Abstract
We assessed cardiovascular disease (CVD) incidence in 1474 survivors of Hodgkin lymphoma (HL) younger than 41 years at treatment (1965-1995). Multivariable Cox regression and competing risk analyses were used to quantify treatment effects on CVD risk. After a median follow-up of 18.7 years, risks of myocardial infarction (MI) and congestive heart failure (CHF) were strongly increased compared with the general population (standardized incidence ratios [SIRs] = 3.6 and 4.9, respectively), resulting in 35.7 excess cases of MI and 25.6 excess cases of CHF per 10 000 patients/year. SIRs of all CVDs combined remained increased for at least 25 years and were more strongly elevated in younger patients. Mediastinal radiotherapy significantly increased the risks of MI, angina pectoris, CHF, and valvular disorders (2- to 7-fold). Anthracyclines significantly added to the elevated risks of CHF and valvular disorders from mediastinal RT (hazard ratios [HRs] were 2.81 and 2.10, respectively). The 25-year cumulative incidence of CHF after mediastinal radiotherapy and anthracyclines in competing risk analyses was 7.9%. In conclusion, risks of several CVDs are 3- to 5-fold increased in survivors of HL compared with the general population, even after prolonged follow-up, leading to increasing absolute excess risks over time. Anthracyclines further increase the elevated risks of CHF and valvular disorders from mediastinal radiotherapy.
Introduction
Over the past decades, survival of patients treated for Hodgkin lymphoma (HL) has improved dramatically, as a result of the development of multiagent chemotherapy (CT), more accurate radiotherapy (RT), and enhanced possibilities to reduce treatment complications.1 Unfortunately, the improved prognosis of HL has been accompanied by long-term toxicity, such as elevated risks of second primary malignancies,2–9 cardiovascular disease (CVD),2,3,8–10 and infections.2,8,9 Increased mortality of cardiac disease after mediastinal radiotherapy for HL has been reported in several studies.2,3,8–10 Dose-dependent anthracycline-induced cardiotoxicity has been observed in survivors of malignancies other than HL, who were usually treated with higher anthracycline doses.11,12 It is not known, therefore, whether anthracyclines add to the increased risk of CVD from mediastinal RT for survivors of HL. This is an important clinical question because most patients with HL now receive anthracycline-containing chemotherapy. Although a few studies reported on nonfatal cardiac events, comparisons with the general population were usually not made, because in most countries CVD incidence rates are not available.13–18 The purpose of our study was to assess the long-term risk of various CVDs in a cohort of 1474 five-year survivors of HL treated between 1965 and 1995.
Unique features of this study include long and near complete follow-up and the availability of complete treatment data, including radiation fields and chemotherapeutic agents. In addition, we compared the incidence of various CVDs with population-based reference rates from the general population, we accounted for competing risk of death from any cause, and we incorporated cardiac risk factors in the analyses.
Patients and methods
Data collection procedures
We included all 5-year survivors of HL diagnosed before age 41 years (n = 1486) from our late-effects HL cohort comprising 2689 patients with HL as the first malignancy.6,9,19 Patients were treated between 1965 and 1995 and identified through the hospital-based cancer registries of The Netherlands Cancer Institute, Amsterdam, or the Erasmus MC–Daniel den Hoed Cancer Center, Rotterdam. Patient selection and methods of data collection have been described in detail previously.6,9,19 Data were collected on date of birth, date of HL diagnosis, histology, clinical stage, cytostatic agents in primary and salvage treatment, radiation fields in primary and salvage treatment, dates and treatments of relapses, dates of diagnoses of cardiovascular events, cardiovascular risk factors at HL diagnosis and at end of follow-up, date of most recent medical information or date of death, vital status, and cause of death. Smoking was scored positive when the patient was smoking at the end of follow-up or had stopped smoking less than 1 year before the end of follow-up. Hypertension, hypercholesterolemia, and diabetes mellitus were scored positive when stated in the medical information or when treated. Data were collected directly from the medical records, through general practitioners (GPs) and attending physicians. Questionnaires on specific cardiovascular diagnoses and risk factors were sent to the patients' GPs and/or the patients' last known attending physicians in case the information could not be obtained from the medical record. When there was ambiguous information on CVDs, additional information was requested from the patient's cardiologist (n = 43). Patients were not routinely screened for CVDs. For patients who died from acute CVD, without prior evidence of preceding CVDs, date of death was recorded as date of diagnosis of CVD and cause of death as CVD diagnosis.
Twelve patients were excluded from the original cohort, because medical records could not be obtained and no information on CVD was received from the GP, leaving 1474 patients for analysis.
Treatment
Patients were usually treated in or according to EORTC trials.20 The distribution of radiotherapy fields is given in Table 1 and Figure 1, based on individual treatment data. Radiotherapy techniques have changed over the years. In the 1960s, patients were treated with cobalt-60 or orthovoltage therapy; from the 1970s onward, linear accelerators were used (usually 8 MV photons). Individual blocks were used to shield normal tissues as much as possible. Shielding of the distal part of the mediastinum was sometimes performed from the late 1980s onward in case there was no spread of disease below the aortic notch. The vast majority of mediastinally irradiated patients (n = 1241) has received a classical mediastinal field, including a relatively large part of the coronary arteries and the heart muscle. In addition, most patients were treated with one field per day only. The procedure of using 2 fields per day was gradually introduced in the late 1980s. Patients usually received 40 Gy in fractions of 2.0 Gy when they were treated with RT only and 30 to 36 Gy in fractions of (1.5-)2.0 Gy when they also received chemotherapy. Detailed information on radiation doses and fractionation schedules for individual patients was not collected.
Patient characteristics
| Patient characteristics . | HL patients, no. (%) . |
|---|---|
| All patients | 1474 (100) |
| Sex | |
| Male | 790 (53.6) |
| Female | 684 (46.4) |
| Age at start of treatment | |
| No older than 20 ya | 314 (21.3) |
| 21-25 y | 363 (24.6) |
| 26-30 y | 296 (20.1) |
| 31-35 y | 266 (18.0) |
| Older than 35 y | 235 (15.9) |
| Attained age at end of follow-up | |
| No older than 35 y | 244 (16.6) |
| 36-40 y | 214 (14.5) |
| 41-45 y | 221 (15.0) |
| 46-50 y | 247 (16.8) |
| 51-55 y | 252 (17.1) |
| Older than 55 years | 296 (20.1) |
| Treatment period | |
| Before 1974 | 416 (28.2) |
| 1974-1982 | 477 (32.4) |
| 1983-1995 | 581 (39.4) |
| Treatmentb | |
| RT only | 406 (27.5) |
| CT only | 71 (4.8) |
| RT + CT, anthracylines | 435 (29.5) |
| RT + CT, no anthracylines | 559 (37.9) |
| Unknown | 3 (0.2) |
| Radiotherapyc | |
| RT mediastinumd | 1241 (84.2) |
| RT PAO or inverted Y with spleene | 410 (27.8) |
| RT PAO or inverted Y without spleenf | 280 (19.0) |
| Type of chemotherapy | |
| MOPPg | 255 (23.9) |
| ABVDg | 38 (3.6) |
| MOPP/ABVg | 189 (17.7) |
| Other combined CTh | 496 (46.6) |
| Unknown | 87 (8.2) |
| Vital status at date of last contact | |
| Alive | 1017 (69.0) |
| Dead | 457 (31.0) |
| Follow-up interval | |
| 6-10 y | 197 (13.4) |
| 11-15 y | 322 (21.8) |
| 16-20 y | 292 (19.8) |
| 21-25 y | 268 (18.2) |
| 26-30 y | 200 (13.6) |
| More than 30 y | 195 (13.2) |
| Risk factorsi | |
| Smokingj | |
| Recent | 253 (17.2) |
| Ever | 675 (45.8) |
| Never | 541 (36.7) |
| Unknown | 258 (17.5) |
| Hypertension | |
| Yes | 147 (10.0) |
| No | 1292 (87.7) |
| Unknown | 35 (2.4) |
| Diabetes mellitus | |
| Yes | 73 (5.0) |
| No | 1381 (93.7) |
| Unknown | 20 (1.4) |
| Hypercholesterolemia | |
| Yes | 126 (8.5) |
| No | 1316 (89.3) |
| Unknown | 32 (2.2) |
| Patient characteristics . | HL patients, no. (%) . |
|---|---|
| All patients | 1474 (100) |
| Sex | |
| Male | 790 (53.6) |
| Female | 684 (46.4) |
| Age at start of treatment | |
| No older than 20 ya | 314 (21.3) |
| 21-25 y | 363 (24.6) |
| 26-30 y | 296 (20.1) |
| 31-35 y | 266 (18.0) |
| Older than 35 y | 235 (15.9) |
| Attained age at end of follow-up | |
| No older than 35 y | 244 (16.6) |
| 36-40 y | 214 (14.5) |
| 41-45 y | 221 (15.0) |
| 46-50 y | 247 (16.8) |
| 51-55 y | 252 (17.1) |
| Older than 55 years | 296 (20.1) |
| Treatment period | |
| Before 1974 | 416 (28.2) |
| 1974-1982 | 477 (32.4) |
| 1983-1995 | 581 (39.4) |
| Treatmentb | |
| RT only | 406 (27.5) |
| CT only | 71 (4.8) |
| RT + CT, anthracylines | 435 (29.5) |
| RT + CT, no anthracylines | 559 (37.9) |
| Unknown | 3 (0.2) |
| Radiotherapyc | |
| RT mediastinumd | 1241 (84.2) |
| RT PAO or inverted Y with spleene | 410 (27.8) |
| RT PAO or inverted Y without spleenf | 280 (19.0) |
| Type of chemotherapy | |
| MOPPg | 255 (23.9) |
| ABVDg | 38 (3.6) |
| MOPP/ABVg | 189 (17.7) |
| Other combined CTh | 496 (46.6) |
| Unknown | 87 (8.2) |
| Vital status at date of last contact | |
| Alive | 1017 (69.0) |
| Dead | 457 (31.0) |
| Follow-up interval | |
| 6-10 y | 197 (13.4) |
| 11-15 y | 322 (21.8) |
| 16-20 y | 292 (19.8) |
| 21-25 y | 268 (18.2) |
| 26-30 y | 200 (13.6) |
| More than 30 y | 195 (13.2) |
| Risk factorsi | |
| Smokingj | |
| Recent | 253 (17.2) |
| Ever | 675 (45.8) |
| Never | 541 (36.7) |
| Unknown | 258 (17.5) |
| Hypertension | |
| Yes | 147 (10.0) |
| No | 1292 (87.7) |
| Unknown | 35 (2.4) |
| Diabetes mellitus | |
| Yes | 73 (5.0) |
| No | 1381 (93.7) |
| Unknown | 20 (1.4) |
| Hypercholesterolemia | |
| Yes | 126 (8.5) |
| No | 1316 (89.3) |
| Unknown | 32 (2.2) |
HL indicates Hodgkin lymphoma; RT, radiotherapy; CT, chemotherapy; PAO, para-aortic lymph nodes; inverted Y, para-aortic and iliac lymph nodes; MOPP, mechlorethamine, vincristine, procarbazine, prednisone; ABVD, doxorubicin, bleomycin, vinblastine, and dacarbazine; MOPP/ABV, mechlorethamine, vincristine, procarbazine, prednisone/doxorubicin, bleomycin, vinblastine; EBVP, epirubicine, bleomycin, vinblastine, prednisone.
Patients (n = 111) were 16 years or younger at HL diagnosis.
Included primary and salvage therapies. RT includes all radiotherapy fields, not only mediastinal radiotherapy.
Describing the most frequently used radiation fields.
1114 during primary treatment.
Patients (n = 372) received RT to the mediastinum, para-aortic lymph nodes (or inverted Y), and spleen.
Patients (n = 240) received RT to both the mediastinum and the para-aortic lymph nodes (or inverted Y) without RT to the spleen.
No other cytostatic drugs.
Among those combinations, including MOPP (n = 167), MOPP/ABV (n = 51), ABVD (n = 73), and EBVP (n = 43).
Data from oncology records and general practitioners, not from screening on cardiovascular risk factors.
No mutually exclusive categories.
Flow chart of applied radiation fields. RT indicates radiotherapy; PAO, para-aortic; med, mediastinal; *Med RT, supradiaphragmatic radiotherapy including mediastinal radiotherapy; inverted Y, para-aortic and iliac nodes. Overall, 21% of the patients received radiotherapy including iliac nodes.
Flow chart of applied radiation fields. RT indicates radiotherapy; PAO, para-aortic; med, mediastinal; *Med RT, supradiaphragmatic radiotherapy including mediastinal radiotherapy; inverted Y, para-aortic and iliac nodes. Overall, 21% of the patients received radiotherapy including iliac nodes.
From the 1960s to the 1980s chemotherapy consisted mainly of MOPP (mechlorethamine, vincristine, procarbazine, prednisone). In the 1980s, anthracycline-containing regimens such as MOPP/ABV (mechlorethamine, vincristine, procarbazine, prednisone/doxorubicin, bleomycin, vinblastine) and ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) were introduced as a part of the primary treatment (Table 1).
Statistical analysis
The incidence of CVDs in the study population was compared with the Netherlands population, using age-, sex-, and calendar period–specific incidence rates for the period from 1972 through 2000 from the Continuous Morbidity Registration Nijmegen (CMRN) from several Dutch GP practices.21 Comparison of recent incidence rates of coronary heart disease (CHD), acute myocardial infarction (MI), and angina pectoris (AP) from the CMRN with incidence rates of several new registries in the Netherlands showed that incidence rates of the CMRN were similar to the mean of all registries combined, indicating that the CMRN is representative of the Netherlands.22 Data on the incidence of MI, AP, and congestive heart failure (CHF) were registered by the CMRN, allowing for multiple separate diagnoses per person, but only the first of a given diagnosis was recorded.23,24
Because we included only 5-year survivors, time at risk started 5 years from HL diagnosis and ended at date of diagnosis of a specific cardiovascular event, date of emigration, date of death, or date of most recent medical information, whichever came first. When analyzing one specific cardiovascular diagnosis, observed numbers were based on all first events of this given diagnosis occurring at least 5 years after HL diagnosis, because the expected numbers of events were recorded correspondingly. Patients who were diagnosed with a specific cardiovascular event before HL diagnosis or within 5 years after HL diagnosis were excluded. In the analyses on CHD, comprising MI and AP, we excluded patients who were diagnosed with MI or AP before HL diagnosis or within 5 years after HL diagnosis. Two different approaches were used to assess risks for this combined diagnostic group. First, an analysis was performed in which both MI and AP were counted corresponding with the reference rates, which were also based on events rather than persons. Because different cardiovascular diagnoses may be more strongly correlated among our patients than in the general population, we also performed an analysis in which patients with both MI and AP contributed only one event to the observed numbers, with person-time at risk ending at the date of diagnosis of the first event, yielding conservative risk estimates.
For only 6.7% of patients, information on CVDs was not complete until at least July 1, 2002, date of death, or date of emigration. These patients contributed person-time and cardiovascular events until the date of most recent CVD information in the analyses. The standardized incidence ratios (SIRs) of the observed (O) and expected (E) numbers of MI, AP, CHF, and combined diagnostic groups in the study population were determined and the confidence intervals of the SIRs were calculated using exact Poisson probabilities of O numbers.25 In addition, observed cumulative incidences of MI and AP in the study population were compared with expected cumulative incidences in their peers in the general population. P values for tests of heterogeneity and tests for trend were calculated according to standard methods.26 Absolute excess risk (AER) was calculated as the observed number of CVDs in our cohort minus the number expected, divided by number of person-years at risk, multiplied by 10 000. The attributable risk (AR) was calculated as SIR minus 1 divided by SIR, multiplied by 100. Attained age was defined as the age of patients during follow-up and was calculated to assess at what ages patients experienced increased risk compared with their peers in the general population. Overall cardiovascular and diagnosis-specific risks were estimated using the Kaplan-Meier method.27 Additionally, cumulative incidences of cardiovascular diseases were calculated with death from any cause as competing risk using S-plus statistical software (Insightful, Seattle, WA), including user-written functions.28 Multivariable Cox regression analysis was performed to quantify the effects of different treatments on CVD risk within the patient group, adjusting for confounders. Cox models were fitted using SPSS statistical software (SPSS, Chicago, IL).
Results
General
Patient characteristics and distribution of risk factors for CVDs are described in Table 1. Twenty-eight percent of patients received RT only, 5% received CT alone, 38% received RT and CT not containing anthracyclines, and 29% received RT and CT including anthracyclines. Overall, 84% of patients received radiotherapy including the mediastinum. The median age at start of treatment was 25.7 years; median follow-up time was 18.7 years for the whole cohort (a total of 28 669 person-years) and 20.1 years for the 1017 patients alive at the end of follow-up. Thirty percent of the patients recently smoked cigarettes and 10% were diagnosed with hypertension.
Cardiovascular disease risk
We observed 619 CVDs at least 5 years from HL diagnosis in 354 of 1474 five-year survivors; 157 patients developed multiple CVDs (Table 2)Valvular disorders, AP and MI were the most common CVDs with 160, 134, and 102 events, respectively. The median time between start of treatment for HL and the diagnosis of CVDs was almost 19 years (Table 2).
Standardized incidence ratios of specific cardiac diseases in 5-year survivors of Hodgkin lymphoma
| Diagnosis . | ICD-9 codes . | O . | E . | SIR . | 95% CI . | AER* . | Median interval, y (range) . |
|---|---|---|---|---|---|---|---|
| Combined diagnostic group | |||||||
| Coronary heart disease† | 410, 413 | 233 | 57.7 | 4.0 | 3.5-4.6 | 87.0 | 20.2 (5.0-37.2) |
| Coronary heart disease‡ | 410, 413 | 182 | 57.7 | 3.2 | 2.7-3.7 | 61.7 | 20.2 (5.0-37.2) |
| Specific heart diseases | |||||||
| Acute myocardial infarction | 410 | 102§ | 28.5 | 3.6 | 2.9-4.4 | 35.7 | 19.5 (7.0-37.5) |
| Angina pectoris | 413 | 134‖ | 32.4 | 4.1 | 3.5-4.9 | 49.6 | 20.7 (5.1-37.2) |
| Congestive heart failure | 428 | 52¶ | 10.7 | 4.9 | 3.6-6.4 | 25.6 | 18.5 (5.0-39.0) |
| Pericarditis# | 420, 423 | 23 | — | — | — | — | 13.6 (6.4-31.1) |
| Valvular disorders# | 424 | 160 | — | — | — | — | 23.3 (5.0-37.8) |
| Cardiomyopathy# | 425 | 33 | — | — | — | — | 18.0 (5.8-34.0) |
| Dysrhythmia# | 427 | 89 | — | — | — | — | 18.3 (5.2-39.6) |
| All heart diseases# | 410-429 | 619** | — | — | — | — | 18.8 (5.0-39.6) |
| Diagnosis . | ICD-9 codes . | O . | E . | SIR . | 95% CI . | AER* . | Median interval, y (range) . |
|---|---|---|---|---|---|---|---|
| Combined diagnostic group | |||||||
| Coronary heart disease† | 410, 413 | 233 | 57.7 | 4.0 | 3.5-4.6 | 87.0 | 20.2 (5.0-37.2) |
| Coronary heart disease‡ | 410, 413 | 182 | 57.7 | 3.2 | 2.7-3.7 | 61.7 | 20.2 (5.0-37.2) |
| Specific heart diseases | |||||||
| Acute myocardial infarction | 410 | 102§ | 28.5 | 3.6 | 2.9-4.4 | 35.7 | 19.5 (7.0-37.5) |
| Angina pectoris | 413 | 134‖ | 32.4 | 4.1 | 3.5-4.9 | 49.6 | 20.7 (5.1-37.2) |
| Congestive heart failure | 428 | 52¶ | 10.7 | 4.9 | 3.6-6.4 | 25.6 | 18.5 (5.0-39.0) |
| Pericarditis# | 420, 423 | 23 | — | — | — | — | 13.6 (6.4-31.1) |
| Valvular disorders# | 424 | 160 | — | — | — | — | 23.3 (5.0-37.8) |
| Cardiomyopathy# | 425 | 33 | — | — | — | — | 18.0 (5.8-34.0) |
| Dysrhythmia# | 427 | 89 | — | — | — | — | 18.3 (5.2-39.6) |
| All heart diseases# | 410-429 | 619** | — | — | — | — | 18.8 (5.0-39.6) |
ICD-9 indicates International Classification of Diseases, 9th revision; CVD, cardiovascular disease; O, observed number of specific CVDs; E, expected number of specific CVDs; AER, absolute excess risk; —, not available; and NA, not applicable.
Per 10 000 person-years. The absolute incidence rate/10 000 can be calculated with the following formula: AER + AER/(SIR − 1) × 10 000.
Acute myocardial infarction and angina pectoris combined allowing both diagnoses per person; 51 patients had both diagnoses.
Acute myocardial infarction and angina pectoris combined allowing only 1 event per person.
Includes 84 men and 18 women.
Includes 86 men and 48 women.
Congestive heart failure was observed in 59 patients but because reference rates are available from 1986, analyses were based on person-years and events since 1986; 7 patients with CHF before 1986 are excluded from this analysis.
No reference rates available.
In addition to the CVDs mentioned in the table: endocarditis (n = 6), cardiac aneurysm (n = 2) and sudden cardiac death (n = 11). Among patients with more than one CD frequently observed combinations were: CHF with valvular disorders (n = 29), CHF with AP (n = 22), CHF with MI (n = 14), CHF with cardiomyopathy (n = 10), dysrhythmia with MI (n = 14), dysrhythmia with AP (n = 31) and dysrhythmia with valvular disorders (n = 43). Among men in total, 356 cardiac events were observed consisting of MI (23.6%), AP (24.2%), valvular disorders (18.6%), dysrhythmia (12.9%), CHF (7.9%), cardiomyopathy (5.9%), pericarditis (3.4%), endocarditis (<1%), and cardiac aneurysm (<1%). Among women in total, 263 cardiac events were observed consisting of MI (6.8%), AP (18.3%), valvular disorders (35.7%), dysrhythmia (16.3%), CHF (11.8%), cardiomyopathy (4.6%), and pericarditis (4.2%).
The overall SIR for CHD was 4.0 (95% CI, 3.5-4.6) when all multiple events per patient were included (n = 233); and 3.2 (95% CI, 2.7-3.7) when only the first event was included (n = 182). SIRs were significantly elevated for MI (SIR = 3.6), AP (SIR = 4.1), and CHF (SIR = 4.9), resulting in 35.7 excess cases of MI, 49.6 excess cases of AP, and 25.6 excess cases of CHF per 10 000 person-years (Table 2). We observed significantly higher cumulative incidences of MI and AP in the study population compared with the general population (Figure 2).
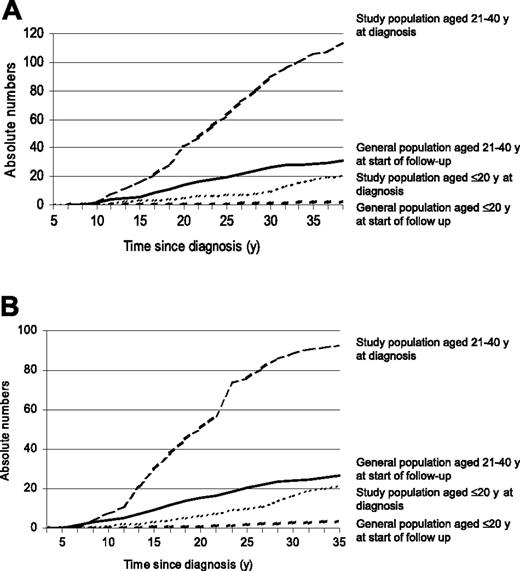
Observed and expected incidences of AP and MI by age among study population and peers from the general population. Observed and expected incidences of AP (A) and MI (B).
Observed and expected incidences of AP and MI by age among study population and peers from the general population. Observed and expected incidences of AP (A) and MI (B).
The SIR for MI was significantly elevated beginning 10 years after treatment and remained increased with longer follow-up duration (Table 3)The AERs, however, increased with longer follow-up duration, because of the increasing incidence of CVD with age. After a follow-up of 25 years or more, 7 excess cases of MI were observed per 1000 person-years (Table 3). The SIRs of AP and CHF were more strongly elevated in patients treated for HL before the age of 20 years (Table 3). The decreasing trend of SIRs for CHF with older attained age was significant (P < .001).
Risk of important cardiovascular diseases by sex, age at start of treatment, attained age, treatment, and follow-up interval
| . | Person-y† . | Myocardial infarction . | Angina pectoris . | Congestive heart failure* . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O . | SIR . | 95% CI . | AER‡ . | O . | SIR . | 95% CI . | AER‡ . | O . | SIR . | 95% CI . | AER‡ . | ||
| Sex | |||||||||||||
| Men | 10 528 | 84 | 4.2 | 3.4-5.2 | 60.7 | 86 | 3.7 | 3.0-4.6 | 59.4 | 25 | 3.9 | 2.5-5.7 | 21.7 |
| Women | 10 034 | 18 | 2.1 | 1.2-3.3 | 9.4 | 48 | 5.2 | 3.8-6.9 | 39.1 | 27 | 6.4 | 4.2-9.4 | 29.8 |
| Age at start of treatment | |||||||||||||
| No older than 20 y | 4 892 | 9 | 5.4 | 2.4-10.3 | 15.0 | 20 | 11.6 | 7.0-17.9 | 37.2 | 11 | 18.2 | 9.0-32.6 | 27.6 |
| 21-25 y | 5 227 | 25 | 5.9 | 3.8-8.7 | 39.7 | 30 | 6.2 | 4.2-8.8 | 48.1 | 11 | 7.0 | 3.5-12.5 | 23.1 |
| 26-30 y | 4 111 | 22 | 4.1 | 2.6-6.2 | 40.4 | 30 | 4.8 | 3.2-6.9 | 58.6 | 13 | 6.7 | 3.6-11.5 | 34.7 |
| 31-35 y | 3 746 | 24 | 2.7 | 1.8-4.1 | 40.7 | 26 | 2.6 | 1.7-3.9 | 43.5 | 8 | 2.5 | 1.1-4.9 | 16.0 |
| 36-40 y | 2 630 | 22 | 2.6 | 1.6-4.0 | 51.8 | 28 | 2.9 | 1.9-4.2 | 70.2 | 9 | 2.7 | 1.2-5.1 | 26.4 |
| Attained age§ | |||||||||||||
| Younger than 40 y | 10 353 | 13 | 4.4 | 2.3-7.5 | 9.7 | 17 | 6.0 | 3.5-9.6 | 13.7 | 9 | 26.0 | 11.8-49.5 | 12.6 |
| 40-49 y | 6 974 | 44 | 4.0 | 2.9-5.4 | 47.4 | 51 | 3.8 | 2.8-5.0 | 53.8 | 19 | 5.1 | 3.1-8.1 | 25.8 |
| At least 50 y | 3 278 | 45 | 3.1 | 2.3-4.2 | 93.0 | 66 | 4.1 | 3.2-5.2 | 159.4 | 24 | 3.9 | 2.5-5.8 | 55.9 |
| Treatment‖ | |||||||||||||
| Initial RT only | 6 551 | 36 | 3.9 | 2.7-5.4 | 49.9 | 54 | 5.2 | 3.9-6.7 | 66.8 | 18 | 4.8 | 2.8-7.6 | 27.1 |
| RT + CT, no anthracyclines | 8 994 | 50 | 3.9 | 2.9-5.1 | 66.0 | 57 | 3.8 | 2.9-5.0 | 47.0 | 25 | 5.3 | 3.4-7.8 | 31.8 |
| RT + CT, anthracyclines | 3 933 | 14 | 3.5 | 1.9-5.9 | 23.6 | 20 | 4.5 | 2.7-6.9 | 39.9 | 9 | 6.2 | 2.8-11.8 | 21.2 |
| Initial CT only | 1 083 | 2 | 1.0 | 0.1-3.5 | 7.4 | 2 | 0.8 | 0.1-2.9 | −4.6 | 0 | 0.0 | 0.0-5.2 | −8.2 |
| Anthracycline-containing chemotherapy | |||||||||||||
| No | 9 870 | 52 | 3.5 | 2.6-4.6 | 37.7 | 60 | 3.5 | 2.7-4.5 | 43.3 | 25 | 4.6 | 3.0-6.8 | 27.5 |
| Yes | 4 141 | 14 | 3.3 | 1.8-5.5 | 23.5 | 20 | 4.2 | 2.6-6.5 | 37.1 | 9 | 5.8 | 2.6-11.1 | 19.8 |
| Follow-up interval | |||||||||||||
| 5-9 y | 6 872 | 7 | 1.7 | 0.7-3.6 | 4.3 | 12 | 2.6 | 1.3-4.5 | 10.7 | 5 | 7.1 | 2.3-16.8 | 11.0 |
| 10-14 y | 5 457 | 24 | 4.4 | 2.8-6.5 | 33.9 | 21 | 3.3 | 2.0-5.1 | 26.8 | 5 | 3.4 | 1.1-7.9 | 8.7 |
| 15-19 y | 3 861 | 24 | 4.0 | 2.5-5.9 | 46.4 | 25 | 3.5 | 2.2-5.1 | 46.0 | 19 | 8.5 | 5.1-13.2 | 47.3 |
| 20-24 y | 2 443 | 26 | 4.7 | 3.1-7.0 | 84.0 | 30 | 4.6 | 3.1-6.5 | 96.7 | 6 | 2.4 | 0.9-5.2 | 13.7 |
| At least 25 y | 1 973 | 21 | 2.9 | 1.8-4.4 | 69.2 | 46 | 6.0 | 4.4-8.0 | 207.7 | 17 | 4.5 | 2.6-7.3 | 62.5 |
| . | Person-y† . | Myocardial infarction . | Angina pectoris . | Congestive heart failure* . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O . | SIR . | 95% CI . | AER‡ . | O . | SIR . | 95% CI . | AER‡ . | O . | SIR . | 95% CI . | AER‡ . | ||
| Sex | |||||||||||||
| Men | 10 528 | 84 | 4.2 | 3.4-5.2 | 60.7 | 86 | 3.7 | 3.0-4.6 | 59.4 | 25 | 3.9 | 2.5-5.7 | 21.7 |
| Women | 10 034 | 18 | 2.1 | 1.2-3.3 | 9.4 | 48 | 5.2 | 3.8-6.9 | 39.1 | 27 | 6.4 | 4.2-9.4 | 29.8 |
| Age at start of treatment | |||||||||||||
| No older than 20 y | 4 892 | 9 | 5.4 | 2.4-10.3 | 15.0 | 20 | 11.6 | 7.0-17.9 | 37.2 | 11 | 18.2 | 9.0-32.6 | 27.6 |
| 21-25 y | 5 227 | 25 | 5.9 | 3.8-8.7 | 39.7 | 30 | 6.2 | 4.2-8.8 | 48.1 | 11 | 7.0 | 3.5-12.5 | 23.1 |
| 26-30 y | 4 111 | 22 | 4.1 | 2.6-6.2 | 40.4 | 30 | 4.8 | 3.2-6.9 | 58.6 | 13 | 6.7 | 3.6-11.5 | 34.7 |
| 31-35 y | 3 746 | 24 | 2.7 | 1.8-4.1 | 40.7 | 26 | 2.6 | 1.7-3.9 | 43.5 | 8 | 2.5 | 1.1-4.9 | 16.0 |
| 36-40 y | 2 630 | 22 | 2.6 | 1.6-4.0 | 51.8 | 28 | 2.9 | 1.9-4.2 | 70.2 | 9 | 2.7 | 1.2-5.1 | 26.4 |
| Attained age§ | |||||||||||||
| Younger than 40 y | 10 353 | 13 | 4.4 | 2.3-7.5 | 9.7 | 17 | 6.0 | 3.5-9.6 | 13.7 | 9 | 26.0 | 11.8-49.5 | 12.6 |
| 40-49 y | 6 974 | 44 | 4.0 | 2.9-5.4 | 47.4 | 51 | 3.8 | 2.8-5.0 | 53.8 | 19 | 5.1 | 3.1-8.1 | 25.8 |
| At least 50 y | 3 278 | 45 | 3.1 | 2.3-4.2 | 93.0 | 66 | 4.1 | 3.2-5.2 | 159.4 | 24 | 3.9 | 2.5-5.8 | 55.9 |
| Treatment‖ | |||||||||||||
| Initial RT only | 6 551 | 36 | 3.9 | 2.7-5.4 | 49.9 | 54 | 5.2 | 3.9-6.7 | 66.8 | 18 | 4.8 | 2.8-7.6 | 27.1 |
| RT + CT, no anthracyclines | 8 994 | 50 | 3.9 | 2.9-5.1 | 66.0 | 57 | 3.8 | 2.9-5.0 | 47.0 | 25 | 5.3 | 3.4-7.8 | 31.8 |
| RT + CT, anthracyclines | 3 933 | 14 | 3.5 | 1.9-5.9 | 23.6 | 20 | 4.5 | 2.7-6.9 | 39.9 | 9 | 6.2 | 2.8-11.8 | 21.2 |
| Initial CT only | 1 083 | 2 | 1.0 | 0.1-3.5 | 7.4 | 2 | 0.8 | 0.1-2.9 | −4.6 | 0 | 0.0 | 0.0-5.2 | −8.2 |
| Anthracycline-containing chemotherapy | |||||||||||||
| No | 9 870 | 52 | 3.5 | 2.6-4.6 | 37.7 | 60 | 3.5 | 2.7-4.5 | 43.3 | 25 | 4.6 | 3.0-6.8 | 27.5 |
| Yes | 4 141 | 14 | 3.3 | 1.8-5.5 | 23.5 | 20 | 4.2 | 2.6-6.5 | 37.1 | 9 | 5.8 | 2.6-11.1 | 19.8 |
| Follow-up interval | |||||||||||||
| 5-9 y | 6 872 | 7 | 1.7 | 0.7-3.6 | 4.3 | 12 | 2.6 | 1.3-4.5 | 10.7 | 5 | 7.1 | 2.3-16.8 | 11.0 |
| 10-14 y | 5 457 | 24 | 4.4 | 2.8-6.5 | 33.9 | 21 | 3.3 | 2.0-5.1 | 26.8 | 5 | 3.4 | 1.1-7.9 | 8.7 |
| 15-19 y | 3 861 | 24 | 4.0 | 2.5-5.9 | 46.4 | 25 | 3.5 | 2.2-5.1 | 46.0 | 19 | 8.5 | 5.1-13.2 | 47.3 |
| 20-24 y | 2 443 | 26 | 4.7 | 3.1-7.0 | 84.0 | 30 | 4.6 | 3.1-6.5 | 96.7 | 6 | 2.4 | 0.9-5.2 | 13.7 |
| At least 25 y | 1 973 | 21 | 2.9 | 1.8-4.4 | 69.2 | 46 | 6.0 | 4.4-8.0 | 207.7 | 17 | 4.5 | 2.6-7.3 | 62.5 |
P values for trends were as follows: for myocardial infarction, P = .001 for age at start of treatment, P = .17 for attained age, and P = .65 for follow-up interval; for angina pectoris, P < .001 for age at start of treatment, P = .43 for attained age, and P = .001 for follow-up interval; and for congestive heart failure, P < .001 for age at start of treatment, P < .001 for attained age; and P = .29 for follow-up interval.
O indicates observed number of specific CVD; SIR, standardized incidence ratio; AER, absolute excess risk; RT, radiotherapy; CT, chemotherapy.
Because reference rates are available from 1986, analyses were based on person-years and events since 1986. For this calculation the numbers of person-years for the follow-up periods from 20 years after diagnosis were similar as in the other analyses but differed for the follow-up periods until 19 years after diagnosis. The numbers of person-years for follow-up periods until 19 years were 5 to 9 years: 3907 person-years; 10 to 14 years: 4044 person-years; 15 to 19 years: 3541 person-years; 7 patients with CHF before 1986 are excluded from this analysis.
Number of person-years for angina pectoris and congestive heart failure were similar to those for myocardial infarction.
Per 10 000 patients per year. The absolute incidence rate/10 000 can be calculated with the following formula: AER + AER/(SIR − 1) × 10 000.
Attained age was defined as the age of patients at diagnosis of a given cardiovascular event or at the end of follow-up and was calculated to assess at what ages patients experienced increased risk compared with their peers in the general population. Each patient contributed person-years to each consecutive attained-age category that the patient passed through during follow-up.
RT includes all irradiated patients (n = 1400); see flow chart for detailed information. Three patients with incomplete treatment data were excluded from the analyses about treatment effects.
There were no significant differences in SIRs of MI, AP, and CHF according to different treatment schedules (Table 3). The median follow-up after anthracycline-containing chemotherapy, however, was significantly lower (13.3 years) than after radiotherapy alone (22.2 years) or after radiotherapy in combination with chemotherapy not containing anthracyclines (21.9 years) (Table 1).
Comparisons within the study cohort
In the multivariable Cox model analyses, treatment variables were adjusted for age at diagnosis, CVD risk factors, and recent smoking. Mediastinal radiotherapy significantly increased the risks of valvular disorders, CHD and CHF (Table 4). In addition, the risks of CHF and valvular disorders significantly increased when mediastinal radiotherapy was combined with anthracycline-containing chemotherapy (HR = 2.81; 95% CI = 1.44-5.49, and HR = 2.10; 95% CI = 1.27-3.48, respectively; Table 4, model 2). Established cardiovascular risk factors, except hypertension, increased the risk of most CVDs but did not appear to interact with treatment effects (Table 4, model 1).
Multivariable Cox regression analysis of potential risk factors for cardiac diseases
| Risk factor . | MI . | AP . | CHF* . | Valvular disorders . |
|---|---|---|---|---|
| Model 1, no. of events | 102 | 129 | 82 | 159 |
| Treatment, HR (95% CI)† | ||||
| Mediastinal RT (yes vs no) | 2.42 (1.12-5.24) | 4.85 (1.97-11.9) | 7.37 (1.81-30.0) | 7.01 (2.59-18.9) |
| Anthracycline-containing CT (yes vs no) | 0.90 (0.50-1.62) | 1.49 (0.89-2.49) | 2.44 (1.37-4.33) | 2.24 (1.40-3.59) |
| Cardiovascular risk factors, HR (95% CI) | ||||
| Recent smoking (yes vs no/unknown) | 2.04 (1.29-3.23) | 1.35 (0.85-2.16) | 1.96 (1.16-3.30) | 1.23 (0.80-1.88) |
| Hypertension (yes vs no/unknown)‡ | 0.52 (0.29-0.94) | 0.90 (0.58-1.42) | 1.07 (0.59-1.94) | 1.28 (0.86-1.92) |
| Hypercholesterolemia (yes vs no/unknown)‡ | 4.12 (2.68-6.33) | 4.55 (3.10-6.68) | 1.48 (0.85-2.58) | 1.65 (1.11-2.44) |
| Diabetes mellitus (yes vs no/unknown)‡ | 1.44 (0.73-2.83) | 2.43 (1.45-4.09) | 4.45 (2.54-7.81) | 1.81 (1.07-3.04) |
| Model 2, no. of events | 95 | 124 | 80 | 155 |
| Treatment group, HR (95% CI)§ | ||||
| Mediastinal RT only‖ | 1.00 | 1.00 | 1.00 | 1.00 |
| Mediastinal RT + CT, no anthracyclines¶ | 1.17 (0.75-1.83) | 0.78 (0.53-1.15) | 1.33 (0.79-2.24) | 0.85 (0.60-1.21) |
| Mediastinal RT + CT, anthracyclines# | 1.00 (0.52-1.94) | 1.32 (0.76-2.30) | 2.81 (1.44-5.49) | 2.10 (1.27-3.48) |
| Risk factor . | MI . | AP . | CHF* . | Valvular disorders . |
|---|---|---|---|---|
| Model 1, no. of events | 102 | 129 | 82 | 159 |
| Treatment, HR (95% CI)† | ||||
| Mediastinal RT (yes vs no) | 2.42 (1.12-5.24) | 4.85 (1.97-11.9) | 7.37 (1.81-30.0) | 7.01 (2.59-18.9) |
| Anthracycline-containing CT (yes vs no) | 0.90 (0.50-1.62) | 1.49 (0.89-2.49) | 2.44 (1.37-4.33) | 2.24 (1.40-3.59) |
| Cardiovascular risk factors, HR (95% CI) | ||||
| Recent smoking (yes vs no/unknown) | 2.04 (1.29-3.23) | 1.35 (0.85-2.16) | 1.96 (1.16-3.30) | 1.23 (0.80-1.88) |
| Hypertension (yes vs no/unknown)‡ | 0.52 (0.29-0.94) | 0.90 (0.58-1.42) | 1.07 (0.59-1.94) | 1.28 (0.86-1.92) |
| Hypercholesterolemia (yes vs no/unknown)‡ | 4.12 (2.68-6.33) | 4.55 (3.10-6.68) | 1.48 (0.85-2.58) | 1.65 (1.11-2.44) |
| Diabetes mellitus (yes vs no/unknown)‡ | 1.44 (0.73-2.83) | 2.43 (1.45-4.09) | 4.45 (2.54-7.81) | 1.81 (1.07-3.04) |
| Model 2, no. of events | 95 | 124 | 80 | 155 |
| Treatment group, HR (95% CI)§ | ||||
| Mediastinal RT only‖ | 1.00 | 1.00 | 1.00 | 1.00 |
| Mediastinal RT + CT, no anthracyclines¶ | 1.17 (0.75-1.83) | 0.78 (0.53-1.15) | 1.33 (0.79-2.24) | 0.85 (0.60-1.21) |
| Mediastinal RT + CT, anthracyclines# | 1.00 (0.52-1.94) | 1.32 (0.76-2.30) | 2.81 (1.44-5.49) | 2.10 (1.27-3.48) |
Forward stepwise confounder selection, in which the effect of adding one confounder at a time was evaluated, was based on a more than 10% change in the risk estimate of the exposure variable of interest, irrespective of significance values. Cardiovascular risk factors (hypertension, hypercholesterolemia, and diabetes mellitus) were included as confounders in the model, although they did not change the risk estimate for the treatment variables with more than 10%, because these are established confounders in the literature and informative as a comparison with the estimates for the treatment variables. All data were adjusted for recent smoking (yes versus no), age at diagnosis (continuous), and cardiovascular risk factors (hypertension, hypercholesterolemia, and diabetes mellitus). No interaction was found between RT and CT, or between treatment and recent smoking (investigated for RT and CT separately).
MI indicate myocardial infarction; AP, angina pectoris; CHF, congestive heart failure; HR, hazard ratio; RT, radiotherapy; CT, chemotherapy.
Cardiomyopathy was included (n = 33, of whom 10 also had CHF).
Includes primary and salvage treatment.
Patients were not screened for cardiovascular risk factors; data have been obtained from medical records and general practitioners.
Patients who had not received mediastinal irradiation were excluded (n = 233).
Reference values.
Patients (n = 196) have been treated with the MOPP regimen only, 87 patients have been treated with the MOPP regimen and (an)other CT regimen(s).
Patients (n = 153) have been treated with MOPP/ABV regimen only, 43 patients have been treated with the MOPP regimen and (an)other CT regimen(s), and 34 patients have been treated with the ABVD regimen only and 58 patients have been treated with the ABVD regimen and (an)other CT regimen(s).
Of the 457 patients who died, the main causes of death were HL (n = 135), second primary malignancies (n = 137), and CVDs (n = 73; including 22 from MI). The overall 30-year cumulative incidence in mediastinally irradiated patients, using the competitive risk method, was 34.5% for any CVD, 12.9% for MI, and 19.7% for valvular disorders (Figure 3).
Cumulative incidences of various CVDs by treatment group, with death from any cause other than CVD as competing risk. (A) Cumulative incidence of all CVDs combined by treatment group with death from any cause as competing risk. Med RT indicates mediastinal RT; CT, chemotherapy; anthra, anthracyclines; MI, myocardial infarction; AP, angina pectoris; and CHF, congestive heart failure. †Thirteen patients were excluded from this analysis because they developed MI, AP, or CHF before or within 5 years after HL diagnosis. (B) Cumulative incidence of CHF and cardiomyopathy combined by treatment group with death from any cause as competing risk. Med RT, mediastinal RT; CT, chemotherapy; anthra, anthracyclines; CHF, congestive heart failure. *One patient was excluded from this analysis because CHF developed before or within 5 years after HL diagnosis. (C) Cumulative incidence of valvular disorders by treatment group, with death from any cause as competing risk. Med RT indicates mediastinal RT; CT, chemotherapy; anthra, anthracyclines. #Four patients were excluded from this analysis because they developed valvular disorders before or within 5 years after HL diagnosis.
Cumulative incidences of various CVDs by treatment group, with death from any cause other than CVD as competing risk. (A) Cumulative incidence of all CVDs combined by treatment group with death from any cause as competing risk. Med RT indicates mediastinal RT; CT, chemotherapy; anthra, anthracyclines; MI, myocardial infarction; AP, angina pectoris; and CHF, congestive heart failure. †Thirteen patients were excluded from this analysis because they developed MI, AP, or CHF before or within 5 years after HL diagnosis. (B) Cumulative incidence of CHF and cardiomyopathy combined by treatment group with death from any cause as competing risk. Med RT, mediastinal RT; CT, chemotherapy; anthra, anthracyclines; CHF, congestive heart failure. *One patient was excluded from this analysis because CHF developed before or within 5 years after HL diagnosis. (C) Cumulative incidence of valvular disorders by treatment group, with death from any cause as competing risk. Med RT indicates mediastinal RT; CT, chemotherapy; anthra, anthracyclines. #Four patients were excluded from this analysis because they developed valvular disorders before or within 5 years after HL diagnosis.
We compared actuarial risks of CHF or cardiomyopathy according to the Kaplan-Meier method with cumulative incidence with death from all causes as competing risk. The 25-year actuarial risks of CHF after mediastinal RT alone and mediastinal RT in combination with anthracycline-containing chemotherapy were 7.5% and 10.7%, respectively, whereas the cumulative incidences were lower (ie, 6.8% and 7.9%, respectively; Table 5)
Actuarial risk of CHF and cardiomyopathy combined in 5-year survivors of HL according to the Kaplan-Meier method versus cumulative incidence with death from any cause as competing risk
| Treatment group . | Time since diagnosis† . | |||||
|---|---|---|---|---|---|---|
| 10 y, % . | 15 y, % . | 20 y, % . | 25 y, % . | 30 y, % . | 35 y, % . | |
| Actuarial risk according to the Kaplan-Meier method* | ||||||
| No mediastinal RT | 0.4 | 0.4 | 0.4 | 0.4 | 2.5 | 2.5 |
| Mediastinal RT only | 0.0 | 0.6 | 4.5 | 7.5 | 13.9 | 13.9 |
| Mediastinal RT + CT, no anthracyclines | 0.7 | 1.7 | 3.5 | 6.5 | 11.3 | 24.8 |
| Mediastinal RT + anthracyclines | 1.5 | 2.8 | 10.7 | 10.7 | 29.8 | NA |
| All treatments | 0.7 | 1.5 | 4.4 | 6.7 | 12.2 | 20.0 |
| Cumulative incidence with death from any cause as competing risk* | ||||||
| No mediastinal RT | 0.4 | 0.4 | 0.4 | 0.4 | 2.0 | 2.0 |
| Mediastinal RT only | 0.0 | 0.6 | 4.2 | 6.8 | 11.7 | 11.7 |
| Mediastinal RT + CT, no anthracyclines | 0.6 | 1.5 | 2.8 | 4.9 | 7.6 | 14.3 |
| Mediastinal RT + anthracyclines | 1.4 | 2.5 | 7.9 | 7.9 | 15.7 | NA |
| All treatments | 0.6 | 1.3 | 3.7 | 5.4 | 8.9 | 13.1 |
| Treatment group . | Time since diagnosis† . | |||||
|---|---|---|---|---|---|---|
| 10 y, % . | 15 y, % . | 20 y, % . | 25 y, % . | 30 y, % . | 35 y, % . | |
| Actuarial risk according to the Kaplan-Meier method* | ||||||
| No mediastinal RT | 0.4 | 0.4 | 0.4 | 0.4 | 2.5 | 2.5 |
| Mediastinal RT only | 0.0 | 0.6 | 4.5 | 7.5 | 13.9 | 13.9 |
| Mediastinal RT + CT, no anthracyclines | 0.7 | 1.7 | 3.5 | 6.5 | 11.3 | 24.8 |
| Mediastinal RT + anthracyclines | 1.5 | 2.8 | 10.7 | 10.7 | 29.8 | NA |
| All treatments | 0.7 | 1.5 | 4.4 | 6.7 | 12.2 | 20.0 |
| Cumulative incidence with death from any cause as competing risk* | ||||||
| No mediastinal RT | 0.4 | 0.4 | 0.4 | 0.4 | 2.0 | 2.0 |
| Mediastinal RT only | 0.0 | 0.6 | 4.2 | 6.8 | 11.7 | 11.7 |
| Mediastinal RT + CT, no anthracyclines | 0.6 | 1.5 | 2.8 | 4.9 | 7.6 | 14.3 |
| Mediastinal RT + anthracyclines | 1.4 | 2.5 | 7.9 | 7.9 | 15.7 | NA |
| All treatments | 0.6 | 1.3 | 3.7 | 5.4 | 8.9 | 13.1 |
CHF indicates congestive heart failure; RT, radiotherapy; CT, chemotherapy; NA, not applicable because there were no patients at risk in this subgroup.
The median age at diagnosis was similar for the different treatment groups.
Because only patients who survived at least 5 years after HL diagnosis and patients without previous CVDs are included, the cumulative incidence at 5 years is 0% for all patients.
Discussion
In our population of 5-year survivors of HL, we observed 3- to 5-fold increased incidences of several CVDs as compared with the general population, even after a follow-up of more than 25 years. This suggests that 66% to 80% of all CVDs in our population were due to HL treatment. CHD contributed most to the CVD burden, with 62 excess cases per 10 000 persons per year. The stably increased SIRs over prolonged follow-up are concerning because they imply increasing AERs over time, as a result of the rising incidence of CVD with age. The importance of assessing cardiovascular morbidity rather than mortality is illustrated by the fact that MI was nonfatal in 78% of our patient population.
When evaluating specific treatment effects, we observed 2- to 7-fold increased risks of cardiotoxicity after irradiation, including part of the heart. Increased risks of death from radiation-associated CVD have been frequently described.8–10,29–36 Radiation-induced CVD includes a wide spectrum of pathologies.37,38 Damage of the vascular endothelium of arteries of different sizes is probably important in the explanation of radiation-induced heart disease.37,39,40 The cause of valvular fibrosis, however, is yet unknown.
Radiation-induced cardiotoxicity is usually observed 5 to 10 years after radiotherapy. Now the standard therapy for most patients with HL includes anthracycline-containing chemotherapy. Anthracycline-related toxicity may be observed at different intervals after therapy. Despite the relatively short median observation time of 13 years after anthracycline-containing therapy, we observed a 2-fold increased risk of CHF and valvular disorders, on top of the effect of mediastinal radiotherapy.
The 25-year cumulative incidence of CHF and cardiomyopathy combined was 7.9% after mediastinal radiotherapy and anthracycline-containing chemotherapy. The risks of CVDs may further increase with prolonged follow-up. Anthracycline-associated cardiotoxicity is caused by direct damage to the myoepithelium and strongly related to the cumulative dose.41,42 Although we did not record the cumulative dose of anthracyclines for individual patients, it is expected to be below 280 mg/m2 because treatment for HL in both study centers usually consisted of maximally 8 cycles of MOPP/ABV. With the current shift to anthracycline-containing chemotherapy in HL, the doxorubicin dose will vary between 200 and 400 mg/m2, possibly increasing the chemotherapy-related incidence of cardiotoxicity.
We observed higher SIRs of MI, AP, and CHF in patients treated at a young age, especially among those treated before age 20. In our publication on long-term mortality, largely concerning the same cohort as currently described, we demonstrated a 6-fold increased standardized mortality ratio from CVDs in patients with HL treated before age 41.9 Other investigators also found age at irradiation to be a major determinant of mortality from CVD.10,29,30 The higher risks in the patients treated at a younger age may partly be explained by low background incidence rates of CVDs. Furthermore, immature cardiovascular tissue may be more vulnerable to radiation and chemotherapy.
Possibilities for primary and secondary prevention of chemotherapy-associated cardiotoxicity have been examined, using, for instance, altered infusion schedules and drugs such as iron chelators (dexrazoxane).43 Dexrazoxane seems promising, but some investigators are concerned that it might diminish the efficacy of chemotherapy.44
The value of secondary prevention for CVDs is debatable. Subclinical cardiac damage has been shown in up to 57% of children or adults after treatment with anthracyclines and/or radiotherapy including part of the heart.11,38,45,46 However, identification of patients at high risk to develop clinically important cardiotoxicity is not possible yet.47,48 We showed that classical risk factors for cardiovascular diseases, except hypertension, increased the incidence of CVDs. Possibly hypertension did not increase CVD risk because patients with HL diagnosed with hypertension were adequately treated, whereas the reference group of patients without known hypertension may include undiagnosed hypertension. Patients with newly diagnosed HL and survivors of HL, especially when treated at young ages, should strongly be advised to refrain from smoking, to maintain a healthy body weight, and to exercise regularly. Furthermore, established risk factors for CVDs should be optimally treated in the patient population at increased risk of developing CVDs.49 Screening may be considered, because the patient population at risk usually has a considerable life expectancy,38,45 and these diagnostic procedures are noninvasive and relatively cheap. In the future, N-terminal pro–B-type natriuretic peptide (NT-proBNP) may also be used as a marker.50,51 The role of prevention, using for instance anticoagulants, ACE-inhibitors, and statins, remains to be determined.
Our study has important strengths. To the best of our knowledge, we are the first to compare the incidences of several heart diseases in a large group of patients with HL treated with anthracycline-containing chemotherapy with the general population. A Swiss group compared the incidence of fatal and nonfatal cardiac disease in their study population (a relatively small group of mediastinally irradiated patients with HL treated with or without chemotherapy) with the incidence in the US Framingham population. A significantly increased risk of ischemic heart disease was observed only in patients with established cardiovascular disease risk factors.14 Incidence rates of hospitalization for ischemic heart disease35 and of utilization of valve surgery, percutaneous interventions, and coronary bypass graft surgery among patients with HL,52 compared with general population rates, have been used as surrogate markers for CVD incidence. Furthermore, we achieved complete follow-up on both cardiac morbidity and mortality, whereas most studies assessed cardiac mortality only, thus underestimating the importance of CVD as a long-term complication of HL treatment. In addition, we calculated not only actuarial risks27 but also cumulative incidences with death from any cause as competing risk.28 The results show that the Kaplan-Meier actuarial risk method overestimates CVD risk, because it wrongly assumes that dead patients, had they lived longer, would have had the same CVD risk as surviving patients.28 Potential weaknesses of our study are the relatively short follow-up after anthracycline-containing chemotherapy, the lack of the possibility to study the effects of (anthracycline-containing) chemotherapy only, and the lack of more detailed information on chemotherapy and radiation doses.
During the study period, treatment strategies and prognosis for patients with HL have changed tremendously.20 In the current study, 84% of the patients received mediastinal radiation, whereas in the future only a minority of patients will need this. Approximately two thirds of patients with HL present with mediastinal localizations, but not all of them will need to be irradiated, and, if so, the target volume will be much more limited than before. Decline of the increased risk of death from CVDs other than MI has already been reported after partial shielding of the heart and a reduction of the total radiation dose to the mediastinum below 30 Gy.10 In addition, radiotherapy techniques have greatly improved, leading to more homogeneous dose distributions and therefore to a lower chance of toxicity.53 Partial shielding of the heart10 was applied in our cohort, depending on tumor localization. We could not examine possible dose-effect or dose-volume relations because details on radiation dose and volume were not collected.
In summary, patients with HL experience a strongly increased risk of various CVDs for a prolonged period after treatment. Mediastinal radiotherapy and anthracycline-containing chemotherapy importantly contribute to cardiac late effects. Especially in young survivors of HL at increased risk of CVD, physicians should consider appropriate risk-reducing strategies such as treatment of hypertension and hypercholesterolemia, and lifestyle advice such as refraining from smoking.
Authorship
Contribution: B.M.P.A., A.W.v.d.B.-D., and F.E.v.L. contributed to the design of the study. B.M.P.A., A.W.v.d.B.-D., M.L.D.B., A.A.M.H., W.J.K., and F.E.v.L. were involved with the data analysis and interpretation. M.A.K. and G.M.O. contributed to collection of data and administrative support. B.M.P.A., A.W.v.d.B.-D., M.L.D.B., M.B.v.V., M.H.A.B., J.P.d.B., A.A.M.H., W.J.K., H.B., and F.E.v.L. contributed to the writing of the report. All authors approved the final manuscript.
Conflict of interest disclosure: The authors declare no competing financial interests.
B.M.P.A. and A.W.v.d.B.-D. contributed equally to the study.
Correspondence: Flora E. van Leeuwen, Department of Epidemiology, The Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX Amsterdam, The Netherlands; e-mail: F.v.Leeuwen@nki.nl.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked advertisement in accordance with 18 USC section 1734.
Acknowledgments
We thank S. Grivell, J. Huisbrink, and L. D. Dorresteijn for collecting data. We thank E. H. van de Lisdonk from CMR-Nijmegen for supplying us with incidence rates. We are indebted to thousands of physicians from throughout the Netherlands who provided follow-up data for the study.
This work was supported by the Dutch Cancer Society, Amsterdam, The Netherlands (grants NKI 98-1833 and NKI 04-3068).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal