done in a humidity chamber, and samples were washed 3 times with PBS after each antibody reaction. Retinas were mounted in PBS:glycerol (2:1) and scanned on a Leica SP2 AOBS or a Zeiss 510 confocal microscope using a 40×/1.25 NA Apochromat objective. Animal experiments were approved by the IACUC Committee at University of North Carolina.
Quantitative image analysis
Quantitative image analysis was performed using Metamorph software (Universal Imaging). Cell division planes were easily identified in H2B-GFP–labeled vessels by bisection of the separating chromosomes during anaphase, and in antiphosphohistone H3–stained vessels by visualization of the metaphase or anaphase chromosomes. Lines were drawn along the division plane and along the long axis of the blood vessel for each mitotic division, and the angle between these 2 lines was calculated. Angles of 0 degree are divisions whose cleavage planes are parallel to the long axis of the blood vessel, whereas angles of 90 degrees are divisions whose cleavage planes are perpendicular to the long axis of the blood vessel. The angles were then grouped to every 10 degrees, ranging from 0 degree to 90 degrees. Microsoft Office Word (version 2003; Microsoft, Redman, WA) was used to generate line drawings of the angles. Daughter cell separation was tracked for at least 60 minutes after division using time lapse imaging and Metamorph software (Universal Imaging).
Results
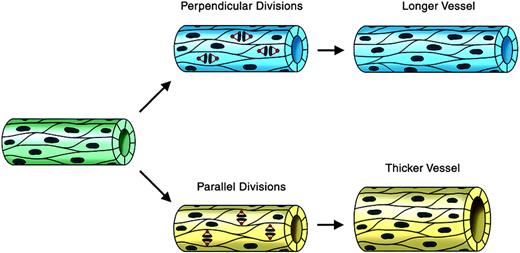
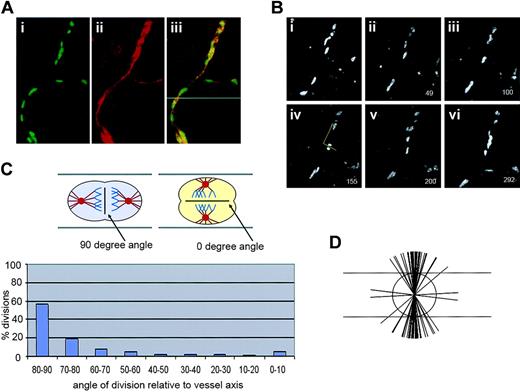
We reasoned that oriented endothelial cell divisions during angiogenic expansion of a vascular plexus might affect vessel morphogenesis by increasing either vessel length or vessel diameter (Figure 1). The cleavage plane was identified as a line between and parallel to the 2 groups of anaphase chromosomes (Figure 2C). Thus, a division oriented so that the plane of cytokinesis is perpendicular to the long axis of the vessel would effectively lengthen the vessel, whereas a division plane oriented parallel to the vessel long axis would increase the vessel diameter. To determine whether endothelial cell divisions are oriented in a flow-independent manner, we used a model of vascular development in which mouse ES cells undergo a programmed differentiation. This process generates multiple embryonic cell types, including endothelial cells that form 3-dimensional vessels and undergo sprouting angiogenesis.47,51 Although primitive hematopoietic cells also differentiate and the vessels form lumens, there is no flow through the vessels.46 WT ES cells were generated with a stably integrated transgene encoding histone H2B fused to GFP (H2B-GFP), linked to the PECAM enhancer/promoter (Tg PECAM-H2B-GFP). Upon differentiation, these cultures expressed H2B-GFP in the developing primitive vessels, as shown by overlay with PECAM antibody stain (Figure 2A). The cultures were imaged for short periods (3-6 hours) on day 7 or 8 of differentiation, when sprouting angiogenesis peaks, then fixed and stained for PECAM reactivity. Endothelial divisions were scored during anaphase, and the angle of cleavage relative to the vessel long axis was determined (Figure 2B). Analysis of the movies showed that the majority (56%) of the endothelial divisions were oriented within 10 degrees of perpendicular, and that 76% of divisions were within 20 degrees of perpendicular to the long axis of the vessel (Figure 2 B-D). This result shows that the endothelial cleavage plane is oriented relative to the long axis of the vessel, suggesting that oriented endothelial cell division participates in net lengthening of vessels in a developing vascular plexus.
Regulated endothelial cell division orientation may affect vessel morphogenesis: a model. This model shows how endothelial cell divisions whose orientation is regulated relative to the long axis of the vessel could affect vessel shape. Endothelial divisions oriented perpendicular to the vessel long axis (blue vessel on top) would effectively lengthen the vessel, whereas divisions oriented parallel to the vessel long axis (yellow vessel on bottom) would effectively increase the vessel diameter. Microtubules and spindles are shown in red, and DNA/chromosomes are brown.
Regulated endothelial cell division orientation may affect vessel morphogenesis: a model. This model shows how endothelial cell divisions whose orientation is regulated relative to the long axis of the vessel could affect vessel shape. Endothelial divisions oriented perpendicular to the vessel long axis (blue vessel on top) would effectively lengthen the vessel, whereas divisions oriented parallel to the vessel long axis (yellow vessel on bottom) would effectively increase the vessel diameter. Microtubules and spindles are shown in red, and DNA/chromosomes are brown.
Endothelial cell divisions are oriented perpendicular to the vessel long axis in ES cell–derived vessels. Mouse ES cells (WT; Tg PECAM-H2B-GFP) differentiated to day 7 to 8 were imaged for several hours prior to fixation and staining for PECAM-1. (A) A confocal image showing H2B-GFP signal in green (i), the same field with PECAM-1 stain in red after fixation (ii), and the overlay of the 2 images (iii), showing the H2B-GFP signal and PECAM-1 stain in the same cells. (B) The top portion of the images in panel A, showing time lapse images from 0 minute (i) to 292 minutes (vi). Panel iv was used to calculate the angle of division relative to the vessel axis, according to the drawn yellow lines. The numbers in the lower right represent elapsed time in minutes. (C) Calculation of division angles relative to the vessel long axis. Top panel was colorized as in Figure 1, except the chromosomes are blue. The 90-degree division angle is perpendicular to the vessel long axis, and the 0-degree division angle is parallel to the vessel long axis; n = 125 divisions. (D) Representation of endothelial division angles, with the vessel long axis diagrammed by the long horizontal lines. Each shorter line represents 3 angle measurements that were close or equivalent to each other. For a video of panel B, see Video S1 (available on the Blood website; see the Supplemental Videos link at the top of the online article).
Endothelial cell divisions are oriented perpendicular to the vessel long axis in ES cell–derived vessels. Mouse ES cells (WT; Tg PECAM-H2B-GFP) differentiated to day 7 to 8 were imaged for several hours prior to fixation and staining for PECAM-1. (A) A confocal image showing H2B-GFP signal in green (i), the same field with PECAM-1 stain in red after fixation (ii), and the overlay of the 2 images (iii), showing the H2B-GFP signal and PECAM-1 stain in the same cells. (B) The top portion of the images in panel A, showing time lapse images from 0 minute (i) to 292 minutes (vi). Panel iv was used to calculate the angle of division relative to the vessel axis, according to the drawn yellow lines. The numbers in the lower right represent elapsed time in minutes. (C) Calculation of division angles relative to the vessel long axis. Top panel was colorized as in Figure 1, except the chromosomes are blue. The 90-degree division angle is perpendicular to the vessel long axis, and the 0-degree division angle is parallel to the vessel long axis; n = 125 divisions. (D) Representation of endothelial division angles, with the vessel long axis diagrammed by the long horizontal lines. Each shorter line represents 3 angle measurements that were close or equivalent to each other. For a video of panel B, see Video S1 (available on the Blood website; see the Supplemental Videos link at the top of the online article).
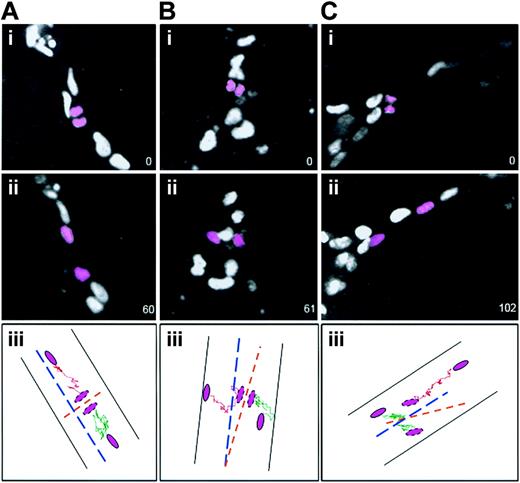
To determine whether the initial relationship of daughter cells following cleavage was maintained, the daughter nuclei produced by endothelial divisions were tracked for the duration of the movies (Figure 3). Divisions whose cleavage angle was 70 degrees to 90 degrees relative to the long axis of the vessel all had daughter nuclei that moved away from the cleavage plane along the long axis of the vessel (100%, 61of 61) (Figure 3A). Of the few divisions whose cleavage plane was 0 degree to 45 degrees relative to the vessel long axis, 70% (7 of 10) of daughter nuclei moved essentially perpendicular to the long axis of the vessel (Figure 3B), whereas 30% (3 of 10) reoriented and moved along the vessel long axis (Figure 3C). These results indicate that the orientation of endothelial cell divisions in vessels affects the subsequent spatial relationship of the daughter cells and thus can modulate vessel morphogenesis.
Daughter cells maintain their division orientation as they migrate after most divisions. ES cell–derived vessels (WT; Tg PECAM H2B-GFP) were imaged, and after divisions were scored (i panels) the 2 daughter nuclei (pink) were followed for 1 to 2 hours further. The numbers in the lower right represent elapsed time in minutes. In all cases ii panels show the final image scored. The iii panels diagram the movement of each daughter nucleus (shown in pink) after division: one daughter nucleus is tracked with a green line and the other with a pink line. The vessel is shown by black lines, the vessel long axis is shown by the broken blue line, and the division angle is shown by the broken orange line. (A) A division perpendicular to the vessel long axis, where the daughter cells maintained the division orientation after 1 hour (n = 61 of 61). (B) A division parallel to the vessel long axis, where the daughter cells maintained the division orientation after 1 hour (n = 7 of 10). (C) A division parallel to the vessel long axis, where the daughter cells changed position relative to the division orientation after 102 minutes (n = 3 of 10).
Daughter cells maintain their division orientation as they migrate after most divisions. ES cell–derived vessels (WT; Tg PECAM H2B-GFP) were imaged, and after divisions were scored (i panels) the 2 daughter nuclei (pink) were followed for 1 to 2 hours further. The numbers in the lower right represent elapsed time in minutes. In all cases ii panels show the final image scored. The iii panels diagram the movement of each daughter nucleus (shown in pink) after division: one daughter nucleus is tracked with a green line and the other with a pink line. The vessel is shown by black lines, the vessel long axis is shown by the broken blue line, and the division angle is shown by the broken orange line. (A) A division perpendicular to the vessel long axis, where the daughter cells maintained the division orientation after 1 hour (n = 61 of 61). (B) A division parallel to the vessel long axis, where the daughter cells maintained the division orientation after 1 hour (n = 7 of 10). (C) A division parallel to the vessel long axis, where the daughter cells changed position relative to the division orientation after 102 minutes (n = 3 of 10).
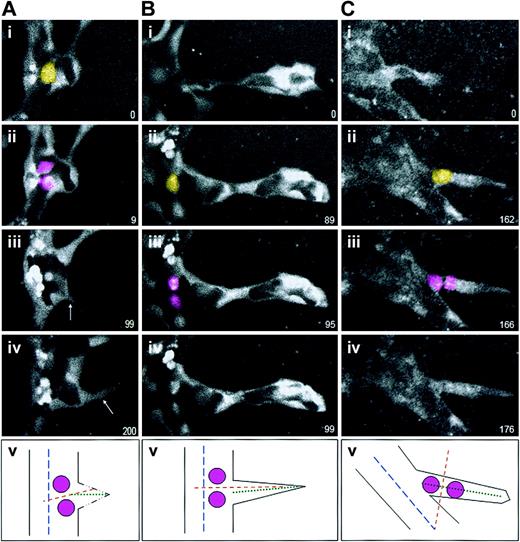
We next asked whether the orientation of endothelial cell division is altered in and near forming sprouts. To analyze sprout divisions, we used ES cell–derived vessels that contain a PECAM-eGFP transgene.12 This GFP reporter localized to the cytoplasm of endothelial cells, and it efficiently outlined the developing sprouts and endothelial cell divisions (Figure 4). We found that the orientation of endothelial division remained perpendicular to the vessel long axis when the division occurred in the parent vessel, whether prior to initiation of sprout formation (2 of 2; Figure 4A) or subsequent to the initiation of sprout formation (6 of 6; Figure 4B). However, divisions within the sprout oriented perpendicular to the long axis of the sprout (5 of 5; Figure 4C). Although the number of scored events was small, the uniform cleavage orientations suggest that endothelial divisions are normally oriented perpendicular to the current vessel axis, and not to a former or future vessel axis.
Endothelial cell divisions orient perpendicular to the nearest long axis. ES cell–derived vessels (WT; Tg PECAM-eGFP) were imaged for various lengths of time, and divisions in or around sprouts were scored for division orientation. In all series, the yellow cell is the scored endothelial cell just prior to division, the pink cells are the daughter cells, and the numbers in the lower right represent elapsed time in minutes. In all cases panel v diagrams the division that was scored, with the parent vessel long axis the broken blue line, the sprout long axis the dotted green line, and the division angle the broken orange line. (A) An endothelial division that occurred prior to nearby sprout formation. Although the sprout (arrows in panels iii and iv) migrated almost perpendicular to the vessel long axis, the division was oriented perpendicular to the parent vessel long axis (n = 2 of 2). (B) An endothelial division that occurred in the sprout field, at the base of a formed sprout but in the parent vessel. These divisions also oriented perpendicular to the parent vessel long axis (n = 6 of 6). (C) In contrast, an endothelial division that occurred within a formed sprout oriented perpendicular to the sprout vessel long axis (n = 5 of 5).
Endothelial cell divisions orient perpendicular to the nearest long axis. ES cell–derived vessels (WT; Tg PECAM-eGFP) were imaged for various lengths of time, and divisions in or around sprouts were scored for division orientation. In all series, the yellow cell is the scored endothelial cell just prior to division, the pink cells are the daughter cells, and the numbers in the lower right represent elapsed time in minutes. In all cases panel v diagrams the division that was scored, with the parent vessel long axis the broken blue line, the sprout long axis the dotted green line, and the division angle the broken orange line. (A) An endothelial division that occurred prior to nearby sprout formation. Although the sprout (arrows in panels iii and iv) migrated almost perpendicular to the vessel long axis, the division was oriented perpendicular to the parent vessel long axis (n = 2 of 2). (B) An endothelial division that occurred in the sprout field, at the base of a formed sprout but in the parent vessel. These divisions also oriented perpendicular to the parent vessel long axis (n = 6 of 6). (C) In contrast, an endothelial division that occurred within a formed sprout oriented perpendicular to the sprout vessel long axis (n = 5 of 5).
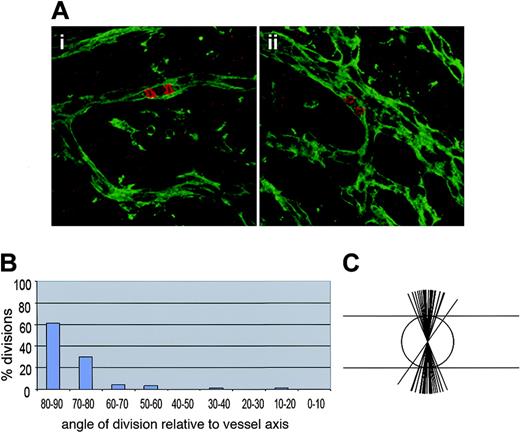
To test the hypothesis that endothelial cell division orientation is also oriented in vivo, we examined postnatal rat retinas. Vessel development occurs in a circumferential wave from the optic nerve in a single plane during the early postnatal stages, and the leading edges of this vascular plexus were associated with high levels of division in cat retinas.49 Retinas were double stained with the Griffonia B4 isolectin to visualize vessels, and antiphosphohistone H3, which reacts with a histone epitope present on mitotic cells.52 This allows for visualization of condensed chromosomes during metaphase and anaphase, and by inference the division cleavage plane (Figure 5). Analysis of mitotic endothelial cells in which the cleavage plane could be scored (Figure 5A) showed that 62% of the divisions were within 10 degrees of perpendicular to the vessel long axis, and a total of 92% were within 20 degrees of perpendicular (Figure 5B-C). These results indicate that the orientation of endothelial cell divisions perpendicular to the vessel long axis is recapitulated in vivo, in a developing vascular bed exposed to blood flow.
Endothelial cell divisions are oriented perpendicular to the vessel long axis in retinal vessels in vivo. Rat retinas were harvested on days P3 to P5 and processed for staining as described with Griffonia B4 isolectin (green) to visualize vessels and phosphohistone H3 (red) to visualize DNA in mitotic cells. (A) Several examples of divisions in retinal vessels that were scored for division angle. (B) Calculation of division angles relative to vessel long axis. Ninety degrees is perpendicular to the vessel long axis, and 0 degree is parallel to the vessel long axis; n = 86 divisions. (C) Representation of endothelial division angles, with the vessel long axis diagrammed by the long horizontal lines. Each shorter line represents 3 angle measurements that were close or equivalent to each other.
Endothelial cell divisions are oriented perpendicular to the vessel long axis in retinal vessels in vivo. Rat retinas were harvested on days P3 to P5 and processed for staining as described with Griffonia B4 isolectin (green) to visualize vessels and phosphohistone H3 (red) to visualize DNA in mitotic cells. (A) Several examples of divisions in retinal vessels that were scored for division angle. (B) Calculation of division angles relative to vessel long axis. Ninety degrees is perpendicular to the vessel long axis, and 0 degree is parallel to the vessel long axis; n = 86 divisions. (C) Representation of endothelial division angles, with the vessel long axis diagrammed by the long horizontal lines. Each shorter line represents 3 angle measurements that were close or equivalent to each other.
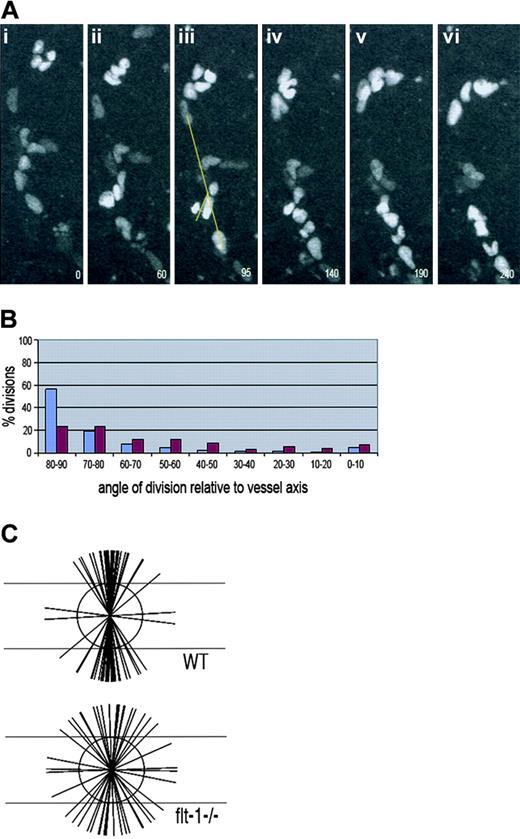
Growth factor signaling is a key mediator of morphogenesis, and the HGF/scatter factor signaling pathway affects the division orientation of MDCK cells undergoing chain extension in 3-dimensional cultures.36 Thus, we reasoned that VEGF signaling might be involved in the regulation of endothelial cell division orientation in developing vessels. Proper regulation of signaling through VEGF-A is crucial to proper vessel morphogenesis, and excessive signaling through the VEGF-A receptor flk-1 leads to vessel dysmorphogenesis. We analyzed the plane of endothelial cell division in ES cell–derived vessels mutant for flt-1, which is a gain-of-function for VEGF signaling through flk-1.53 These vessels also expressed PECAM-H2B-GFP to allow for visualization of cleavage orientation (Figure 6). Although the vessel dysmorphogenesis associated with the mutation produces endothelial sheets with time, the edges of the sheets have distinguishable vessels whose long axis can be scored12 (Figure 6A). This analysis showed that the orientation of endothelial divisions in flt-1−/− vessels was randomized relative to WT vessels (Figure 6B-C). Only 24% of divisions were within 10 degrees of perpendicular, as opposed to 56% of WT divisions. Moreover, tracking of nuclei showed that 50% (8 of 16) of parallel divisions resulted in daughter nuclei that maintained the relationship they had at the time of division, indicating that these divisions can increase vessel diameter over length (data not shown). These data show that perturbation of VEGF signaling leads to increased randomization of endothelial cell division orientation in developing vessels.
Endothelial cell divisions are randomly oriented flt-1−/− ES cell–derived vessels. Mouse ES cells (flt-1−/−; Tg PECAM-H2B-GFP) differentiated to day 7 to 8 were imaged for several hours prior to fixation and staining for PECAM-1. (A) Time lapse images of a representative movie from 0 minute (i) to 240 minutes (vi), showing the H2B signal in vessels. Panel iii was used to calculate the angle of division relative to the vessel axis, according to the drawn yellow lines. The numbers in the lower right represent elapsed time in minutes. (B) Calculation of division angles relative to the vessel long axis. WT endothelial division angles are shown in blue, and flt-1−/− endothelial division angles are shown in purple. Ninety degrees is perpendicular to the vessel long axis, and 0 degree is parallel to the vessel long axis; n = 125 divisions for WT vessels (same data as shown in Figure 2) and n = 93 divisions for flt-1−/− vessels. (C) Representation of endothelial division angles, with the vessel long axis diagrammed by the long horizontal lines. Each shorter line represents 3 angle measurements that were close or equivalent to each other. For the video of panel A, see Video S2.
Endothelial cell divisions are randomly oriented flt-1−/− ES cell–derived vessels. Mouse ES cells (flt-1−/−; Tg PECAM-H2B-GFP) differentiated to day 7 to 8 were imaged for several hours prior to fixation and staining for PECAM-1. (A) Time lapse images of a representative movie from 0 minute (i) to 240 minutes (vi), showing the H2B signal in vessels. Panel iii was used to calculate the angle of division relative to the vessel axis, according to the drawn yellow lines. The numbers in the lower right represent elapsed time in minutes. (B) Calculation of division angles relative to the vessel long axis. WT endothelial division angles are shown in blue, and flt-1−/− endothelial division angles are shown in purple. Ninety degrees is perpendicular to the vessel long axis, and 0 degree is parallel to the vessel long axis; n = 125 divisions for WT vessels (same data as shown in Figure 2) and n = 93 divisions for flt-1−/− vessels. (C) Representation of endothelial division angles, with the vessel long axis diagrammed by the long horizontal lines. Each shorter line represents 3 angle measurements that were close or equivalent to each other. For the video of panel A, see Video S2.
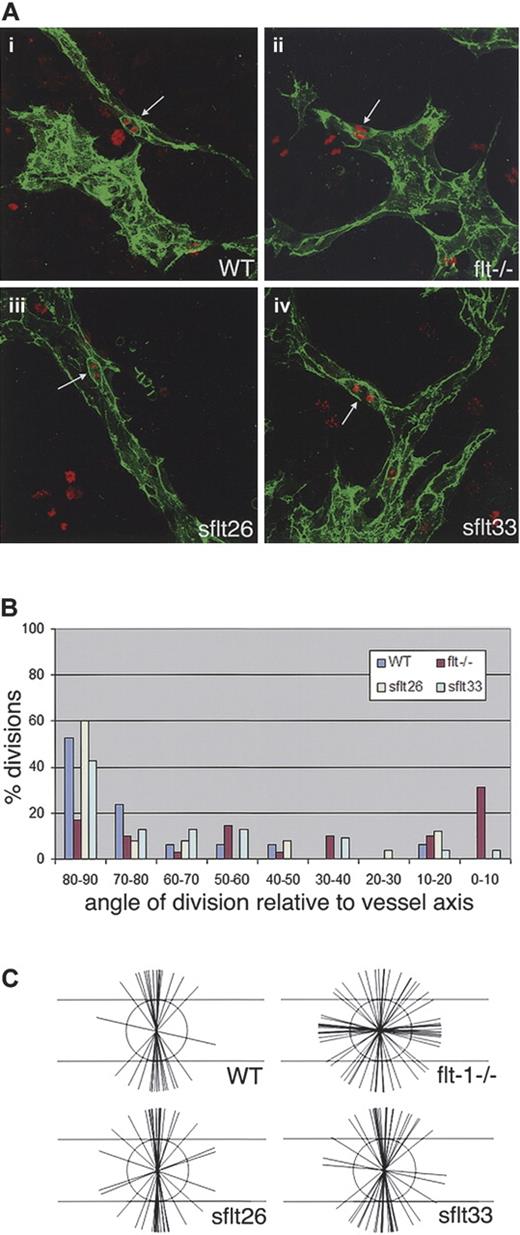
To determine whether the flt-1 mutation was responsible for the orientation phenotype, we asked whether the randomization of endothelial cell division orientation was rescued by a genetic rescue of the flt-1 mutation. We previously showed that addition of a PECAM-sflt-1 transgene, that expresses the soluble isoform of flt-1 in developing vessels, rescued the reduction in branching morphogenesis seen in flt-1−/− mutant vessels.12 We thus examined the orientation of endothelial cell division in 2 of the sflt-1 rescue lines, by double staining fixed cultures with anti–PECAM-1 to visualize vessels and antiphosphohistone H3 to visualize condensed chromosomes for cleavage plane angle calculations (Figure 7). These data show that both flt-1−/−;Tg PECAM-sflt-1 lines rescued the randomized orientation of endothelial cell division seen in flt-1−/− mutant vessels (Figure 7A-C). In the flt-1−/−;Tg PECAM-sflt-1#33 and the flt-1−/−;Tg PECAM-sflt-1#26 vessels, 43% and 60% of endothelial cell divisions were within 10 degrees of perpendicular relative to the vessel long axis (Figure 7B). This compared with WT vessel values of 53% and flt-1−/− mutant vessel values of 17%. Thus, a genetic manipulation to rescue the dysmorphogenesis of flt-1−/− mutant vessels rescues the orientation of endothelial cell division almost to WT levels.
Randomized endothelial division orientation is rescued by a sflt-1 transgene that rescues flt-1−/− vessel dysmorphogenesis. ES cell–derived vessels were wild-type (WT), flt-1−/−, or flt-1−/− with a sflt-1 transgene (flt-1−/−;Tg PECAM sflt-1#26, indicated as sflt26; or flt-1−/−;Tg PECAM sflt-1#33, as sflt33). Cultures were differentiated to day 8, fixed, and stained for PECAM-1 (green) and phosphohistone H3 (red). (A) Representative vessels of the indicated genotypes, with white arrows pointing to endothelial divisions that were scored. (B) Graphic representation of division angles from the different genetic backgrounds. Ninety degrees is perpendicular to the vessel long axis, and 0 degree is parallel to the vessel long axis. WT is blue (n = 19), flt-1−/− is purple (n = 29), sflt26 is yellow (n = 25), and sflt33 is light green (n = 23). (C) Representation of endothelial division angles, with the vessel long axis diagrammed by the long horizontal lines. Each shorter line represents a single angle measurement.
Randomized endothelial division orientation is rescued by a sflt-1 transgene that rescues flt-1−/− vessel dysmorphogenesis. ES cell–derived vessels were wild-type (WT), flt-1−/−, or flt-1−/− with a sflt-1 transgene (flt-1−/−;Tg PECAM sflt-1#26, indicated as sflt26; or flt-1−/−;Tg PECAM sflt-1#33, as sflt33). Cultures were differentiated to day 8, fixed, and stained for PECAM-1 (green) and phosphohistone H3 (red). (A) Representative vessels of the indicated genotypes, with white arrows pointing to endothelial divisions that were scored. (B) Graphic representation of division angles from the different genetic backgrounds. Ninety degrees is perpendicular to the vessel long axis, and 0 degree is parallel to the vessel long axis. WT is blue (n = 19), flt-1−/− is purple (n = 29), sflt26 is yellow (n = 25), and sflt33 is light green (n = 23). (C) Representation of endothelial division angles, with the vessel long axis diagrammed by the long horizontal lines. Each shorter line represents a single angle measurement.
Discussion
This study shows, for the first time, that endothelial cell division is normally oriented in developing vascular beds so that the cleavage plane is usually perpendicular to the long axis of the vessel. This finding, along with evidence that daughter nuclei maintain the spatial relationships set up by the cleavage, indicates that oriented cell division is a novel mechanism contributing to vessel morphogenesis. The oriented divisions can effectively extend vessel length. Because developing vascular beds normally expand rapidly by the formation of many thin vessels, the bias of endothelial cell division orientation toward vessel lengthening is consistent with the overall morphogenetic program.
Our finding that endothelial cell divisions orient perpendicular to the vessel long axis in both ES cell–derived vessels and in the postnatal retina indicates that regulated endothelial division orientation is a common attribute of developing vascular beds. Moreover, the tight linkage between endothelial division orientation and the long axis of ES cell–derived vessels is striking, because there is no blood flow in this model. Numerous studies have linked aspects of endothelial cell polarity to the direction of shear stress produced by flow, including the orientation of the actin cytoskeleton, the microtubule network, and the position of the microtubule organizing center.54-56 Recent work shows that endothelial cells can transduce mechanical shear stress to cell polarity pathways through a sensor that is composed of PECAM-1, VE-cadherin, and VEGFR2.57 Moreover, BrdU-labeled daughter cells are positioned to suggest division with cleavage perpendicular to the flow vector in rabbit carotid arteries, although cleavage orientation was not directly measured.58
Our data show that endothelial cell division orientation is regulated in a flow-independent manner, at least during the beginning stages of vessel extension. Developing vessels and sprouts do not form lumens capable of sustaining blood flow until later in the angiogenic process, and even then the shear stress values are significantly lower than those found in adult arteries,59 so a flow-independent mechanism to regulate endothelial division orientation might be predicted. Additionally, most vessels never experience the levels of shear stress found in major arteries, and the microcirculation has a low flow velocity.60,61 Thus, it seems plausible that a major component of endothelial cell division orientation operates independent of blood flow in vessels other than major arteries. We found that retinal vessels, which have blood flow but not the shear stress of major vessels, had 92% of endothelial divisions within 20 degrees of perpendicular, and ES cell–derived vessels with no flow had 76% of endothelial divisions in the same category. These data are consistent with a model in which flow-independent regulation of endothelial division orientation contributes substantially to vascular pattern formation.
Analysis of the divisions that occur in or near a sprout, the “sprout field,” shows that endothelial cells orient their cleavage plane perpendicular to the long axis of the structure in which they reside. We did not find divisions within a parent vessel that oriented parallel to the long axis, as was seen in MDCK cells exposed to HGF and in mouse skin epithelium.29,36 Moreover, once a sprout formed, we did not score divisions within the parent vessel that oriented with the sprout axis. However, once an endothelial cell was in a sprout, its division oriented to the sprout axis. Thus, it appears that endothelial cells, unlike some other epithelial cells, do not use division orientation to initiate new morphogenetic structures, but rather to reinforce new sprouts once they have initiated.
How is endothelial division orientation regulated? Growth factor signaling is critical to this regulation in other models, and recently Wnt signaling was shown to be a positional cue for spindle orientation in early C elegans embryos.62 Thus, we asked whether VEGF signaling regulated endothelial division orientation in developing vessels, by examining vessels deleted for flt-1, which acts as a gain-of-function mutation in VEGF signaling.9,11,53 Our analysis of flt-1−/− mutant vessels revealed that endothelial division orientation was randomized relative to the vessel long axis, consistent with a role for VEGF signaling in this process. Moreover, introduction of a sflt-1 transgene that rescues branching morphogenesis in the flt-1 mutant background also rescued the increased randomization of endothelial division orientation. These data strongly support a role for VEGF signaling in the regulation of endothelial orientation in vessels. Modulation of VEGF signaling regulates the rate of endothelial cell division,11,13 and our data provide the first evidence showing that VEGF signaling also regulates the orientation of endothelial cell division. It is well established that VEGF affects vessel morphogenesis by regulating migration associated with sprout formation,4,12,17 and our data indicate that VEGF also affects the morphogenetic program by its ability to regulate endothelial cell division orientation.
Our finding that endothelial division orientation is regulated indicates that integration between the endothelial cell division and morphogenesis programs occurs in developing vessels, and it suggests that morphogenetic signals regulate cell division. Although the VEGF signal itself is one point of integration, it is likely that morphogenesis and cell division are coordinated by cross talk at multiple places in the downstream pathways. In other organisms and tissues, division is oriented by the placement of the astral microtubules that emanate from the spindle poles on the cortex. This placement is regulated by polarity determinants, and the polarity determinants in turn are spatially organized by the actin cytoskeleton. Thus, the VEGF signaling pathway is likely to intersect with one or more polarity pathways. It is possible that VEGF signaling leads to differences in gene expression that affect polarity, but this is considered unlikely because most polarity information is imparted by spatial organization within the cell. One likely intersection point is the actin cytoskeleton itself, because VEGF regulates actin dynamics.14,15 Thus, the ability of VEGF to locally influence polymerization/depolymerization of the actin filaments could lead to spatial organization of polarity cues at the cortex. The VEGF signaling pathway is also likely to intersect a planar cell polarity pathway, which orients cells in the plane of an epithelial sheet, because planar axis orientation must be regulated to obtain the cleavage angles that we scored. Moreover, one such pathway involving noncanonical Wnt signaling is implicated in the regulation of division orientation during zebra fish gastrulation.42 This intersection might occur at the level of the small GTPase Rho, because Rho is downstream of both pathways.63,64 Interestingly, preliminary data show that endothelial division orientation is randomized by treatment with a Rho kinase inhibitor (S.M.T. and V.L.B., unpublished results, June 2006), suggesting that VEGF may act through Rho to regulate endothelial cell division orientation.
We have shown that endothelial cell division orientation is regulated early in vascular development in a flow-independent manner, and that this regulation can affect vessel morphogenesis. This indicates that endothelial morphogenesis and cell division are integrated in developing vessels. Moreover, disruption of the integration by perturbed VEGF signaling correlates with vessel dysmorphogenesis, suggesting that cross talk between morphogenesis and division orientation is critical to proper vessel morphogenesis. Thus, endothelial cell division orientation represents another cellular process that is disrupted in dysmorphogenic vessels and could therefore be a therapeutic target.
Authorship
Contribution: G.Z. and S.M.T. designed and performed the research and collected and analyzed the data; J.R.M., N.C.K., J.B.K., and M.E.H. contributed vital reagents and tools; J.R.M. and L.H.W. performed the research; V.L.B. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Victoria L. Bautch, Department of Biology, CB#3280, University of North Carolina, Chapel Hill, NC 27599; e-mail: bautch@med.unc.edu.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Mark Peifer and members of the Bautch laboratory for discussion and Bob Goldstein, Mark Majesky, Dave Roberts, and Eleni Tzima for comments on the manuscript and discussion. We thank Guo-Hua Fong for the flt-1−/− ES cells and H. Scott Baldwin for the PECAM promoter/enhancer. We thank Rebecca Rapoport, Pete Geisen, and William Smith for technical assistance and Susan Whitfield for artwork. Confocal fluorescence microscopy was performed at UNC-CH in the Department of Biology Microscopy Facility and in the Michael Hooker Microscopy Facility.
This work was supported by the National Institutes of Health (R01HL43174) (V.L.B.), (R21 HL71993) (V.L.B.), (R01EY015130) (M.E.H.), and American Heart Association Mid-Atlantic Affiliate Predoctoral Fellowship (315331U) (N.C.K.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal