Abstract
The combination of imatinib with chemotherapy has been recently reported as very promising in patients with Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). During 2004 and 2005, 45 patients with newly diagnosed Ph+ ALL were treated in the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAAPH) 2003 study, in which imatinib was started with HAM (mitoxantrone with intermediate-dose cytarabine) consolidation in good early responders (corticosensitive and chemosensitive ALL) or earlier during the induction course in combination with dexamethasone and vincristine in poor early responders (corticoresistant and/or chemoresistant ALL). Imatinib was then continuously administered until stem cell transplantation (SCT). Overall, complete remission (CR) and BCR-ABL real-time quantitative polymerase chain reaction (RQ-PCR) negativity rates were 96% and 29%, respectively. All of the 22 CR patients (100%) with a donor actually received allogeneic SCT in first CR. At 18 months, the estimated cumulative incidence of relapse, disease-free survival, and overall survival were 30%, 51%, and 65%, respectively. These 3 end points compared very favorably with results obtained in the pre-imatinib LALA-94 trial. This study confirms the value of the combined approach and encourages prospective trials to define the optimal chemotherapy that has to be combined with imatinib and to carefully reevaluate the place of allogeneic SCT in this new context.
Introduction
Before imatinib, the prognosis of adult patients with Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL) treated with chemotherapy only was poor, with an expected long-term survival of less than 20%.1-5 Even if complete remission (CR) rates after 1 or 2 courses of chemotherapy were often close to the rates achieved in Ph− ALL patients, most, if not all, patients who were only treated with chemotherapy relapsed, with very few long-term survivors. In this context, allogeneic stem cell transplantation (SCT) was and is still considered as the treatment of choice in adults with this disease, even if their higher median age leads to a significant transplantation-related mortality (TRM) and if post-SCT relapses are relatively frequent.
Our previous LALA-94 trial from the Leucémie Aiguë Lymphoblastique de l'Adulte (LALA) group was conducted during this pre-imatinib period.5,6 In patients with Ph+ ALL, we reported the value of a second course of HAM consolidation/salvage. This HAM (mitoxantrone with intermediate-dose cytarabine) course allowed us to increase the hematologic response rate from 53% to 71%. All patients reaching hematologic CR after 2 courses (standard induction followed by HAM) were allocated to receive allogeneic SCT if a matched donor was identified or autologous SCT if not. The most important result was the good prognostic value of molecular remission achievement after these 2 courses independently of the presence of a donor. Patients combining both bad-prognostic factors (no molecular remission and no donor) did very poorly, leading us to consider molecular response as a good surrogate end point in this disease and to not recommend autologous SCT in patients with persistent minimal residual disease (MRD).
Despite poor results of single-agent imatinib in patients with advanced Ph+ ALL,7 the combination of imatinib with chemotherapeutic agents appears to be associated with a very good response rate and better outcome. Preliminary results of frontline combinations with standard intensive induction and consolidation chemotherapy in relatively limited series of patients with Ph+ ALL are very promising.8-13 CR rates are about 95% and, more importantly, short-term survival compares favorably with previous historic controls. Imatinib combined with less intensive chemotherapy also provides encouraging results, as we have recently reported using a combination with vincristine and dexamethasone (DIV regimen) in relapsed/refractory patients.14
During this first imatinib period, the strategy followed by the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL) in its GRAAPH-2003 study was slightly different, because imatinib was not systematically added from day 1 of the first induction course. In early responders with corticosensitive and chemosensitive leukemia, imatinib was introduced later in combination with the HAM consolidation according to the HAMI (HAM with imatinib) regimen.15 In patients with corticoresistant and/or chemoresistant leukemia, imatinib was introduced earlier between day 8 and day 15 of the induction course according to the DIV regimen.14 We report here the results of this GRAAPH-2003 strategy in 45 younger patients with newly diagnosed Ph+ ALL treated between January 2004 and October 2005.
Patients and methods
Eligibility
All patients aged 15 to 59 years with newly diagnosed ALL (ALL-L3 excluded) included in the GRAALL-2003 phase 2 trial were eligible for the GRAAPH-2003 study if they were diagnosed with a Ph+ ALL. Ph+ ALL was defined as ALL carrying the t(9;22) translocation on standard karyotype and/or fluorescent in situ hybridization (FISH) analysis and/or positivity for BCR-ABL fusion transcript detection by real-time quantitative polymerase chain reaction (RQ-PCR) analysis. Patients with antecedent myeloproliferative disorders including chronic myeloid leukemia (CML) were not included. All patients gave their written informed consent. The study was approved in March 2003 from the institutional review board (IRB) of Hôpital Purpan, Toulouse, France, and conducted in accordance of the Declaration of Helsinki.
Treatments
Treatments are summarized in Table 1 Induction therapy was stratified after the first 2 weeks of treatment according to Ph+ diagnosis and early response (corticosensitivity and chemosensitivity). The first 2 weeks (prephase and first induction week) were thus similar in all patients whether they had Ph+ or Ph− ALL and were good or poor early responders.
Induction and consolidation therapy
| . | Dose . | Time, d . |
|---|---|---|
| Prephase | ||
| Prednisone | 60 mg/m2/d PO | Between −7 and −1 |
| Methotrexate | 15 mg IT | Between −7 and −4 |
| Standard induction, wks 1-2 | ||
| Daunorubicin | 50 mg/m2/d IV | 1 to 3 |
| Cyclophosphamide | 750 mg/m2/d IV | 1 |
| Vincristine | 2 mg IV | 1, 8 |
| Prednisone | 60 mg/m2/d PO | 1 to 14 |
| L-asparaginase | 6000 IU/m2/d IV | 8, 10, 12 |
| Triple IT | —‡ | 1, 8 |
| Standard induction, wks 3-4 | ||
| Daunorubicin | 30 mg/m2/d IV | 15 to 16 |
| Cyclophosphamide | 750 mg/m2/d IV | 15 |
| Vincristine | 2 mg IV | 15, 22 |
| L-asparaginase | 6000 IU/m2/d IV | 20, 22, 24, 26, 28 |
| G-CSF lenograstim | 150 μg/m2/d SC or IV | From 17 |
| DIV combination* | ||
| Vincristine | 2 mg IV | 1, 8, 15, 22 |
| Dexamethasone | 40 mg PO or IV | 1-2, 8-9, 15-16, 22-23 |
| Imatinib | 800 mg/d PO | 1 until SCT |
| Triple IT | —‡ | 1, 8, 15, 22 |
| HAMI combination† | ||
| Mitoxantrone | 10 mg/m2/d, IV | 1 to 3 |
| Cytarabine | 2000 mg/m2/12h, IV | 1 to 4 |
| Imatinib | 600 mg/d PO | 1 until SCT |
| Triple IT | —‡ | 8, 15 |
| G-CSF filgrastim | 5 μg/kg/d SC or IV | From 9 |
| . | Dose . | Time, d . |
|---|---|---|
| Prephase | ||
| Prednisone | 60 mg/m2/d PO | Between −7 and −1 |
| Methotrexate | 15 mg IT | Between −7 and −4 |
| Standard induction, wks 1-2 | ||
| Daunorubicin | 50 mg/m2/d IV | 1 to 3 |
| Cyclophosphamide | 750 mg/m2/d IV | 1 |
| Vincristine | 2 mg IV | 1, 8 |
| Prednisone | 60 mg/m2/d PO | 1 to 14 |
| L-asparaginase | 6000 IU/m2/d IV | 8, 10, 12 |
| Triple IT | —‡ | 1, 8 |
| Standard induction, wks 3-4 | ||
| Daunorubicin | 30 mg/m2/d IV | 15 to 16 |
| Cyclophosphamide | 750 mg/m2/d IV | 15 |
| Vincristine | 2 mg IV | 15, 22 |
| L-asparaginase | 6000 IU/m2/d IV | 20, 22, 24, 26, 28 |
| G-CSF lenograstim | 150 μg/m2/d SC or IV | From 17 |
| DIV combination* | ||
| Vincristine | 2 mg IV | 1, 8, 15, 22 |
| Dexamethasone | 40 mg PO or IV | 1-2, 8-9, 15-16, 22-23 |
| Imatinib | 800 mg/d PO | 1 until SCT |
| Triple IT | —‡ | 1, 8, 15, 22 |
| HAMI combination† | ||
| Mitoxantrone | 10 mg/m2/d, IV | 1 to 3 |
| Cytarabine | 2000 mg/m2/12h, IV | 1 to 4 |
| Imatinib | 600 mg/d PO | 1 until SCT |
| Triple IT | —‡ | 8, 15 |
| G-CSF filgrastim | 5 μg/kg/d SC or IV | From 9 |
PO indicates per os (orally); IT, intrathecally; IV, intravenously; SC, subcutaneously.
Administered at day 15 of the standard induction course in patients with corticoresistant and/or chemoresistant ALL.
Administered after hematologic CR achievement as consolidation in patients with corticosensitive and chemosensitive ALL.
Consisted of 15 mg methotrexate, 40 mg cytarabine, and 40 mg dexamethasone, all administered intrathecally.
At that time, all patients with Ph+ ALL entered the GRAAPH-2003 study. Good early responders with corticosensitive and chemosensitive ALL continued with standard induction, which did not include imatinib. Those achieving hematologic CR then received imatinib combined with HAM consolidation (HAMI regimen). Imatinib was given from day 1 of the consolidation until SCT at the daily dosage of 600 mg for a planned period of 90 days (Table 1). Poor early responders with corticoresistant and/or chemoresistant ALL did not continue with standard induction. They switched between day 8 and day 15 of the induction course to receive imatinib at the daily dosage of 800 mg in combination with vincristine and dexamethasone according to the DIV regimen14 (Table 1). Imatinib was then administered daily until SCT at the same 800 mg/d dosage for a planned period of 90 days.
Stem cell transplantation
In the absence of acquired contraindication, all CR patients aged 55 years or less with an identified donor—either matched familial donor (MFD) or matched unrelated donor (MUD) matched at 9 or 10 of 10 HLA antigens—were eligible for allogeneic SCT after HAMI or DIV whatever their molecular response. Patients without a donor as well as patients aged more than 55 years were eligible for autologous SCT but only if they had reached a low PCR level. Autologous graft consisted of peripheral blood stem cells (PBSCs) collected at steady state after granulocyte colony-stimulating factor (G-CSF) mobilization and under continuous imatinib administration. PBSC harvesting was performed after assessment of response to HAMI or DIV combination. Patients without a donor who failed to achieve a low PCR level after HAMI or DIV did not receive transplants and generally were treated with combinations of imatinib and various chemotherapeutic agents. Results are given according to this intent-to-treat SCT strategy.
For allogeneic as well as autologous SCT, the conditioning regimen was standard high-dose cyclophosphamide and total body irradiation. For allogeneic SCT, graft versus host disease (GVHD) prophylaxis consisted of a standard methotrexate and cyclosporine A combination. No maintenance therapy was planned after SCT, but some patients received postautologous SCT maintenance with imatinib, as detailed in “Results.”
Safety and response evaluation
All adverse events were prospectively collected and graded according to the World Health Organization (WHO) classification. During HAMI and DIV administration, imatinib therapy was interrupted only in case of severe (grade 3 or 4) noninfectious nonhematologic toxicity. For hepatic toxicity, only serum bilirubin and alanine aminotransferase (ALAT) levels were taken into account.
Early response criteria included corticosensitivity after a pediatric-like prephase and chemosentitivity after the first week of induction chemotherapy. Corticosensitivity was assessed by PB examination after the 1-week prephase and defined as less than 1.0 × 109/L circulating blasts, while chemosensitivity was assessed by PB and marrow examination at day 8 of chemotherapy and defined by the absence of circulating blasts and less than 5% marrow blasts. In patients who received standard induction, marrow response was evaluated at CR and then at day 45 of HAMI consolidation under continuous imatinib. In those who received the DIV combination, post-DIV marrow response was evaluated between day 35 and day 49 of this regimen under continuous imatinib. Hematologic CR was defined as a normal marrow cytology (less than 5% blasts and more than 25% cellularity), neutrophil counts higher than 1.5 × 109/L, platelet counts higher than 100 × 109/L, and no extramedullary disease. Marrow minimal BCR-ABL residual disease was monitored using RQ-PCR. PCR negativity was defined as no BCR-ABL transcript detection with a minimum level of sensitivity of 10−5. Low PCR level was defined as a BCR-ABL/ABL ratio between 10−5 and 10−4. Molecular monitoring was performed in predefined centralized reference laboratories using a common RQ-PCR methodology according to the Europe Against Cancer program.16
Statistical methods
Binary variables were compared with the Fisher exact test. For continuous variables, the t test was used for mean comparisons and the Mann-Whitney test for median comparisons. Multivariate analyses for response were tested using the maximum-likelihood model. Disease-free survival (DFS) was calculated as survival without relapse or death from the date of first CR. Outcome was updated at the point date of December 31, 2005. The actuarial median follow-up was 11 months. Failure time data but not cumulative incidence of relapse were estimated by the Kaplan-Meier method and then compared by the log-rank test. By contrast, in estimating cumulative incidence of relapse, we took into account deaths in first CR as competing risk using the cumulative incidence curves and then compared by the Gray test while the Fine and Gray model was used to estimate specific hazard ratio (HR). A P value of less than .05 was considered to indicate statistical significance. All calculations were performed using the STATA software, version 7.0E, (Stata, College Station, TX) and the R software, version 1.5.1 (The R Development Core Team, 2002).
Results
Patients
Between January 2004 and October 2005, 45 patients (25 males and 20 females) entered the GRAAPH-2003 study. Their median age was 45 years (range, 16-59 years). Six patients were aged more than 55 years. Median white blood cell count (WBC) was 11.1 × 109/L (range, 1.1-159 × 109/L), and median PB blast count was 5.7 × 109/L (range, 0-133 × 109/L). Four patients had central nervous system (CNS) involvement at diagnosis. They received additional triple intrathecal infusions as well as pre-SCT cranial irradiation. Twenty-two percent of these patients had a major bcr fusion transcript (p210 ALL subtype). Seven patients had a Ph chromosome in the context of a complex karyotype. One patient had ALL with a double Philadelphia chromosome.
Hematologic and MRD response
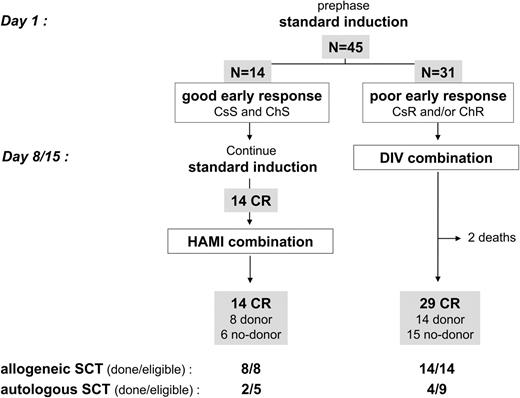
The overall CR rate was 96% (43 of 45 patients). Fourteen patients (31%) were good early responders. They all achieved hematologic CR after standard induction, then received the planned HAMI consolidation, and were still alive in CR at post-HAMI evaluation time (Figure 1; Table 2) Thirty-one patients (69%) were poor early responders (6 corticoresistant, 17 chemoresistant, and 8 corticoresistant and chemoresistant ALL). They all received the planned DIV combination. Two of them died early, while the 29 remaining achieved hematologic CR at post-DIV evaluation time (Figure 1; Table 2). There was no significant difference in age or BCR-ABL bcr subtype between good and poor early responders, but the WBC was significantly lower in the good-responder group (median WBC, 7.5 × 10,9/L versus 17.5 × 10,9/L; P = .05 by the Mann-Whitney test).
Response to induction and consolidation therapy
| . | Good early responders . | Poor early responders . | All patients . |
|---|---|---|---|
| No. patients | 14 | 31 | 45 |
| Early response, no. | |||
| CsS and ChS | 14 | 0 | 14 |
| CsR and ChS | 0 | 6 | 6 |
| CsS and ChR | 0 | 17 | 17 |
| CsR and ChR | 0 | 8 | 8 |
| After standard induction, no. (%) | |||
| Hematologic CR | 14 (100) | NA | NA |
| PCR negativity* | 4 (29) | NA | NA |
| Overall response to combined imatinib/chemotherapy, no. (%)† | |||
| Hematologic CR | 14 (100) | 29 (94) | 43 (96) |
| Low PCR level* | 10 (71) | 18 (58) | 28 (62) |
| PCR negativity* | 9 (64) | 8 (26) | 17 (38) |
| . | Good early responders . | Poor early responders . | All patients . |
|---|---|---|---|
| No. patients | 14 | 31 | 45 |
| Early response, no. | |||
| CsS and ChS | 14 | 0 | 14 |
| CsR and ChS | 0 | 6 | 6 |
| CsS and ChR | 0 | 17 | 17 |
| CsR and ChR | 0 | 8 | 8 |
| After standard induction, no. (%) | |||
| Hematologic CR | 14 (100) | NA | NA |
| PCR negativity* | 4 (29) | NA | NA |
| Overall response to combined imatinib/chemotherapy, no. (%)† | |||
| Hematologic CR | 14 (100) | 29 (94) | 43 (96) |
| Low PCR level* | 10 (71) | 18 (58) | 28 (62) |
| PCR negativity* | 9 (64) | 8 (26) | 17 (38) |
CsS indicates corticosensitive; ChS, chemosensitive; CsR, corticoresistant; ChR, chemoresistant; NA, not applicable.
As defined in “Patients and methods.”
After HAMI consolidation in good early responders and after DIV combination in poor early responders.
At marrow evaluation time, the median duration of imatinib administration was 45 days (range, 35-52 days) and 35 days (range, 24-53 days) for patients receiving the HAMI and DIV combination, respectively (P = .003 by the Mann-Whitney test). However, given the different daily dosage between both patient subgroups, the median total dose of imatinib received was similar (27 000 mg versus 28 000 mg for HAMI and DIV patients, respectively; P = .21 by the Mann-Whitney test).
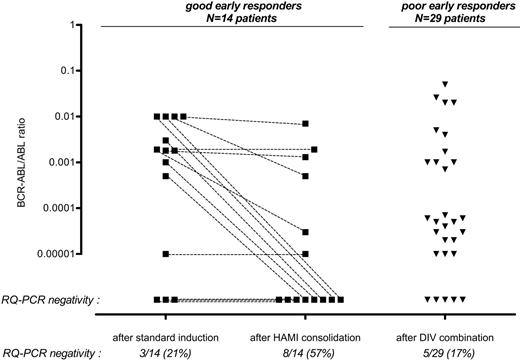
Marrow MRD evolution is shown in Figure 2 and Table 2. In good early responders, the rate of PCR negativity was higher after HAMI than after the first standard induction course that did not include imatinib (8 of 14 and 3 of 14, respectively; P = .06 by the 1-sided Fisher exact test). The rate of PCR negativity reached after DIV in poor early responders was significantly lower than after HAMI in good early responders (5 of 29 versus 8 of 14; P = .01 by the 2-sided Fisher exact test). This was not surprising, because patients who received DIV had hematologic resistance at the time of DIV initiation and received a total dose of imatinib equivalent to that in patients treated with HAMI who were in hematologic CR at the time of HAMI initiation. After adjustment on these 2 subgroups, no other covariate, including age, WBC, peripheral blast count, complex karyotype, and bcr subtype, was found as predictive of PCR negativity achievement.
Marrow MRD evolution according to early response. Individual RQ-PCR MRD monitoring is shown for good (corticosensitive and chemosensitive) and poor (corticoresistant and/or chemoresistant) early responders, respectively. Not surprisingly, the rate of PCR negativity was higher after HAMI in good early responders than after DIV in poor early responders (57% versus 17%; P = .01).
Marrow MRD evolution according to early response. Individual RQ-PCR MRD monitoring is shown for good (corticosensitive and chemosensitive) and poor (corticoresistant and/or chemoresistant) early responders, respectively. Not surprisingly, the rate of PCR negativity was higher after HAMI in good early responders than after DIV in poor early responders (57% versus 17%; P = .01).
Interestingly, however, a subset of 10 additional patients reached a low PCR level after DIV with a BCR-ABL/ABL ratio between 10−5 and 10−4 (Table 2; Figure 2), leading to similar rates of patients who achieved either PCR negativity or a low PCR level among the 2 subgroups of patients (18 of 29 after DIV versus 10 of 14 after HAMI; P = .73 by the 2-sided Fisher exact test). Overall, the PCR negativity rate was thus 29% (13 of 45), while the rate of patients who achieved at least a low PCR level (less than 10−4) was 56% (25 of 45).
Toxicity of HAMI and DIV combinations
Simultaneous administration of imatinib and chemotherapy was well tolerated in patients who received DIV as well as the HAMI combination. During DIV, main noninfectious nonhematologic grade 3/4 toxicities possibly or probably related to the treatment were the following: constipation (17%), nausea/vomiting (10%), peripheral neuropathy (7%), liver enzyme increase (7%), mucositis (3%), and pleural effusion (3%). Imatinib interruption or dose reduction never occurred in these patients. In those who presented grade 3/4 constipation or neuropathy, vincristine was replaced by vindesine. The 2 early deaths observed in the DIV subgroup were related to severe infectious events (1 septic shock and 1 pulmonary aspergillosis). During HAMI, main noninfectious nonhematologic grade 3/4 toxicities possibly or probably related to the treatment were the following: nausea/vomiting (36%), diarrhea (14%), mucositis (7%), headaches (7%), arterial hypertension (7%), and liver enzyme increase (7%). Imatinib was interrupted during 2 days in the patient who had a grade 3 liver enzyme increase. All other HAMI patients received imatinib continuously at the planned daily dosage. One additional HAMI patient presented delayed thrombocytopenia leading to transient imatinib interruption after myeloid recovery.
Stem cell transplantation
Among the 39 CR patients aged 55 years or less, 22 had an identified donor (15 MFD, 7 MUD). Eight were good early responders, and 14 were poor early responders (Figure 1). All of these 22 patients actually received allogeneic SCT in first CR. The median time between CR achievement and SCT was 92 days (range, 18-159 days). Fourteen of these 22 patients had achieved at least a low PCR level prior to SCT (5 after HAMI and 9 after DIV). Among the 21 other CR patients (6 good and 15 poor early responders; Figure 1), 14 had achieved at least a low PCR level and were thus eligible for autologous SCT, but only 6 actually received an autograft, mostly because some investigators preferred to wait longer for an MUD than perform autologous SCT. Among these 6 patients, 4 received posttransplantation maintenance with imatinib. Of note, one patient eligible for autologous SCT received cord blood transplantation and died 3 months later from TRM, while 3 other patients without a donor but positive MRD eventually received autologous SCT, 2 of them after late low PCR level achievement. Overall, 28 of 43 CR patients (65%) thus received SCT (22 allogeneic and 6 autologous) according to the protocol design.
Outcome
Among the 45 patients, 10 died, including 2 early deaths during DIV therapy, 3 deaths after relapse, and 5 deaths in first CR (4 after allogeneic SCT). At 18 months, overall survival was estimated at 65% (95% confidence interval, 43 to 81) (Figure 3A).
Outcome. (A) Overall survival. (B) DFS. (C) Cumulative incidence of relapse.
Among the 43 CR patients, 8 relapsed. Two patients relapsed early, while still under imatinib therapy. Four relapses occurred after allogeneic SCT. The 2 remaining relapses occurred after autologous SCT in patients receiving imatinib maintenance. Of note, one of these patients received an autograft despite a persistent BCR-ABL/ABL ratio of more than 10−4, while the other one received a late autologous SCT after delayed achievement of a low PCR level. At 18 months, cumulative incidence of relapse and DFS were estimated at 30% (95% confidence interval, 15 to 57) and 51% (95% confidence interval, 29 to 69), respectively (Figure 3B-C). Only one patient with initial CNS disease presented a CNS relapse (combined with marrow relapse after allogeneic SCT).
Possibly due to the relatively low number of patients and short follow-up, no prognostic factor could be identified among WBC, PB blast count, complex karyotype, BCR-ABL bcr subtype, early response, achievement of PCR negativity, and allogeneic donor availability. Advanced age was nevertheless associated with a trend for shorter overall survival (P = .07 using the 45-year cutoff).
Discussion
This study confirms that the introduction of imatinib in combination with fist-line chemotherapy seems to deeply modify the prognosis associated with adult Ph+ ALL, as already shown by recent single-institution or multicenter reports.8-13 Despite relatively short follow-up and variations in the design of imatinib administration (time of onset, sequential versus continuous administration, daily dosage), significantly higher response rate and better outcome were observed in all of these studies when compared with historic controls.
One characteristic of the present study was that imatinib administration was stratified on early response to conventional therapy. Time of imatinib onset and chemotherapeutic agents that were combined with imatinib was different in good versus poor early responders. The rationale for the design chosen in good early responders was (1) to administer a conventional 5-drug induction course including l-asparaginase in patients who presented a good early response to conventional agents and (2) to not expose these patients to an excessive toxicity during this first course by adding imatinib at that time. Actually, all of these good responders achieved hematologic CR without imatinib. They received imatinib later in combination with the HAM consolidation course, which has previously been shown as effective in Ph+ ALL.5 We had tested this HAMI combination in the AFR03 phase 1/2 study, which demonstrated an excessive toxicity in the imatinib 800 mg/d cohort while toxicity was manageable in the imatinib 600 mg/d cohort.15 In this context, dose-limiting toxicities of imatinib were severe facial edema and severe hypokalemia. The rationale for the design chosen in poor early responders was (1) to introduce imatinib earlier in patients who presented a poor early response to conventional induction and (2) to base remission induction in these patients on a “more imatinib – less chemotherapy” schedule such as the DIV regimen used. Imatinib was used at the higher 800 mg daily dosage in this second group of patients, because we have previously demonstrated the absence of limiting toxicity at that dose with this regimen.14 Interestingly, the rate of poor early responders who achieved PCR negativity or at least a low PCR level after this DIV combination was quite high and the outcome of these poor early responders quite good. The question of whether such a “more imatinib – less chemotherapy” approach could be better than a “less imatinib – standard chemotherapy” approach will be addressed in the recently activated GRAAPH-2005 trial, which randomly compares DIV with a standard imatinib–hyper-CVAD (cyclophosphamide/vincristine/doxorubicin/dexamethasone) induction.
Using this strategy, we also observed that results compared very favorably with those obtained during the pre-imatinib era. Table 3 and Figure 3 illustrate the comparison between the 45 patients of the present study and the 198 Ph+ ALL patients previously treated in the LALA-94 study from the Leucémie Aiguë Lymphoblastique de l'Adulte (LALA) group.5,6 As indicated, the CR rate has significantly increased from 71% to 96% (P < .001). Response to a steroid prephase was not evaluated in the LALA-94 study, but approximately half of the Ph+ ALL patients had a marrow evaluation for chemosensitivity at day 8. In this pre-imatinib period, early chemoresistance actually influenced the CR rate (60% versus 94%; P = .009). Even if treatment preceding early marrow evaluation differed between both studies, the CR rate obtained in patients with chemoresistant ALL has also significantly increased from 60% to 96% (P < .001). As a consequence of these gains in response rate, the ratio of CR patients with a donor who actually received allogeneic SCT in first CR has increased from 85% to 100%. Finally, cumulative incidence of relapse, DFS, and overall survival were significantly longer in the present study as compared with the pre-imatinib study (P = .02, .02, and .05, respectively) (Table 3; Figure 3).
Comparison between GRAAPH-2003 and LALA-94 studies
| . | Present GRAAPH-2003 . | LALA-94 . | P . |
|---|---|---|---|
| No. patients | 45 | 198 | |
| Hematologic CR, no./no. patients tested (%) | |||
| All patients | 43/45 (96) | 140/198 (71) | <.001 |
| Chemoresistant patients* | 24/25 (96) | 63/105 (60) | <.001 |
| Allogeneic transplantation rate, no./no. patients tested (%) | |||
| All CR patients | 22/43 (51) | 62/140 (44) | .49 |
| CR patients with a donor | 22/22 (100) | 62/73 (85) | .06 |
| Cumulative incidence of relapse: 18-mo estimates, % (95% CI) | 30 (15-57) | 49 (40-59) | .02 |
| DFS: 18-mo estimates, % (95% CI) | 51 (29-69) | 31 (24-39) | .02 |
| Overall survival: 18-mo estimates, % (95% CI) | 65 (43-81) | 39 (32-46) | .05 |
| . | Present GRAAPH-2003 . | LALA-94 . | P . |
|---|---|---|---|
| No. patients | 45 | 198 | |
| Hematologic CR, no./no. patients tested (%) | |||
| All patients | 43/45 (96) | 140/198 (71) | <.001 |
| Chemoresistant patients* | 24/25 (96) | 63/105 (60) | <.001 |
| Allogeneic transplantation rate, no./no. patients tested (%) | |||
| All CR patients | 22/43 (51) | 62/140 (44) | .49 |
| CR patients with a donor | 22/22 (100) | 62/73 (85) | .06 |
| Cumulative incidence of relapse: 18-mo estimates, % (95% CI) | 30 (15-57) | 49 (40-59) | .02 |
| DFS: 18-mo estimates, % (95% CI) | 51 (29-69) | 31 (24-39) | .02 |
| Overall survival: 18-mo estimates, % (95% CI) | 65 (43-81) | 39 (32-46) | .05 |
CI indicates confidence interval.
Considering that early treatments were different in both studies (no prephase, no L-asparaginase, different anthracycline types and dosages in the LALA-94 trial).
Due to better response rates and outcome and probably to the targeted mechanism of action of imatinib, some of the bad-prognostic factors previously established in Ph+ ALL patients might disappear with a treatment combining imatinib and chemotherapy. We report here the lack of prognostic value of early response to conventional agents in this new setting. In a large cohort of 90 patients, the Japan Adult Leukemia Study Group has recently reported a trend to lower survival in patients who underwent allogeneic SCT as compared with those who did not,8 indicating that the availability of an allogeneic donor might no longer confer any significant benefit in outcome in Ph+ ALL patients in this new context of combined therapy. The numbers of patients and median follow-up are certainly too low to definitely conclude, because late relapses might still occur in patients who did not undergo transplantation. In addition, the quite high incidence of BCR-ABL kinase domain mutations in patients with Ph+ ALL developing clinical resistance to imatinib leads us to be particularly cautious.17 At the present time, the best recommendation is probably to still perform transplantation in younger patients with a matched donor after using imatinib concurrently with induction and consolidation chemotherapy13 for a period long enough to reach a good molecular response, especially in patients with a high initial WBC. Frequent interim reanalysis taking into account patient age and donor type (MFD versus MUD) may also be recommended in order to periodically compare the results of allogeneic SCT versus combined imatinib and chemotherapy or autologous SCT.
Authorship
Contribution: The study was conceived by the GRAALL scientific board, including P.R., F.H.-R., E.D., F.W., S.M., J.-M.C., M.-C.V., O.R., A.B., A.P., Y.C., E.M.I., V.L., J.-P.V., X.T., N.I., and H.D.; the statistical analysis was undertaken by H.D.; the manuscript was written by A.d.L. and H.D.; D.R. and M.E. were significant clinical contributors to the study; and P.R., D.R., X.T., and N.I. reviewed the manuscript. A.d.L. and P.R. contributed equally to study analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hervé Dombret, Maladies du Sang, Hôpital Saint-Louis 1, avenue Claude Vellefaux, 75010 Paris, France; e-mail: herve.dombret@sls.ap-hop-paris.fr.
A complete list of the members of GRAAL appears in Document S1, available on the Blood website; see the Supplemental Document link at the top of the online version of this article.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants P0200701 and P030425/AOM03081 from Le Programme Hospitalier de Recherche Clinique, Ministère de l'Emploi et de la Solidarité, France.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal