Abstract
B-1b cells produce IgM natural antibodies against α1-3Galβ1-4GlcNAc (αGal). These can be tolerized by nonmyeloablative induction of mixed chimerism using αGal-positive (αGal+) donor marrow. We assessed the role of CR1/2 in this model for induction of tolerance of B-1b cells. Mixed hematopoietic chimerism was induced in α1-3galactosyltransferase (GalT−/−) and GalT−/−Cr2−/− mice with αGal+ BALB/c marrow donors. Anti-αGal Ab and anti-αGal Ab–producing B cells became undetectable in GalT−/− chimeras, whereas they persisted in chimeric GalT−/−Cr2−/− mice. To determine whether CR1/2 expression on stromal cells and/or hematopoietic cells was critical for B-1–cell tolerance, we generated GalT−/− radiation chimeras in which CR1/CR2 was expressed on either stromal cells, hematopoietic cells, neither, or both. After induction of mixed chimerism from αGal+ allogeneic bone marrow (BM) donors, anti-αGal–producing B cells were rendered tolerant in reconstituted recipients expressing only stromal CR1/CR2. Our results suggest a possible role for follicular dendritic cells that pick up immune complexes via CR1/CR2 receptors in the tolerization of B-1b cells.
Introduction
Humans and old-world primates lack functional α1-3galactosyltransferase (GalT) and produce natural antibodies (NAbs) that recognize Galα1-3Galβ1-4GlcNAc (αGal). Pigs express high levels of αGal antigen on their cell surfaces, and hyperacute rejection is induced by anti-αGal NAb in pig-to-primate xenotransplantation.1 GalT−/− mice lack the ability to produce αGal and, like humans, produce anti-αGal NAb. These mutant mice thereby provide a model for the investigation of anti-αGal B-cell responses and tolerance.2,3
Using myeloablative and nonmyeloablative conditioning regimens, we have previously shown that NAb-producing B cells in GalT−/− mice can be tolerized via mixed chimerism induction.4-6 Both pre-existing and newly developing B cells producing anti-αGal Ab are tolerized by the achievement of chimerism from αGal-positive (αGal+) donors. Using nonmyeloablative bone marrow transplantation (BMT), permanent acceptance of αGal+ heart allografts and xenografts was achieved in GalT−/− mice.7 We have observed that B-1b cells bearing anti-αGal receptors in the peritoneal cavity (PerC), which are IgMhigh CD5−CD43+Mac1+ and lack CD21 (CR2) expression, are the main progenitors of anti-αGal Ab–producing cells. These PerC cells lose Mac1 expression in association with migration into the spleen and transition to Ab-secreting cells.8,9
Complement receptor 1 (CR1, CD35), the receptor for C3b and C4b, and CR2 (CD21), the receptor for iC3b and C3d, g, are coexpressed on B cells and follicular dendritic cells (FDCs) in both humans and mice.10-12 CD21 is a splice variant of CD35, and Cr2−/− mice therefore lack both CD21 and CD35. Coupled with CR1 or CD19, CR2 regulates B-cell activation and differentiation and promotes memory generation and immunoglobulin class switching.13 CR1/CR2 on marginal zone B cells also contributes an initial step toward adaptive immunity by transporting immune complexes onto FDCs.14-17 Impaired B-cell responses to T-dependent and T-independent antigens have been observed using Cr2−/− mice.13,17-22
Complement and complement receptors have also been implicated in self-tolerance of B cells in animals and humans.23 Humans lacking C1q or C4 are predisposed to develop systemic lupus erythematosus (SLE).24 Cr2−/− mice transgenic for soluble antigen and a high-affinity Ig receptor showed increased accumulation of autoreactive B cells in the peripheral tissues and a failure of anergy.25,26 In Fas-deficient Cr2−/− mice on either a mixed B6 × 129 or pure B6 background, an SLE-like syndrome developed, with increased production of antinuclear and anti-dsDNA Abs and increased IgG deposition in the glomeruli compared with Cr2+ animals.25,26 Moreover, a dysfunctional Cr2 allele was mapped to the Sle-1c locus in the NZM2410 autoimmune strain.27
The mechanisms of tolerance of B-1b cells, which can produce NAbs and autoantibodies,28-30 are unclear. We have assessed the role of CR1/CR2 in the induction of NAb-producing B-cell tolerance via mixed chimerism. We found that CR1/CR2 expression on nonhematopoietic stromal cells promotes the production of anti-αGal natural and induced Abs. Moreover, CR1/CR2 expression on nonhematopoietic stromal cells promotes the induction of B-cell tolerance in mixed chimeras.
Materials and methods
Mice
GalT−/− mice were kindly provided by Dr John Lowe (University of Michigan) and were bred in our colony.3 Age-matched (10- to 12-week old) GalT−/− mice on the C57BL/6 (B6) background (backcrossed 7 times to B6) were used for the experiments. BALB/c mice were obtained from Jackson ImmunoResearch Laboratories (Bar Harbor, ME). GalT−/− mice on the C57BL/6 background were crossed with Cr2−/− (CD21/CD35-deficient) mice on the C57BL/6 background.18 Offspring were then intercrossed to generate GalT−/−Cr2−/− mice. All animals were maintained in a specific pathogen-free microisolator environment.
Immunization
To enhance anti-αGal Ab production, recipient mice were immunized with αGal-bearing xenogeneic cells (ie, 109 rabbit red blood cells [RRBCs]; Cocalico Biologicals, Reamstown, PA) 8 days before enzyme-linked immunospot (ELISpot) assays. RRBCs were washed twice and resuspended at 109/mL in PBS before intraperitoneal injection.
Nonmyeloablative conditioning regimen and BMT
Mixed allogeneic BM chimerism was induced in GalT−/− mice and GalT−/−Cr2−/− double-knockout mice using a nonmyeloablative conditioning regimen and BMT from BALB/c donor mice as previously described.31 In brief, recipient mice were injected intraperitoneally with rat IgG2b anti–mouse CD4 monoclonal antibody (mAb) GK1.5 (1.8 mg)32 and anti–mouse CD8 mAb 2.43 (1.4 mg)33 on day −5. They received 3 Gy total body irradiation (TBI) and 7 Gy selective thymic irradiation (TI) prior to allogenic BMT on day 0. The mAbs for in vivo use were produced at the National Cell Culture Center, Minneapolis, MN.
Construction of GalT−/− mice expressing CR1/2 selectively on hematopoietic or nonhematopoietic cells
BM was prepared as previously described.6 Twelve-week-old GalT−/− or GalT−/−Cr2−/− recipient mice were lethally irradiated with 10.5 Gy TBI and reconstituted with 5 × 106GalT−/− or GalT−/−Cr2−/− BM cells by intravenous injection. Analysis of peripheral blood chimerism was performed 6 weeks after BMT. Secondary nonmyeloablative BMT was performed 8 weeks after the initial BMT.
FCM analysis and detection of B cells bearing receptors for αGal
Chimerism was measured by flow cytometric (FCM) analysis of peripheral blood on a FACScan cytometer (Becton Dickinson Immunocytometry Systems, Mountain View, CA) as described previously.34 White blood cells (WBCs) were purified by H2O lysis and were stained with FITC-conjugated anti-CD21/CD35, FITC- or PE-conjugated anti-CD4, FITC- or PE-conjugated anti-CD8, FITC- or PE-conjugated anti-CD19, FITC- or PE-conjugated anti-Mac1, and/or biotin-conjugated anti–H-2Dd (34-2-12) (all from PharMingen, San Diego, CA). The biotinylated mAb was visualized with PE-streptavidin (for 3-color FCM analysis) or APC-streptavidin (for 4-color FCM analysis). Cells with anti-Gal receptors were detected with FITC-conjugated Gal-BSA and prepared and used as described.4 Dead cells were excluded from data by gating out propidium iodide (PI)–positive cells.
ELISA assay for detecting anti-αGal Ab
Anti-αGal or total IgM or IgG Ab levels in sera were quantified by enzyme-linked immunosorbent assay (ELISA), according to procedures previously described.6 Briefly, ELISA plates were coated with 5 μg/mL Gal-BSA (Alberta Research Council, Edmonton, AB, Canada), BSA (IgG free; Jackson ImmunoResearch Labs, West Grove, PA), goat anti–mouse IgM (Southern Biotech, Birmingham, AL), or goat anti–mouse IgG (Southern Biotech) and blocked with Starting Block Buffer (Pierce, Rockford, IL). Diluted serum samples were incubated in the plates, and bound Abs were detected using horseradish peroxidase (HRP)–conjugated goat anti–mouse IgM- or IgG-specific Ab (Southern Biotech). Color development was achieved using 0.2 mg/mL o-phenylenediamine dihydrochloride ([OPD] Sigma, St Louis, MO) in substrate buffer. The OPD reaction was stopped using 3 M NH2SO4, and absorbance at 490 nm was measured. Serum was diluted serially, and the optical density (OD) value that corresponded to 2 times the background OD value was set as the titer. In all experiments, binding of serum Abs to BSA was examined in parallel, and data were subtracted from binding of serum Abs to Gal-BSA. Sera were tested twice, and 1 of the representative data is shown.
ELISpot for detecting anti-αGal– and total IgM-producing cells
The ELISpot assay was performed as described previously.6 Briefly, nitrocellulose membranes of a 96-well filtration plate (Millipore, Bedford, MA) were coated with 5 μg/mL Gal-BSA (Alberta Research Council), BSA (IgG free; Jackson ImmunoResearch Labs), or 5 μg/mL goat anti–mouse IgM and IgG (Southern Biotech) for detecting anti-αGal– or total IgM- and IgG-producing cells, respectively. Nonspecific binding was blocked with Iscove modified Dulbecco medium (IMDM) supplemented with 15% FCS, 50 μM β-mercaptoethanol, and 4 μg/mL gentamicin. Serial dilutions of cell suspensions in complete IMDM were added to wells in duplicate. After a 24-hour culture at 37°C, bound Abs were detected using HRP-conjugated goat anti–mouse IgM and IgG Abs (Southern Biotech) followed by color development with 3-amino-9-ethylcarbazole (Sigma). After the membranes were dried, red spots in each well were counted using an automated ELISpot reader (CTL, Cleveland, OH). In all experiments, the number of Ab-producing cells against BSA was examined in parallel, and data were subtracted from the number of antibody-producing cells against Gal-BSA.
LPS stimulation of PerC B cells
PerC cells obtained from recipient mice were cultured at 5 × 105/mL in RPMI 1640 supplemented with 10% FCS (Sigma), 50 μM 2-mercaptoethanol, 1% HEPES buffer, 0.09 mM nonessential amino acids, 1 mM sodium pyruvate, 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10 μg/mL LPS as previously described.9 After in vitro LPS stimulation for 8 days, cells were used for ELISpot assay.
Statistics
The results were analyzed by the unpaired or paired Student t test. A P value of less than .05 was considered to be statistically significant.
Results
Similar phenotype of anti-Gal receptor-bearing cells in GalT−/− and GalT−/−Cr2−/− mice
To compare the phenotypes of B cells recognizing Gal in GalT−/−Cr2−/−and GalT−/− mice, we used multicolor flow cytometry with a fluorochrome conjugate of Gal-BSA to detect B cells with surface Ig receptors that bind to αGal. Because the numbers of Gal-binding cells are very low at baseline and are enhanced by RRBC immunization,9 these studies were performed 8 days after immunization with RRBCs. As shown in Figure 1, PerC Gal-binding cells in both groups of mice were CD21−, Mac1+/low, and largely CD5−, consistent with a B-1b phenotype. In spleen, the Gal-binding cells in both groups showed a similar phenotype except that most were Mac1− (data not shown), consistent with our previous results in GalT−/− mice.9 No differences in the proportions of B cells with surface receptors for αGal were detected in GalT−/−Cr2−/−and GalT−/− mice. Thus, similar B-1b–like cell populations recognizing αGal are present in GalT−/−Cr2−/− and GalT−/− mice. Moreover, the proportions of PerC B-1b cells (CD5−/Mac1+/CD19+) were not significantly different between GalT−/− (28.8% ± 2.7%) and GalT−/−Cr2−/− mice (34.6% ± 8.3%, not significant [NS]) (data not shown). Consistent with a previous report,18 significantly lower proportions of B-1a cells (CD5+/Mac1+/CD19+) were detected in the PerC of GalT−/−Cr2−/− mice compared with GalT−/− mice (not shown).
Phenotypic similarity of anti-αGal receptor-bearing B cells in GalT−/− and GalT−/−Cr2−/− mice. PerC cells were stained with the indicated antibodies and Gal-BSA (top) or BSA (bottom) conjugated with Cy5 fluorochrome. Shown are representative FACS plots of PerC cells gated for CD19+ lymphocytes. Anti-αGal receptor-bearing cells in both GalT−/− and GalT−/−Cr2−/− mice were CD19+CD5−CD21−Mac1+/low.
Phenotypic similarity of anti-αGal receptor-bearing B cells in GalT−/− and GalT−/−Cr2−/− mice. PerC cells were stained with the indicated antibodies and Gal-BSA (top) or BSA (bottom) conjugated with Cy5 fluorochrome. Shown are representative FACS plots of PerC cells gated for CD19+ lymphocytes. Anti-αGal receptor-bearing cells in both GalT−/− and GalT−/−Cr2−/− mice were CD19+CD5−CD21−Mac1+/low.
Failure to achieve B-cell tolerance via mixed chimerism in GalT−/−Cr2−/− mice
We investigated the possible role of CR1/CR2 in the induction of tolerance of anti-αGal–producing B cells via mixed chimerism. With the exception of a small increase in donor B-cell chimerism in GalT−/−Cr2−/− mice at one time point (16 weeks), similar levels of stable, multilineage mixed chimerism were achieved in GalT−/− and GalT−/−Cr2−/− mice (Table 1) receiving αGal+ BALB/c allogeneic BMT with the nonmyeloablative regimen. On average, close to one half of the circulating B cells in both groups were of recipient origin. In a separate experiment performed to compare chimerism in PerC and splenic B-1b–cell populations (CD19+CD5−Mac1+ and CD19+CD21−CD23− lymphocytes, respectively) of mixed chimeric GalT−/−Cr2−/−and GalT−/− mice, no significant differences were detected between the 2 groups (data not shown).
Similar levels of mixed chimerism in GalT−/− and GalT−/−CR2−/− mice after nonmyeloablative conditioning and H-2-mismatched BMT
| Group (donor and recipient)* . | No. . | Percentage of donor cells† . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD4 . | B cells . | Monocytes . | ||||||||
| 4 wk after preparation . | 8 wk after preparation . | 16 wk after preparation . | 4 wk after preparation . | 8 wk after preparation . | 16 wk after preparation . | 4 wk after preparation . | 8 wk after preparation . | 16 wk after preparation . | ||
| A (BALB/c and GalTKO) | 9 | 33.30 ± 27.70 | 24.70 ± 20.30 | 25.80 ± 17.60 | 41.30 ± 28.40 | 44.20 ± 18.60 | 42.90 ± 14.30 | 58.3 ± 17.7 | 48.6 ± 18.6 | 44.5 ± 12.8 |
| B (BALB/c and GalTCR2DKO) | 7 | 12.80 ± 5.2‡ | 42.40 ± 19.3‡ | 40.90 ± 18.4‡ | 56.40 ± 22.5‡ | 59.00 ± 20.7‡ | 62.40 ± 17.2§ | 53.8 ± 14.5‡ | 39.6 ± 15.1‡ | 40.5 ± 12.4‡ |
| Group (donor and recipient)* . | No. . | Percentage of donor cells† . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD4 . | B cells . | Monocytes . | ||||||||
| 4 wk after preparation . | 8 wk after preparation . | 16 wk after preparation . | 4 wk after preparation . | 8 wk after preparation . | 16 wk after preparation . | 4 wk after preparation . | 8 wk after preparation . | 16 wk after preparation . | ||
| A (BALB/c and GalTKO) | 9 | 33.30 ± 27.70 | 24.70 ± 20.30 | 25.80 ± 17.60 | 41.30 ± 28.40 | 44.20 ± 18.60 | 42.90 ± 14.30 | 58.3 ± 17.7 | 48.6 ± 18.6 | 44.5 ± 12.8 |
| B (BALB/c and GalTCR2DKO) | 7 | 12.80 ± 5.2‡ | 42.40 ± 19.3‡ | 40.90 ± 18.4‡ | 56.40 ± 22.5‡ | 59.00 ± 20.7‡ | 62.40 ± 17.2§ | 53.8 ± 14.5‡ | 39.6 ± 15.1‡ | 40.5 ± 12.4‡ |
Recipient GalT−/− or GalT−/−Cr2−/− mice were treated with anti-CD4 and anti-CD8 mAbs on day –5, TI and TBI on day 0, and BALB/c BMT on day 0 with 2 × 107 cells.
Chimerism was detected by flow cytometric analysis after staining with FITC-conjugated anti-CD4, anti-CD19, and anti-Mac 1 mAbs, biotinylated anti-H-2Dd mAb, and PE-streptavidin. To analyze CD4 and B-cell chimerism, a lymphocyte forward scatter/side scatter (FSC/SSC) gate was set. To analyze monocyte chimerism, a monocyte FSC/SSC gate was set. The mean ± SD percentage of donor cells in each lineage is shown.
NS compared with GalT−/− chimeras at the same time point.
P < .05 compared with GalT−/− chimeras at the same time point.
As shown at 6 weeks in Figure 2A, GalT−/− recipients of allogeneic BMT showed significantly reduced levels of anti-αGal IgM in their sera compared with conditioned control GalT−/− recipients. Following immunization with RRBCs at 9 weeks after BMT, anti-αGal IgM levels increased markedly in GalT−/−Cr2+/+ conditioned controls (Figure 2A, control GalT−/−). In contrast, the levels of anti-αGal Ab remained low after immunization in GalT−/−(Cr2+/+) mixed chimeras (Figure 2A, BMT GalT−/−), demonstrating unresponsiveness to αGal, consistent with our previous results.4
Failure of tolerance induction of anti-Gal–producing B cells inGalT−/−Cr2−/− mice. (A) Anti-αGal IgM titers of GalT−/− or GalT−/−Cr2−/− mice receiving conditioning alone or conditioning with wild-type BMT. Serum levels of anti-αGal IgM were measured in GalT+/+ control mice, conditioned (control; ▴, n=5) or chimeric (BMT; ▵, n=5) GalT−/− mice, and conditioned (control; ▪, n=4) or chimeric (BMT; □, n=6) GalT−/−Cr2−/− mice 6 weeks after BMT and at 10 weeks, 8 days following immunization with RRBCs. P < .05 comparing chimeric and control GalT−/−; P > .05 (NS) comparing chimeric and control GalT−/−Cr2−/− mice, chimeric GalT−/− and GalT−/−Cr2−/− mice, or conditioned GalT−/− and GalT−/−Cr2−/− mice at 6 weeks after BMT. P > .05 comparing chimeric and control GalT−/−Cr2−/− mice after rabbit RRBC immunization. P < .02 comparing chimeric GalT−/−Cr2−/− mice and GalT−/− mice and comparing conditioned GalT−/−Cr2−/− mice and GalT−/− mice after RRBC immunization. Data shown are individual animals in a single experiment. (B) ELISpot assay was used to detect anti-αGal (IgM + IgG)–producing cells. PerC cells prepared from conditioned and chimeric GalT−/− mice (□, n=3; ▪, n=4; respectively) and conditioned and chimeric (BMT) GalT−/−Cr2−/− mice (▵, n=3; ▴, n=4; respectively) were cultured with LPS for 8 days and used in ELISpot assays. P < .001 comparing chimeric and control GalT−/− mice (†) and P < .05 comparing chimeric GalT−/− and GalT−/−Cr2−/− mice (*) at 8 × 105 and 1.6 × 105 cells-per-well dilutions. There was no statistical difference between chimeric and control GalT−/−Cr2−/− mice (‡) at the 8 × 105 cells-per-well dilution, but P < .05 at 1.6 × 105 cells per well. The frequencies obtained from BSA-coated plates were subtracted from those obtained with Gal-BSA–coated plates. The average frequencies of spot-forming cells detected on BSA-coated plates at 8 × 105 cells per well were 5.2 ± 3.0, 7.1 ± 1.5, 12.6 ± 4.2, and 10.0 ± 5.0 in BMT GalT−/−, BMT GalT−/−Cr2−/−, conditioned GalT−/−, and conditioned GalT−/−Cr2−/− groups, respectively. Data shown are mean ± SD in each group.
Failure of tolerance induction of anti-Gal–producing B cells inGalT−/−Cr2−/− mice. (A) Anti-αGal IgM titers of GalT−/− or GalT−/−Cr2−/− mice receiving conditioning alone or conditioning with wild-type BMT. Serum levels of anti-αGal IgM were measured in GalT+/+ control mice, conditioned (control; ▴, n=5) or chimeric (BMT; ▵, n=5) GalT−/− mice, and conditioned (control; ▪, n=4) or chimeric (BMT; □, n=6) GalT−/−Cr2−/− mice 6 weeks after BMT and at 10 weeks, 8 days following immunization with RRBCs. P < .05 comparing chimeric and control GalT−/−; P > .05 (NS) comparing chimeric and control GalT−/−Cr2−/− mice, chimeric GalT−/− and GalT−/−Cr2−/− mice, or conditioned GalT−/− and GalT−/−Cr2−/− mice at 6 weeks after BMT. P > .05 comparing chimeric and control GalT−/−Cr2−/− mice after rabbit RRBC immunization. P < .02 comparing chimeric GalT−/−Cr2−/− mice and GalT−/− mice and comparing conditioned GalT−/−Cr2−/− mice and GalT−/− mice after RRBC immunization. Data shown are individual animals in a single experiment. (B) ELISpot assay was used to detect anti-αGal (IgM + IgG)–producing cells. PerC cells prepared from conditioned and chimeric GalT−/− mice (□, n=3; ▪, n=4; respectively) and conditioned and chimeric (BMT) GalT−/−Cr2−/− mice (▵, n=3; ▴, n=4; respectively) were cultured with LPS for 8 days and used in ELISpot assays. P < .001 comparing chimeric and control GalT−/− mice (†) and P < .05 comparing chimeric GalT−/− and GalT−/−Cr2−/− mice (*) at 8 × 105 and 1.6 × 105 cells-per-well dilutions. There was no statistical difference between chimeric and control GalT−/−Cr2−/− mice (‡) at the 8 × 105 cells-per-well dilution, but P < .05 at 1.6 × 105 cells per well. The frequencies obtained from BSA-coated plates were subtracted from those obtained with Gal-BSA–coated plates. The average frequencies of spot-forming cells detected on BSA-coated plates at 8 × 105 cells per well were 5.2 ± 3.0, 7.1 ± 1.5, 12.6 ± 4.2, and 10.0 ± 5.0 in BMT GalT−/−, BMT GalT−/−Cr2−/−, conditioned GalT−/−, and conditioned GalT−/−Cr2−/− groups, respectively. Data shown are mean ± SD in each group.
Baseline anti-αGal IgM levels were slightly (but not significantly) lower in GalT−/−Cr2−/− conditioned controls (Figure 2A, control GalT−/−Cr2−/−) than in GalT−/−Cr2+/+ controls and were detectable above the background seen in GalT+/+ mice (Figure 2A, GalT+/+ control). In contrast to GalT−/−Cr2+/+ recipients, achievement of mixed chimerism in GalT−/−Cr2−/− mice did not lead to a significant reduction in anti-αGal IgM levels (Figure 2A, left panel BMT, GalT−/−Cr2−/−). Following immunization with RRBCs at 9 weeks, anti-αGal IgM levels increased above baseline levels in control GalT−/−Cr2−/− recipients (P < .05 using the paired test), indicating that these mice were capable of responding, albeit weakly, to αGal (Figure 2A, left versus right panel, control GalT−/−Cr2−/−). Importantly, GalT−/−Cr2−/− BMT recipients also showed a significant response to αGal following immunization (Figure 2A, left versus right panel, BMT GalT−/−Cr2−/−; P < .05 compared with baseline using the paired t test), and these levels were significantly higher than those in GalT−/−Cr2+/+ mixed chimeric mice (Figure 2A, right panel). These results suggested that αGal+ BMT may have failed to tolerize anti-αGal–producing cells in GalT−/−Cr2−/− mice, despite the successful achievement of mixed chimerism.
To directly assess tolerance, we quantified anti-αGal Ab–producing LPS-stimulated PerC cells by ELISpot assay of recipients killed 10 weeks after BMT (Figure 2B). Corresponding to the ELISA data and consistent with our previous studies,4 numbers of anti-αGal Ab–producing B cells were significantly decreased in the LPS-stimulated PerC of GalT−/− BMT recipients compared with conditioned control GalT−/− mice. In contrast, the numbers of anti-αGal Ab–producing cells in mixed chimeric GalT−/−Cr2−/− recipients were generally similar to those in conditioned GalT−/−Cr2−/− controls (except for a small but significant reduction in the frequency of anti-αGal–producing cells at 1 cell dilution in the chimeras versus controls; Figure 2B, ▴ versus ▵). Significantly, despite the anti-αGal hyporesponsiveness of GalT−/−Cr2−/− controls (compare □ and ▵ in Figure 2B), frequencies of anti-Gal–producing cells were significantly greater in the PerC of GalT−/−Cr2−/− mixed chimeras than in those of GalT−/−Cr2+/+ mixed chimeras (both at the 8 × 105 and 1.6 × 105 cells-per-well dilutions in Figure 2B). Thus, while induced anti-αGal IgM responses are lower in GalT−/−Cr2−/−compared with GalT−/−Cr2+/+ controls, our data show that GalT−/−Cr2−/− mice can respond to αGal. Comparison of chimeric and control GalT−/−Cr2−/− mice clearly shows a failure of tolerance induction in chimeric animals, indicating that CR1/CR2 plays an important role in the induction of anti-αGal–producing B-cell tolerance by BMT.
Construction of GalT−/− mice with selective expression of CR1/CR2 on hematopoietic and/or nonhematopoietic cells and induction of mixed chimersim
GalT−/− mice and GalT−/−Cr2−/− mice received lethal TBI and either 5 × 106GalT−/−(Cr2+/+) or GalT−/−Cr2−/− syngeneic BM cells, resulting in animals that lacked αGal expression on both hematopoietic and nonhematopoieitic cells, and expressed CR1/CR2 on hematopoietic or nonhematopoietic stromal cells, neither, or both. GalT−/−Cr2−/− mice receiving GalT−/−Cr2+/+ marrow should express CR1/CR2 only on hematopoietic cells, including B cells. In contrast, GalT−/−Cr2+/+ mice that received GalT−/−Cr2−/− marrow should express CR1/CR2 only on nonhematopoietic stromal cells, including FDCs. Appropriate CR1/CR2 expression in reconstituted mice was confirmed 6 weeks after the initial BMT by assaying CR1/CR2 expression on WBCs by FCM (data not shown).
To test the role of stromal versus hematopoietic CR1/CR2 in B-cell tolerance induction, we treated the Cr2 chimeric GalT−/− mice with our nonmyeloablative conditioning protocol 8 weeks after the original irradiation and reconstitution. Animals in each group served as conditioned controls or received transplants with H2-mismatched BALB/c (H-2d) BM cells. Long-lasting multilineage mixed chimerism was induced after secondary BALB/c BMT (Table 2). Although there were some transient differences at 4 weeks, there were no statistical differences 8 weeks after BALB/c BMT in lymphocyte, monocyte, or granulocyte chimerism between stromal CR1/CR2–positive (S+)/hematopoietic CR1/CR2–positive (H+), S+/H−, S−/H+, and S−/H− chimeric GalT−/− mice.
Similar levels of mixed chimerism in GalT−/− mice regardless of CR2 expression on hematopoietic or nonhematopoietic cells
| Group . | . | No. . | Chimerism,* percentage of donor cells . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Phenotype of CR2 chimera . | CD4 . | B220 . | Monocytes . | ||||||
| CR2 expression on stroma . | CR2 expression on BM . | 4 wk after second BMT . | 8 wk after second BMT† . | 4 wk after second BMT . | 8 wk after second BMT† . | 4 wk after second BMT† . | 8 wk after second BMT† . | ||
| GalT KO → GalT KO | + | + | 8 | 27.6 ± 14.4† | 30.2 ± 12.5 | 62.5 ± 10.1‡ | 61.2 ± 11.5 | 87.5 ± 4.9 | 90.2 ± 5.1 |
| GalT KO → GalT Cr2 DKO | + | − | 5 | 32.7 ± 12.6§ | 34.5 ± 11.5 | 61.1 ± 14.2† | 58.2 ± 11.5 | 82.3 ± 4.6 | 89.2 ± 12.2 |
| GalT Cr2 DKO → GalT KO | − | + | 4 | 16.3 ± 5.2§ | 25.4 ± 12.4 | 39.5 ± 14.6‡ | 55.4 ± 14.2 | 86.0 ± 0.9 | 92.1 ± 3.4 |
| GalT Cr2 DKO → GalT Cr2 DKO | − | − | 8 | 14.7 ± 14.7§ | 24.5 ± 13.5 | 43.9 ± 15.8‡ | 52.1 ± 14.2 | 86.6 ± 22.0 | 91.2 ± 8.5 |
| Group . | . | No. . | Chimerism,* percentage of donor cells . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Phenotype of CR2 chimera . | CD4 . | B220 . | Monocytes . | ||||||
| CR2 expression on stroma . | CR2 expression on BM . | 4 wk after second BMT . | 8 wk after second BMT† . | 4 wk after second BMT . | 8 wk after second BMT† . | 4 wk after second BMT† . | 8 wk after second BMT† . | ||
| GalT KO → GalT KO | + | + | 8 | 27.6 ± 14.4† | 30.2 ± 12.5 | 62.5 ± 10.1‡ | 61.2 ± 11.5 | 87.5 ± 4.9 | 90.2 ± 5.1 |
| GalT KO → GalT Cr2 DKO | + | − | 5 | 32.7 ± 12.6§ | 34.5 ± 11.5 | 61.1 ± 14.2† | 58.2 ± 11.5 | 82.3 ± 4.6 | 89.2 ± 12.2 |
| GalT Cr2 DKO → GalT KO | − | + | 4 | 16.3 ± 5.2§ | 25.4 ± 12.4 | 39.5 ± 14.6‡ | 55.4 ± 14.2 | 86.0 ± 0.9 | 92.1 ± 3.4 |
| GalT Cr2 DKO → GalT Cr2 DKO | − | − | 8 | 14.7 ± 14.7§ | 24.5 ± 13.5 | 43.9 ± 15.8‡ | 52.1 ± 14.2 | 86.6 ± 22.0 | 91.2 ± 8.5 |
Recipient mice were treated with anti-CD4 and anti-CD8 mAbs on day –5, TI and TBI on day 0, and BALB/c BMT on day 0. All groups received 2 × 107 BALB/c cells at their second BMT.
Chimerism was detected by FCM analysis and analyzed as in Table 1. The mean and SD percentage of H-2d–positive BALB/c donor cells is shown for the indicated lineages.
NS compared with other groups at the same time point.
P < .02 comparing GalT KO → GalT KO to GalT Cr2 DKO → GalT KO or GalT Cr2 DKO → GalT Cr2 DKO at the same time point.
P < .05 comparing GalT KO → GalT Cr2 DKO to GalT Cr2 DKO → GalT KO or GalT Cr2 DKO → GalT Cr2 DKO at the same time point.
Expression of CR1/CR2 on nonhematopoietic cells promotes the production of anti-αGal natural antibodies
As shown in Figure 3, conditioned control S+/H+ mice demonstrated significant anti-αGal IgM levels in their sera 10 weeks after conditioning (18 weeks after initial reconstitution). While anti-αGal Ab was detected in sera of S+/H− conditioned mice at similar levels as S+/H+ mice, it was almost undetectable in S−/H+ and S−/H− conditioned mice (Figure 3). Anti-αGal IgG was undetectable in all mice before sensitization with RRBCs (data not shown). Thus, CR1/CR2 expression on nonhematopoietic stromal cells promotes anti-αGal NAb production.
Anti-αGal IgM levels inGalT−/−Cr2−/−/GalT−/− radiation chimeras that received nonmyeloablative conditioning with or without BMT from αGal+ BALB/c mice 8 weeks later. Serum after secondary nonmyeloablative conditioning of control and mixed chimeric (BALB/c secondary BMT donors) GalT−/−Cr2+/+ → GalT−/−Cr2+/+ (S+H+, groups 1 and 2), GalT−/−Cr2−/− → GalT−/−Cr2−/− (S−/H−, groups 3 and 4), GalT−/−Cr2−/− → GalT−/−Cr2+/+ (S+H−, groups 5 and 6), and GalT−/−Cr2+/+ → GalT−/−Cr2−/− (S−/H+, groups 7 and 8) radiation chimeras (where S denotes the Cr2 gene status of the nonhematopoietic stromal compartment and H denotes the Cr2 gene status of the hematopoietic compartment) was tested by ELISA assay 10 weeks after secondary conditioning. Significant titers of anti-αGal IgM were detected in conditioned S+/H+ and S+/H− GalT−/− mice but were undetectable in conditioned S−/H− and S−/H+ GalT−/− mice. *Statistically significant differences. There were 4 mice in group 1, 6 in group 2, 5 in group 3, 4 in group 4, 3 in group 5, 3 in group 6, 3 in group 7, and 3 in group 8. Data shown are mean ± SD.
Anti-αGal IgM levels inGalT−/−Cr2−/−/GalT−/− radiation chimeras that received nonmyeloablative conditioning with or without BMT from αGal+ BALB/c mice 8 weeks later. Serum after secondary nonmyeloablative conditioning of control and mixed chimeric (BALB/c secondary BMT donors) GalT−/−Cr2+/+ → GalT−/−Cr2+/+ (S+H+, groups 1 and 2), GalT−/−Cr2−/− → GalT−/−Cr2−/− (S−/H−, groups 3 and 4), GalT−/−Cr2−/− → GalT−/−Cr2+/+ (S+H−, groups 5 and 6), and GalT−/−Cr2+/+ → GalT−/−Cr2−/− (S−/H+, groups 7 and 8) radiation chimeras (where S denotes the Cr2 gene status of the nonhematopoietic stromal compartment and H denotes the Cr2 gene status of the hematopoietic compartment) was tested by ELISA assay 10 weeks after secondary conditioning. Significant titers of anti-αGal IgM were detected in conditioned S+/H+ and S+/H− GalT−/− mice but were undetectable in conditioned S−/H− and S−/H+ GalT−/− mice. *Statistically significant differences. There were 4 mice in group 1, 6 in group 2, 5 in group 3, 4 in group 4, 3 in group 5, 3 in group 6, 3 in group 7, and 3 in group 8. Data shown are mean ± SD.
In contrast to anti-αGal, total IgM levels did not appear to be influenced by the presence or absence of CR1/CR2 on stromal cells. In addition, the induction of mixed chimerism from allogeneic αGal+ BALB/c donors did not influence total IgM levels (data not shown). Similarly, no effect of hematopoietic or nonhematopoietic cell CR1/CR2 expression on total IgM levels could be discerned upon analysis of sera obtained 6 weeks following lethal TBI and reconstitution, a time point when significant anti-αGal levels were not yet detectable in any group of reconstituted mice. Likewise, no differences in total IgG levels were detected between the groups (data not shown). These results are consistent with reports in which Cr2−/− mice demonstrated similar levels of serum IgM13,18 and IgG13 compared with Cr2+/+ mice. However, reductions in total IgG of certain isotypes have been detected in Cr2−/− mice.18,35
Expression of CR2 on stromal cells promotes anti-αGal Ab–producing B-cell tolerance via mixed chimerism
Anti-αGal levels were also examined 10 weeks after allogeneic BMT in sera of mixed chimeras (using αGal+ allogeneic donors) prepared simultaneously with the conditioned controls in Figure 3. Chimeric GalT−/− mice with CR1/CR2 expression on hematopoietic and nonhematopoietic tissues (S+/H+) showed marked reductions in anti-Gal IgM levels compared with conditioned controls (Figure 3, group 1 versus 2). Importantly, mixed chimeras with CR1/CR2 expression only on nonhematopoietic cells (S+/H−) also showed marked reductions in the level of anti-Gal compared with similarly reconstituted nonchimeric conditioned controls (group 5 versus 6). Thus, CR1/CR2 expression on nonhematopoietic cells was sufficient to ensure normal baseline anti-αGal levels and marked reductions in anti-αGal IgM levels via induction of mixed chimerism from αGal+ donors. Because baseline anti-αGal IgM levels were very low in conditioned control mice lacking CR1/CR2 expression on nonhematopoietic cells (S−/H−, group 3; S−/H+, group 7), the effect of mixed chimerism on these levels could not be assessed. Chimerism did not influence total IgM or IgG levels in any group (data not shown). Anti-αGal IgG was not observed in any group of mice prior to sensitization.
Ten weeks after αGal+ BALB/c BMT, recipient animals were sensitized by intraperitoneal injection with RRBCs. Serum from recipient animals was examined for anti-αGal titers by ELISA assay 8 days after sensitization (Figure 4A,D). Because serum Ab levels may be affected by absorption by αGal+ cells, we also performed ELISpot assays 8 days after sensitization with RRBCs to directly assess tolerance. After RRBC immunization, splenocytes produce anti-αGal without further stimulation, whereas PerC cells require in vitro stimulation to become antibody-secreting cells.9 Thus, splenocytes were directly assessed for anti-αGal–producing cells (Figure 4B), whereas PerC cells were stimulated with LPS for 8 days prior to ELISpot assay (Figure 4C).
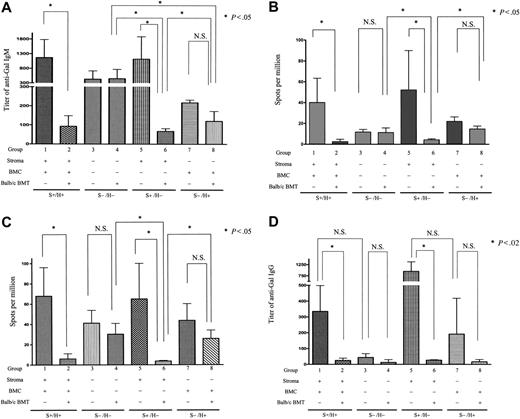
Requirement for nonhematopoietic CR1/CR2 for maximal anti-Gal responses and for suppression of anti-αGal Ab production via mixed chimerism induction. (A,D) ELISA assays. Serum after secondary nonmyeloablative conditioning of control and mixed chimeric (BALB/c secondary BMT donors) GalT−/−Cr2+/+ → GalT−/−Cr2+/+ (S+H+, groups 1 and 2), GalT−/−Cr2−/− → GalT−/−Cr2−/− (S−/H−, groups 3 and 4), GalT−/−Cr2−/− → GalT−/−Cr2+/+ (S+H−, groups 5 and 6), and GalT−/−Cr2+/+ → GalT−/−Cr2−/− (S−/H+, groups 7 and 8) radiation chimeras (where S denotes the Cr2 gene status of the nonhematopoietic stromal compartment and H denotes the Cr2 gene status of the hematopoietic compartment) was tested 8 days after sensitization with RRBCs for anti-αGal IgM (A) and IgG (D). Increased titers of anti-αGal IgM and IgG were detected following immunization in conditioned S+/H+ and S+/H− mice as well as conditioned S−/H− and S−/H+ mice. Levels of anti-αGal IgM Ab were significantly reduced in S+/H+ and S+/H− allogeneic BMT recipients compared with conditioned controls but not in S−/H− and S−/H+ recipients. *Statistically significant differences. There were 4 mice in group 1, 6 in group 2, 4 in group 3, 3 in group 4, 3 in group 5, 3 in group 6, 2 in group 7, and 3 in group 8. Data shown are mean ± SD. (B-C) ELISpot assays. Spleen cells (B) and PerC cells (C) from secondary conditioned and αGal+ BALB/c BMT GalT−/−Cr2−/−/GalT−/− radiation chimeras were tested by ELISpot assay 8 days after RRBC sensitization. PerC cells were cultured with LPS for 8 days before assessment in ELISpot assay. Anti-Gal Ab–producing cells were detected among splenocytes from conditioned control but not αGal+ BMT S+/H+ and S+/H− recipient mice. Anti-Gal Ab–producing B cells were also detected in conditioned S−/H− and S−/H+ recipient mice, but their numbers were not reduced by the presence of αGal+ mixed chimerism in S−/H− and S−/H+ recipient mice. *Statistically significant differences. The spot numbers obtained from BSA-coated plates were subtracted from those obtained from Gal-BSA–coated plates. The average frequencies against BSA were 10.1 ± 6.2, 0.1 ± 0.3, 2.3 ± 1.7, 1.8 ± 1.1, 6.2 ± 6.1, 0.3 ± 0.5, 6.2 ± 2.4, 4.2 ± 0.3, and (C) 16.7 ± 6.4, 1.0 ± 1.3, 12.7 ± 5.1, 9.0 ± 4.3, 17.5 ± 6.5, 0.3 ± 0.5, 10.1 ± 2.0, 9.8 ± 2.0 spots per million in groups 1 to 8, respectively. There were 5 mice in group 1, 8 in group 2, 4 in group 3, 5 in group 4, 3 in group 5, 3 in group 6, 2 in group 7, and 2 in group 8. Data shown are mean ± SD.
Requirement for nonhematopoietic CR1/CR2 for maximal anti-Gal responses and for suppression of anti-αGal Ab production via mixed chimerism induction. (A,D) ELISA assays. Serum after secondary nonmyeloablative conditioning of control and mixed chimeric (BALB/c secondary BMT donors) GalT−/−Cr2+/+ → GalT−/−Cr2+/+ (S+H+, groups 1 and 2), GalT−/−Cr2−/− → GalT−/−Cr2−/− (S−/H−, groups 3 and 4), GalT−/−Cr2−/− → GalT−/−Cr2+/+ (S+H−, groups 5 and 6), and GalT−/−Cr2+/+ → GalT−/−Cr2−/− (S−/H+, groups 7 and 8) radiation chimeras (where S denotes the Cr2 gene status of the nonhematopoietic stromal compartment and H denotes the Cr2 gene status of the hematopoietic compartment) was tested 8 days after sensitization with RRBCs for anti-αGal IgM (A) and IgG (D). Increased titers of anti-αGal IgM and IgG were detected following immunization in conditioned S+/H+ and S+/H− mice as well as conditioned S−/H− and S−/H+ mice. Levels of anti-αGal IgM Ab were significantly reduced in S+/H+ and S+/H− allogeneic BMT recipients compared with conditioned controls but not in S−/H− and S−/H+ recipients. *Statistically significant differences. There were 4 mice in group 1, 6 in group 2, 4 in group 3, 3 in group 4, 3 in group 5, 3 in group 6, 2 in group 7, and 3 in group 8. Data shown are mean ± SD. (B-C) ELISpot assays. Spleen cells (B) and PerC cells (C) from secondary conditioned and αGal+ BALB/c BMT GalT−/−Cr2−/−/GalT−/− radiation chimeras were tested by ELISpot assay 8 days after RRBC sensitization. PerC cells were cultured with LPS for 8 days before assessment in ELISpot assay. Anti-Gal Ab–producing cells were detected among splenocytes from conditioned control but not αGal+ BMT S+/H+ and S+/H− recipient mice. Anti-Gal Ab–producing B cells were also detected in conditioned S−/H− and S−/H+ recipient mice, but their numbers were not reduced by the presence of αGal+ mixed chimerism in S−/H− and S−/H+ recipient mice. *Statistically significant differences. The spot numbers obtained from BSA-coated plates were subtracted from those obtained from Gal-BSA–coated plates. The average frequencies against BSA were 10.1 ± 6.2, 0.1 ± 0.3, 2.3 ± 1.7, 1.8 ± 1.1, 6.2 ± 6.1, 0.3 ± 0.5, 6.2 ± 2.4, 4.2 ± 0.3, and (C) 16.7 ± 6.4, 1.0 ± 1.3, 12.7 ± 5.1, 9.0 ± 4.3, 17.5 ± 6.5, 0.3 ± 0.5, 10.1 ± 2.0, 9.8 ± 2.0 spots per million in groups 1 to 8, respectively. There were 5 mice in group 1, 8 in group 2, 4 in group 3, 5 in group 4, 3 in group 5, 3 in group 6, 2 in group 7, and 2 in group 8. Data shown are mean ± SD.
Following immunization, increased titers of anti-αGal IgM were detected in S+/H+ and S+/H− conditioned controls (Figure 4A, groups 1 and 5). In ELISpot assays, anti-αGal Ab–producing cells were detected in spleens and PerCs of S+/H+ and S+/H− conditioned control mice (Figure 4B-C, groups 1 and 5). Anti-αGal titers were also increased in S−/H− and S−/H+ conditioned control mice (Figure 4A, groups 3 and 7) compared with baseline (Figure 3), but these titers were much lower than those in S+/H+ or S+/H− conditioned mice (P > .05 comparing group 1 versus 5, group 3 versus 7; P < .02 comparing group 1 versus 3, group 1 versus 7, group 3 versus 5, and group 5 versus 7). Consistently, compared with S+/H+ and S+/H− conditioned control mice (groups 1 and 5), reduced numbers of anti-αGal Ab–producing cells were detected in spleens and PerCs of conditioned control S−/H+ and S−/H− mice (Figure 4B-C, groups 7 and 3). Therefore, anti-αGal responses do not require CR1/CR2 but are maximized by the presence of CR1/CR2 on nonhematopoietic cells.
In groups with mixed chimerism induced by αGal+ BMT, anti-αGal IgM levels were significantly reduced in S+/H+ and S+/H− recipients compared with controls (Figure 4A, group 1 versus 2, group 5 versus 6). Moreover, the numbers of anti-αGal Ab–producing cells were dramatically decreased in spleens and PerCs of mixed chimeric S+/H+ and S+/H− mice receiving αGal+ BMT compared with their conditioned controls (Figure 4B-C, group 1 versus 2, group 5 versus 6), demonstrating tolerance induction via mixed chimerism. In contrast, anti-αGal IgM titers, as well as splenic and PerC anti-Gal–producing cell numbers, were not significantly reduced in S−/H− and S−/H+ mice rendered mixed chimeric with αGal+ BMT compared with conditioned controls (group 3 versus 4, group 7 versus 8; P > .05) (Figure 4A-C). Together, these data indicate that CR1/CR2 expression on nonhematopoietic cells is sufficient to permit tolerization of the anti-αGal response by induction of mixed chimerism. Moreover, the failure of S−/H− and S−/H+ mice receiving αGal+ CR1/CR2+ BMT to reduce anti-Gal production indicates that recipient B-cell CR1/CR2 plus donor marrow-derived CR1/CR2 (combined with Gal expression) is insufficient to promote tolerance induction of recipient anti-Gal–producing cells.
Class-switched anti-αGal responses are reduced in the absence of stromal CR1/CR2
Anti-αGal IgG was detected in sera of S+/H+ and S+/H− conditioned control mice after RRBC sensitization (Figure 4D, groups 1 and 5). In S−/H− conditioned control mice, in contrast, anti-αGal IgG was not observed after sensitization (group 3), consistent with data in GalT−/−Cr2−/− mice. In S−/H+ conditioned mice, a variable increase in anti-αGal IgG titers was observed following immunization (group 7). These data indicate that CR1/CR2 expression on nonhematopoietic cells optimizes the induction of a class-switched Ig response to this carbohydrate antigen and that CR1/CR2 expression on hematopoietic cells may have variable capacity to promote such responses.
When αGal+ BMT recipients were compared with the relevant conditioned controls for anti-αGal IgG levels, marked reductions in anti-αGal IgG titers were observed in chimeric S+/H+ and S+/H− mice (Figure 4D, group 1 versus 2, group 5 versus 6). Chimeric S−/H+ mice also showed an absence of anti-αGal IgG, but the high variability in anti-αGal IgG levels in conditioned controls did not permit demonstration of statistically significant differences between the groups. Because S−/H− conditioned controls did not produce significant anti-αGal IgG titers (Figure 4D, group 3), the results do not necessarily implicate CR1/CR2 expression in “tolerizing” the anti-αGal IgG response in mixed chimeras.
Together, our results demonstrate that CR1/ CR2 expression on stromal cells promotes both optimal Ab responses and the induction of B-cell tolerance toward the αGal carbohydrate antigen via induction of mixed chimerism.
Discussion
Both pre-existing and newly developing NAb-producing B cells in GalT−/− mice can be tolerized via mixed chimerism induction using αGal+ donors.4-6 This observation is of considerable importance for the field of xenotransplantation, in which anti-αGal NAbs have presented a major obstacle due to their ability to cause hyperacute and delayed vascular rejection.36 Studies in a nonmyeloablative rat-to-mouse BMT model suggest that B cells producing NAbs against other specificities are also tolerized by induction of mixed chimerism,37 which might be important in the context of xenotransplantation from the recently developed GalT−/− pigs.38-40 We now demonstrate a role for nonhematopoietic cell CR1/CR2 expression in the B-cell tolerance achieved with this approach. Our data implicate a novel pathway involving NAb binding to Gal antigens provided by the donor marrow, complement fixation, and pickup via complement receptors leading to presentation in a tolerogenic fashion by FDCs to Gal-reactive B-1b cells. Furthermore, our data implicate immune complexes and complement receptors on FDCs in maintaining baseline natural anti-Gal Ab levels.
In mice, CR1 and CR2 are splice variants encoded in one locus on chromosome 1, Cr2.12,18 Therefore, Cr2−/− mice have defects in the expression of both CR1 and CR2. On macrophages and FDCs, both CR1 and CR2 promote immune complex transport, phagocytosis, and antigen presentation to B cells.41 Ab responses to T-dependent and T-independent antigens are abrogated by blocking both CR1 and CR2 in vivo,42,43 consistent with studies using Cr2−/− mice.18 Blocking of CR1 alone did not impede Ab responses, suggesting that CR2 is more important than CR1 in B-cell maturation. Additionally, CR1 has a negative regulatory function on human B cells.44
CR2 coupled with CR1 or CD19 on the B-cell surface plays a role in activation signaling of B cells.13,23 B-cell CR1/CR2 signaling also induces Blimp-1 and XBP-1, thereby promoting plasma cell differentiation, and up-regulates Bcl2, promoting plasma cell survival.45 Our studies, which address the role of these receptors on B-1 cells in an antigen-specific response, suggest that neither CR1 nor CR2 on B-1b cells plays a critical role in the baseline or induced IgM or IgG anti-αGal response. These data extend previous studies showing a role for CR1/CR2 in anti-αGal responses.46 We have previously demonstrated that the spleen is the major site of anti-αGal IgM Ab production, which is mediated by B-1b cells that migrate from the PerC in association with loss of Mac1 expression.8 Consistent with the lack of a role for B-cell intrinsic CR2 in anti-Gal Ab production, anti-Gal–producing B-1b cells express only low levels of CD21 (CR2).9
FDCs trap immune complexes via CR1/CR2 receptors, enabling germinal center formation and B-cell maturation.23,47 CR1/CR2 expression on nonhematopoietic cells is required for both the generation and the maintenance of B-cell memory.48,49 While B-1a cells have been shown to localize to B-cell follicles and interact with FDCs and to promote FDC development in neonatal mice,50 complement receptors and FDCs have not previously been implicated in B-1b–dependent Ab responses. Such a pathway is suggested by our observation that expression of CR1/CR2 on nonhematopoietic cells supports normal anti-αGal IgM NAb levels. The role of stromal CR1/2 in inducing these NAb is not readily understood in light of existing data on the origin of B-1b cells.
One hypothesis regarding the origin of B-1 cells posits that these cells arise from B-2 cells that are activated under conditions that mimic T-independent–2-type activation (ie, B-cell–receptor [BCR] cross-linking in the absence of T-cell help or CD40 ligation).51 Antigen presentation by FDCs might play a role in this differentiation process by using CR1/CR2 to trap immune complexes. Indeed, CR1/CR2-deficient mice have reduced numbers of B-1a cells but not B-1b cells,18 a result that we have confirmed in GalT−/−Cr2−/− mice. Alterations in the IgM NAb repertoire have been suggested in Cr2−/− mice.52,53 However, we observed similar frequencies of cells with anti-αGal receptors in GalT−/−Cr2−/− compared with GalT−/− mice, and the same B-1b–cell population appeared to be responsible for anti-αGal production in both groups. The relationship between B-1a and B-1b cells has not been well defined.
Although there is some controversy regarding NAb regulation by T cells,54 we have observed that baseline anti-αGal NAb levels are increased by depletion of T cells.55 In contrast, the induced anti-αGal IgM response is largely T-cell dependent.54,56 Both baseline and induced anti-αGal levels were enhanced by nonhematopoietic CR1/CR2 expression, suggesting a role for FDC CR1/CR2 expression in both T-independent and T-dependent Ab responses.
Total serum IgM levels were unaffected by the presence or absence of CR1/CR2 on hematopoietic and/or nonhematopoietic cells in our model. Our previous studies showing that anti-αGal– and total IgM-producing cells belong to the same B-cell subset9 led to the expectation that total IgM levels would be reduced in mice lacking nonhematopoietic cell CR1/CR2 expression. However, other cell populations (eg, marginal zone cells) might compensate for the inability of B-1b cells to produce NAb in this setting. Further studies are required to address this possibility.
Nonhematopoeitic cell CR1/CR2 expression promoted robust class-switched anti-αGal IgG responses in the absence of hematopoietic cell CR1/CR2 expression, indicating that B-cell CR1/CR2 expression is not required for this response. These data are consistent with the observation that FDC CR1/CR2 expression is necessary for the generation of strong antigen-specific IgG responses but not for germinal center formation.5,49,57 The absence of CR1/CR2 coreceptor signaling on B cells has also been reported to reduce Ab responses to T-dependent antigens.58 Although we did not observe any increase in anti-αGal IgG responses when hematopoietic cells also expressed CR1/CR2 compared with those in mice with CR1/CR2 expressed only on nonhematopoietic cells, a more detailed kinetic analysis might reveal a role for B-cell CR1/CR2 expression in the maintenance of these IgG responses.
Our present studies indicate that CR1/CR2 plays a critical role in the tolerization of the B-1b subset that produces IgM anti-αGal NAbs and mediates induced IgM responses. Moreover, our studies demonstrate that expression of CR1/CR2 on nonhematopoietic cells is critical for the tolerization of these cells. CR1/CR2 is expressed by FDCs but is not known to be expressed on bone marrow stromal cells.59,60 Thus, encounter of B-1b cells, following migration from the PerC, with FDCs in splenic B-cell follicles may be implicated in this tolerance pathway. This, to our knowledge, is the first study to implicate expression of CR1/CR2 on FDCs in B-cell tolerance.
Deficiencies in the classical pathway of complement activation and CR1/CR2 have been associated with autoimmune disease,24,27,61 and CR2 expression is reduced in patients with SLE.62 Murine data show a role for Cr2 (CR1/CR2) and C4 in preventing B-cell–dependent autoimmune disease.25,26 While B-1 cells have been implicated in autoantibody production,28,30 the autoantibodies identified in the murine lupus model were mainly of the IgG class, suggesting they may have undergone T-cell–dependent class switching. Because B-1 cells produce IgM and not IgG NAbs,9 B-1 cells were not particularly implicated by these results.
In transgenic models, encounter of immature B cells with soluble antigen expressed at relatively low levels has been reported to tolerize B cells via anergy, whereas encounter with membrane-bound antigen induced deletion.63 Mature follicular B cells undergo nondeletional tolerance involving Ig receptor down-modulation in response to soluble self-antigen.64,65 Interaction with membrane-bound self-antigen can activate anergic B cells, breaking tolerance.66 Antigen expressed only on cell membranes in the periphery has, in other instances, been associated with tolerance and deletion of B cells.63 Little is known about the mechanisms of tolerization of B-1 cells, particularly of the CD5−B-1b subset, which is the major cell population of IgM NAb-producing cells in mice.9 High-density membrane-bound antigens introduced into the PerC have been reported to lead to B-1–cell deletion,67 but FDCs or other stromal CR1/CR2-expressing populations have not previously been implicated. Our studies have implicated anergy in the initial phase of B-1 tolerance induction via mixed chimerism induction, presumably affecting pre-existing αGal-reactive B cells. In contrast, the later tolerance mechanisms seem to involve deletion and/or receptor editing, probably affecting tolerance of B cells developing from the marrow in the presence of αGal+ cells following BMT.68
In summary, we have shown a major role for CR1/CR2 expression on nonhematopoietic cells for the tolerization of anti-αGal NAb–producing B-1b cells via induction of mixed chimerism. These results have important implications for xenotransplantation and provide novel insights into the mechanisms of tolerance of B-1b cells. Given that B-1 cells also produce autoantibodies,28,30 the results obtained in our mixed chimeric GalT−/− mouse model also have important implications for the mechanisms of self-tolerance.
Authorship
Contribution: I.S. designed, performed, analyzed, and interpreted experiments and wrote the paper; T.K. and P.D.B. designed, performed, analyzed, and interpreted experiments and contributed to the paper; F.H. designed, performed, analyzed, and interpreted experiments; M.C.C. provided CR2 knockout mice, interpreted results, and contributed to the paper; and M.S. designed experiments, interpreted results, wrote the paper, and oversaw all aspects of the work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
F.H. and P.D.B. contributed equally to this study.
Correspondence: Megan Sykes, Bone Marrow Transplantation Section, Transplantation Biology Research Center, Massachusetts General Hospital, MGH East, Bldg 149-5102 13th St, Boston, MA; e-mail: megan.sykes@tbrc.mgh.harvard.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant POI HL 018646.
We thank Dr Shiv Pillai for helpful review of the manuscript, Mr Orlando Moreno for technical assistance and expert animal husbandry, and Ms Luisa Raleza for expert assistance with the manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal