Abstract
New activities of human platelets continue to emerge. One unexpected response is new synthesis of proteins from previously transcribed RNAs in response to activating signals. We previously reported that activated human platelets synthesize B-cell lymphoma-3 (Bcl-3) under translational control by mammalian target of rapamycin (mTOR). Characterization of the ontogeny and distribution of the mTOR signaling pathway in CD34+ stem cell–derived megakaryocytes now demonstrates that they transfer this regulatory system to developing proplatelets. We also found that Bcl-3 is required for condensation of fibrin by activated platelets, demonstrating functional significance for mTOR-regulated synthesis of the protein. Inhibition of mTOR by rapamycin blocks clot retraction by human platelets. Platelets from wild-type mice synthesize Bcl-3 in response to activation, as do human platelets, and platelets from mice with targeted deletion of Bcl-3 have defective retraction of fibrin in platelet-fibrin clots mimicking treatment of human platelets with rapamycin. In contrast, overexpression of Bcl-3 in a surrogate cell line enhanced clot retraction. These studies identify new features of post-transcriptional gene regulation and signal-dependant protein synthesis in activated platelets that may contribute to thrombus and wound remodeling and suggest that posttranscriptional pathways are targets for molecular intervention in thrombotic disorders.
Introduction
Platelets are critical effector cells in hemostasis and inflammation in health and disease.1–3 Previously unrecognized responses of human platelets continue to emerge.1,2,4 One of the most unexpected is synthesis of new proteins from constitutively expressed but silenced messenger RNAs (mRNAs) by activated platelets.4–9 Activation-dependent synthesis of proteins is contrary to conventional expectations because platelets are anucleate. Nevertheless, early10,11 and more recent4,5,8,9,12–14 observations demonstrate that mature circulating human platelets have a significant complement of mRNAs generated by precursor megakaryocytes. Furthermore, platelets translate some of these constitutive transcripts in response to activating signals,9 providing a previously unrecognized mechanism to alter the platelet proteome.15 Activated human platelets also process pre-mRNAs to mature, translatable transcripts.4 The diversity of regulatory mechanisms in the platelet repertoire suggests that post-transcriptional gene expression is an important feature of their biology4–9 and is again contrary to previous dogma that they are functionally simple cells.
B-cell lymphoma-3 (Bcl-3), a member of the Ikβα family of factors,16,17 is an index example of signal-dependent synthesis of a specific protein by human platelets.5–7,9 Its translation from constitutively repressed mRNA, which is rapid and yields new protein within minutes, is triggered by cellular activation via thrombin receptors and is modulated by engagement of integrin αIIbβ3.5–7 Inhibition of synthesis of Bcl-3 by the therapeutic macrolide rapamycin provided evidence that its translation in activated platelets is controlled by mammalian target of rapamycin (mTOR).5 This conserved phosphatidylinositol kinase–related kinase is a key regulator of cell-cycle progression, growth, and nutrient balance.18–20 It was, however, not known to control expression of gene products in nonproliferating cells until its activity in stimulated platelets was identified.5 Initially, the functional significance of de novo synthesis of Bcl-3 by activated platelets was unclear, because it is a transcriptional regulator.17,21,22 A clue was its ability to participate in intracellular protein-protein interactions, suggesting that it influences platelet responses independent of its known activities in nucleated cells.5 Here, we show that Bcl-3 is required for retraction of fibrin by activated platelets. Clot retraction is thought to be important in thrombus consolidation, stabilization, and wound healing, although there are no standard in vivo models, and this process has largely been studied in vitro.23–25 We also characterize features of the ontogeny of the mTOR pathway in megakaryocytes and proplatelets that provide insights into its regulation of synthesis of Bcl-3 and a subset of other protein products in mature, activated platelets. These studies establish new facets of platelet and megakaryocyte biology and previously unrecognized activities of mTOR and Bcl-3. They also identify activities of rapamycin (sirolimus) that may be relevant to its clinical use in drug-impregnated, or “eluting,” intravascular stents and in other therapeutic settings.18,20,26–29
Materials and methods
Studies with human platelets
Platelet isolation.
Washed human platelets were isolated according to protocols that we have previously published in detail.5,30 All studies are approved by the University of Utah's Institutional Review Board (IRB). The cells were resuspended in medium 199 (M199) at 37°C for each experiment. Where indicated, the washed platelets were pretreated with rapamycin (10 nM) or vehicle (DMSO) for 30 minutes prior to start of each study. Rapamycin is highly specific for mTOR,18,20 and we chose a concentration that we have previously shown to be selective in human blood cells.5,31
Characterization of mTOR pathway components in platelets.
Antibodies against mTOR were used for Western analysis and immunocytochemistry using methods that we have described.5,8,30 In additional studies, platelets were pretreated with rapamycin or DMSO and activated with thrombin over a 1-hour time period. Cellular lysates were then separated by SDS–polyacrylamide gel electrophoresis, and examined by Western analysis for S6K1 (p70S6k). Similar assays were accomplished using antibodies against S6 and the mTOR pathway-regulated product, Bcl-3 (Santa Cruz Biotechnology, Santa Cruz, CA). Confocal images were visualized using an Olympus FV 300 IX81 confocal microscope (Olympus, Melville, NY) equipped with a 40×/0.95 numerical aperature (NA) dry or a 60×/1.42 NA oil objective for viewing megakaryocytes or platelets, respectively. No imaging medium or solutions were used. Colors of imaging reagents are indicated in the figure legends. An Olympus FVS-PSU/IX2-UCB camera and scanning unit and Olympus Fluoview FV 300 image acquisition software version 5.0 were used for recording. Adobe Photoshop CS version 8.0 was used for image processing.
Retraction of fibrin polymers in control and rapamycin-treated human platelets.
Retraction of fibrin polymers was conducted using minor modifications of a published method.32,33 Washed human platelets were resuspended in M199 at a final concentration of 1 × 109/mL. The cells were pretreated with 10 nM rapamycin or vehicle, DMSO, for 60 minutes. After this incubation period, 200 μg/mL exogenous fibrinogen labeled with Alexa 647 (Molecular Probes, Eugene, OR) in 2 mM CaCl2 was added to the cell suspensions followed by stimulation of the platelets with thrombin (1.0 U/mL). The Alexa 647–labeled fibrinogen enabled us to globally visualize the platelet-fibrin complex as well as examine fibrin networks microscopically (see “Results”). The cells were examined over 4 hours, at which time photomicrographs of the retracted clot were taken. The platelet-fibrin networks were subsequently placed in an equal volume of 4.0% paraformaldehyde (pH 7.35) and prepared for confocal microscopy. The cells were fixed for 30 minutes at room temperature, deposited onto vectabond-treated glass coverslips by cytocentrifugation, and examined by confocal microscopy (Bio-Rad, Hercules, CA). Imaging of fibrin using fluorescently labeled fibrinogen and of polymerized actin was conducted according to protocols that we have previously described in detail.8
Assays of integrin αIIbβ3 expression and function.
Platelets were pretreated with rapamycin or vehicle as described in “Platelet isolation,” and the conformational change in αIIbβ3 integrin was measured by flow cytometric analysis of binding of PAC-1 antibody.34 Platelet aggregation and adherence to immobilized fibrinogen were also performed as previously described.4,6
Studies of megakaryocytes differentiated from CD34± stem cells and proplatelet development
Umbilical cord blood samples from healthy full-term human newborns were obtained after review and approval by the University of Utah IRB. Hematopoietic progenitors were purified from the mononuclear interphase with magnetic beads that were directly conjugated to an antibody against CD34 (Miltenyi Biotec, Auburn, CA) as previously described.4 The CD34+ cells were resuspended in 2.5 mL X Vivo-20 culture medium (BioWhittaker, Walkersville, MD) that contained thrombopoietin, stem cell factor, and interleukin-3.4 The cell suspension was transferred to tissue-culture plates and incubated in suspension. The liquid cultures were maintained at 37°C in a humidified 5% CO2 atmosphere, and the cells were replenished with fresh growth factors and culture medium every third day. At day 13, the cells were transferred to culture vessels coated with immobilized human fibrinogen (Calbiochem-Novabiochem, San Diego, CA). The megakaryocytic progenitors then differentiated further to mature megakaryocytes that extended proplatelets over a 16-hour incubation period.4 Immunodetection of components of the mTOR pathway was accomplished using reagents detailed in studies of human platelets (“Characterization of mTOR pathway components in platelets”) and previously published approaches.4
Studies using transfected cell systems
Clot retraction in transfected cell models.
Nucleated cell clot retraction assays were performed essentially as described.35 Chinese hamster ovary (CHO) cells that stably overexpress αIIbβ336 were kindly provided by Paul Bray and were transfected with cDNA for Bcl-3 using methods similar to those that we have previously described.37 The cells were harvested by incubation with trypsin-EDTA, washed once with media containing 10% fetal calf serum, and washed twice with serum-free media. The cells were resuspended in serum-free media at 5 × 106/mL and mixed with 200 μg/mL labeled fibrinogen (see “Refraction of fibrin polymers in control and rapamycin-treated human platelets”). Aprotinin (10 μM) was added to the suspensions to inhibit fibrinolysis, and 1 U/mL thrombin was added to initiate cell-fibrin clot formation. Clot retraction was examined as described in “Refraction of fibrin polymers in control and rapamycin-treated human platelets”).
Adhesion to fibrinogen.
CHO cells that stably overexpress αIIbβ3 were transfected with cDNA for Bcl-3, and adhesion to immobilized fibrinogen was determined. In brief, fibrinogen (100 μg/mL) was immobilized on borosilicate chamber slides as we have previously described.4 CHO cells were incubated on the fibrinogen-coated surfaces for 30 minutes. The cells were fixed and examined by confocal microscopy following immunostaining with antibody against Bcl-3 and phalloidin to visualize actin polymerization.
Studies of platelets from mice deficient in Bcl-3 and control animals
Bcl-3–deficient and wild-type mice.
Bcl-3−/− C57B1/6 mice were generously provided by Dr Ulrich Siebenlist.38 These animals were previously characterized in detail and were shown to have no Bcl-3 expression.38 Homozygous−/− littermates were bred at the Utah Care Facility at the University of Utah. Between 10 and 12 weeks of age, the progeny were used for ex vivo platelet function studies as described in the next section. Age- and sex-matched control C57BL/6 mice, purchased from Jackson Laboratories (Bar Harbor, ME), were used in parallel for each study. Bcl-3 protein expression was determined by Western analysis in control and thrombin-activated platelets as previously described.5,6
Platelet isolation.
Mouse platelets were isolated using a minor modification of a published method.39 The animal protocol (98-09014) was approved by the Institutional Animal Care and Use Committee at the University of Utah. Blood was obtained by cardiac puncture under methoxyflurane anesthesia using a heparinized 25-gauge needle connected to a 3-mL syringe that contained 5 U heparin/mL blood. Whole blood from 6 to 8 mice was pooled for most studies. For flow cytometric studies and measurement of circulating fibrinogen, whole blood from one animal was used. For studies with isolated platelets, platelet-rich plasma (PRP) was prepared by centrifugation of the whole blood at 350g for 15 minutes. The PRP was removed and placed into a second tube for subsequent experimental manipulation. When washed platelets were required, 100 μM PGE1 was added to the PRP solution to prevent in vitro activation, and the cells were centrifuged at 500g for 15 minutes. The plasma layer was removed, and the cell pellet was placed in 10 mL pipes/saline/glucose (PSG) containing 100 μM PGE1 and centrifuged at 500g for 15 minutes. The PSG was then discarded, and the cell pellet was gently resuspended in 37°C M199 culture media. The platelets were counted with a Coulter counter (Coulter Electronics, Fullterton, CA), and cell numbers were normalized for each assay.
Retraction of fibrin polymers by murine platelets.
To determine the retraction of fibrin strands, PRP from wild-type and Bcl-3–deficient mice was obtained, the platelet number was determined, and the cell count in each sample was adjusted to 500 000/μL with platelet-poor plasma (PPP). Individual suspensions were placed in separate glass aggregometor tubes and stimulated with thrombin (1.0 U/mL) for 4 hours. After 4 hours, the platelet-fibrin complexes were placed in 2.5% glutaraldehyde, 1.0% paraformaldehyde, and 0.1 M Na Cacodylate (pH 7.35) and prepared for transmission electron microscopy.
Circulating platelet levels.
The number of circulating platelets per milliliter of whole blood was determined using a Coulter counter (Coulter Electronics). In brief, mice were anesthetized, blood was obtained, and, prior to any pooling, 10 μL anticoagulated blood was removed and placed in 10 mL Isoton solution (Coulter, Fullterton, CA). Platelet numbers were determined in triplicate.
Circulating fibrinogen levels.
For measurement of circulating fibrinogen levels, blood from 6 wild-type and 6 Bcl-3–deficient mice was collected in sodium citrate. Fibrinogen levels were measured by commercial determination (Antech Diagnostics, Farmingdale, NY) and reported in milligram per deciliter.
Flow cytometry.
Flow cytometry was conducted as described previously.40 In brief, heparinized blood was obtained as described in “Platelet isolation,” and 20 μL was mixed with 100 μL Tyrode buffer. The diluted cell suspensions were incubated with biotin-conjugated antibody directed against mouse P-selectin or control IgG (Pharmingen, San Diego, CA). The diluted cells were then incubated with GPRP (glycyl-l-prolyl-l-arginyl-l-proline; Calbiochem, San Diego, CA) prior to treatment with thrombin (0.5 U/mL) or its vehicle (Tyrode). After 15 minutes, the cells were incubated with a FITC-conjugated rat antimouse antibody directed against integrin αIIb (Pharmingen, San Diego, CA) and a phycoerythrin-streptavidin conjugate (Sigma, St Louis, MO). Following a 30-minute incubation period, the samples were fixed with paraformaldehyde for an additional 30 minutes at room temperature. They were subsequently diluted in Tyrode buffer and examined using a Becton Dickinson (Franklin Lakes, NJ) flow cytometer.
Platelet aggregation.
For platelet aggregation studies, PRP from wild-type and Bcl-3–deficient mice was obtained as described in “Platelet isolation.” PPP was used as a reference to establish 100% light transmission in a lumi-aggregometer (Sienco, Arvada, CO). The platelets in PRP suspension were counted, and the PRP was mixed with PPP to yield a final platelet concentration of 500 000 platelets/μL. PRP samples were subsequently stimulated with thrombin (0.5 U/mL), and aggregometry waves were recorded.
Tail bleeding time.
Tail bleeding time was measured in age-matched animals as described previously39 after amputating 0.5 cm of the tail tip when the animals were under general anesthesia (see “Platelet isolation”). Blood was blotted onto filter paper every 15 seconds until bleeding stopped.
Results
Megakaryocytes distribute mTOR to platelets
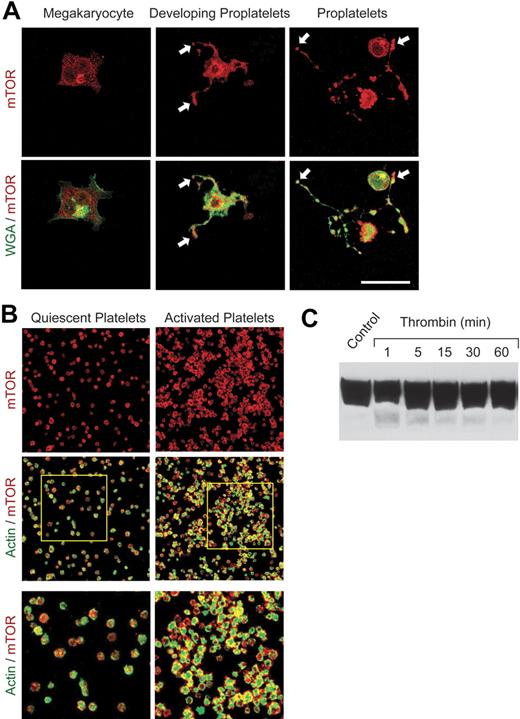
Previous studies demonstrated that rapamycin selectively inhibits signal-induced translation of a subset of proteins in activated human platelets, including Bcl-3.5 Because blockade of translation of specific mRNAs by rapamycin is mediated by its highly selective inhibition of mTOR,18,20 we examined the ontogeny and distribution of mTOR using a human hematopoietic stem cell model of megakaryocyte maturation4 (M.M.D., N.D.T., J.F., H.S., N. B. Chandler, Z. Wang, K.H.A., S. L. Perkins, A.S.W., manuscript submitted, January 2007) and freshly isolated primary blood platelets. The mTOR pathway has not previously been characterized in megakaryocytes and platelets. Under conditions in which CD34+ hematopoietic stem cells develop the megakaryocyte phenotype,4 mTOR is speckled throughout the cytoplasm and is also localized in the perinuclear region of the cell (Figure 1A). When the megakaryocytes are then induced to extend proplatelets, the specialized precursors of mature platelets,4,41 there is intense immunostaining of mTOR in proplatelet shafts and at their tips (Figure 1A). Components of platelets are vectorially transported through proplatelet extensions to the tips, which then separate to form mature platelets.41 mTOR is present in proplatelet tips both when proplatelets begin to form (Figure 1A, middle panels) and when they are fully extended (Figure 1A, right panels). Consistent with this pattern, mTOR is present in mature platelets freshly isolated from human blood when examined by immunocytochemical (Figure 1B) and Western analysis (Figure 1C). The amount of mTOR protein in resting unstimulated platelets is greater than that in freshly isolated unstimulated human monocytes and neutrophils (not shown), cells that also use mTOR to regulate signal-dependant gene expression.31,37,42 Activation of platelets with thrombin, which triggers translation and synthesis of Bcl-3 and additional proteins,5,6 did not obviously alter the levels of mTOR in aggregated cells over a 60-minute time period when examined by Western blotting (Figure 1C). This indicated that the expression level of mTOR itself does not change in response to activation in contrast to the new synthesis of the product that it regulates, Bcl-3.5 The pattern of mTOR staining indicated diffuse cytoplasmic distribution in resting platelets, which is also the topographic localization in proplatelet tips (Figure 1A-B). mTOR is then redistributed to areas subjacent to the plasma membrane when platelets are activated (Figure 1B, right panels). Redistribution of regulatory factors is a spatial mechanism of control of gene expression in activated platelets and other cell types.4,7 Of note, TOR1 is present in both cytoplasmic and submembranous sites in yeast (J.F. and G.A.Z., unpublished experiment, August 2005), the organism in which TOR signaling was first discovered.20
mTOR is expressed in CD34+ stem cell–derived megakaryocytes, proplatelets, and circulating human platelets. Megakaryocytes were cultured from CD34+ stem cells and induced to extend proplatelets as described.4 (A) mTOR was identified by immunostaining (red fluorescence) in megakaryocytes and megakaryocytes with proplatelet extensions. In parallel, the cells were labeled with wheat germ agglutinin (WGA; green immunofluorescence). Arrows indicate proplatelet tips, the sites of platelet budding, in developing and fully extended proplatelets (middle and right panels). Scale bar, 25 μm. (B) mTOR (red) and polymerized actin (green) were imaged in quiescent and activated, aggregated platelets. Immunostaining of mTOR was done with the same antibody as in panel A. Platelets were activated with thrombin for 30 minutes. The bottom panels are enlargements of the areas outlined in the 2 middle panels. Images in panels A and B are each representative of 5 independent experiments. (C) Western analysis of mTOR was done using lysates of freshly isolated human platelets under unstimulated conditions (control) and after activation with thrombin (0.5 U/mL) for the indicated times.
mTOR is expressed in CD34+ stem cell–derived megakaryocytes, proplatelets, and circulating human platelets. Megakaryocytes were cultured from CD34+ stem cells and induced to extend proplatelets as described.4 (A) mTOR was identified by immunostaining (red fluorescence) in megakaryocytes and megakaryocytes with proplatelet extensions. In parallel, the cells were labeled with wheat germ agglutinin (WGA; green immunofluorescence). Arrows indicate proplatelet tips, the sites of platelet budding, in developing and fully extended proplatelets (middle and right panels). Scale bar, 25 μm. (B) mTOR (red) and polymerized actin (green) were imaged in quiescent and activated, aggregated platelets. Immunostaining of mTOR was done with the same antibody as in panel A. Platelets were activated with thrombin for 30 minutes. The bottom panels are enlargements of the areas outlined in the 2 middle panels. Images in panels A and B are each representative of 5 independent experiments. (C) Western analysis of mTOR was done using lysates of freshly isolated human platelets under unstimulated conditions (control) and after activation with thrombin (0.5 U/mL) for the indicated times.
Downstream components of the mTOR pathway are localized to developing proplatelets, are phosphorylated in activated platelets, and are selectively inhibited by rapamycin
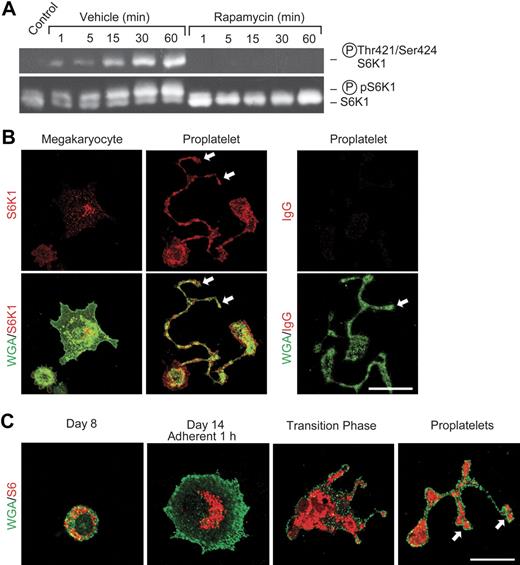
To further define the activities of mTOR in platelets, we examined S6K1, which is downstream of mTOR in its signaling cascade18–20,43 and has key roles in specialized translational control. S6K1 is present in mature human platelets (Figure 2A) as is its target, ribosomal protein S6.9 Consistent with this, S6K1 and S6 are distributed to proplatelet tips during megakaryocyte differentiation (Figure 2B-C). Activation of freshly isolated mature platelets with thrombin induced their aggregation (Figure 1B) and parallel phosphorylation of S6K1 that peaked at 30 minutes and was sustained for at least 60 minutes (Figure 2A). This is consistent with the time course of translation of Bcl-3 mRNA in thrombin-stimulated platelets, which is both rapidly initiated and sustained.5 Pretreatment of platelets with rapamycin blocked S6K1 phosphorylation (Figure 2A). In contrast, rapamycin did not inhibit phosphorylation of p38 map kinase (not shown), consistent with its specificity for mTOR in platelets and other cells.5,7,18,20 We previously found that rapamycin also selectively blocks phosphorylation of eukaryotic initiation factor 4E–binding protein (4E-BP1), a second downstream translation regulator in the mTOR pathway, in activated human platelets.5 Thus, the components of the regulatory cascade distal to mTOR18–20,43 are also present in developing megakaryocytes (Figure 2B-C) and mature platelets (Figure 2A), as is mTOR (Figure 1), and their phosphorylation status is modulated by inhibition of mTOR with rapamycin (Figure 2A) under conditions in which it inhibits synthesis of Bcl-3.5 Developmental activities of mTOR and its pathway members in megakaryocytes and proplatelets may contribute to thrombocytopenia as a complication of systemic rapamycin therapy.44
S6K1 and ribosomal protein S6 are distributed to proplatelets and S6K1 is activated in mature human platelets stimulated with thrombin. (A) Western analysis of total and phosphorylated S6K1 was done using lysates of washed human platelets that were pretreated with rapamycin (10 nM) or vehicle and subsequently stimulated with thrombin as in Figure 1. The bottom panel indicates mobility shift of S6K1 when phosphorylated in response to thrombin activation. The top panel illustrates results of incubations using an antibody that recognizes S6K1 phosphorylated on residues 421 and 424. (B) S6K1 is developmentally expressed in proplatelets and proplatelet tips. Megakaryocytes were cultured from CD34+ hematopoietic stem cells as in Figure 1. S6K1 is indicated by red fluorescence and is present in proplatelet tips (arrows). WGA localization is identified by green fluorescence. S6K1 was detected in proplatelet shafts and in proplatelet tips (middle panels, arrows) as proplatelets were extended during developmental transition (also see panel C). In the right panels, nonimmune IgG was substituted for anti-S6K1. (C) Ribosomal protein S6 is distributed to proplatelets and proplatelet tips in megakaryocyte development. S6 protein and WGA are indicated by red and green fluorescence, respectively. CD34+ hematopoietic stem cells were differentiated to the megakaryocytic stage.4 After 8 days in suspension culture S6 was diffusely distributed in the cytoplasm. One hour after adhesion to immobilized fibrinogen on day 14, S6 was centrally located in the perinuclear cytoplasm. During the transition phase, in which adherent megakaryocytic cells begin to form proplatelets, S6 was diffusely distributed in the central cytoplasm and in early proplatelet shafts. As proplatelets were extended, S6 was detected in early proplatelet tips (bottom right panel, arrows). (B-C) Scale bar, 20 μm.
S6K1 and ribosomal protein S6 are distributed to proplatelets and S6K1 is activated in mature human platelets stimulated with thrombin. (A) Western analysis of total and phosphorylated S6K1 was done using lysates of washed human platelets that were pretreated with rapamycin (10 nM) or vehicle and subsequently stimulated with thrombin as in Figure 1. The bottom panel indicates mobility shift of S6K1 when phosphorylated in response to thrombin activation. The top panel illustrates results of incubations using an antibody that recognizes S6K1 phosphorylated on residues 421 and 424. (B) S6K1 is developmentally expressed in proplatelets and proplatelet tips. Megakaryocytes were cultured from CD34+ hematopoietic stem cells as in Figure 1. S6K1 is indicated by red fluorescence and is present in proplatelet tips (arrows). WGA localization is identified by green fluorescence. S6K1 was detected in proplatelet shafts and in proplatelet tips (middle panels, arrows) as proplatelets were extended during developmental transition (also see panel C). In the right panels, nonimmune IgG was substituted for anti-S6K1. (C) Ribosomal protein S6 is distributed to proplatelets and proplatelet tips in megakaryocyte development. S6 protein and WGA are indicated by red and green fluorescence, respectively. CD34+ hematopoietic stem cells were differentiated to the megakaryocytic stage.4 After 8 days in suspension culture S6 was diffusely distributed in the cytoplasm. One hour after adhesion to immobilized fibrinogen on day 14, S6 was centrally located in the perinuclear cytoplasm. During the transition phase, in which adherent megakaryocytic cells begin to form proplatelets, S6 was diffusely distributed in the central cytoplasm and in early proplatelet shafts. As proplatelets were extended, S6 was detected in early proplatelet tips (bottom right panel, arrows). (B-C) Scale bar, 20 μm.
In additional experiments rapamycin did not block activation-induced conformational changes in integrin αIIbβ3 detected by specific antibody binding, thrombin-stimulated platelet aggregation, or adhesion of platelets to immobilized fibrinogen (not shown). Each of these rapid responses uses constitutive integrin αIIbβ3 and other proteins that do not require new synthesis. These results further demonstrate selective inhibitory activity of rapamycin (Figure 2A), consistent with previous studies.5,7
Rapamycin inhibits retraction of fibrin polymers by activated human platelets
We then examined a model of clot retraction in which platelet aggregates condense fibrin in a platelet-fibrin complex.8 Activation of platelets in media containing fluorescently labeled fibrinogen resulted in platelet-fibrin clots that could be monitored macroscopically and microscopically and that retracted into a tight cell-fibrin matrix within 2 hours under control conditions (Figure 3, tubes A and B). Rapamycin inhibited consolidation of the platelet-fibrin complex and tight fibrin mesh formation but not formation of platelet aggregates (Figure 3, tubes C and D; data not shown), suggesting that mTOR-dependent synthesis of Bcl-3 influences clot retraction. Bcl-3 accumulates in the actin-rich cytoskeleton of platelets (not shown) and binds Fyn,5 a tyrosine kinase that regulates cytoskeletal responses,45–47 consistent with this conclusion. Of interest, a defect in fibrin organization was reported in a patient treated with systemic rapamycin.44
Rapamycin inhibits retraction of fibrin clots by activated human platelets. Washed human platelets were pretreated with buffer, rapamycin (10 nM), or the vehicle for rapamycin for 1 hour. Exogenous labeled fibrinogen was then added to the cells, and they were left unstimulated or activated with thrombin (1.0 U/mL) for 2 hours. Appearance of the platelet suspensions under each experimental condition is shown in the middle panel. The stir bar in each tube is indicated by an arrow. The panels labeled A to D are photomicrographs from tubes shown in the same order in the middle panel. Actin was stained in unactivated and activated platelets (green fluorescence). Red fluorescence indicates fibrin strands. The platelet suspension in the first tube (control) is opaque, indicating dispersed, single unactivated platelets as seen by microscopy (A). No polymerized fibrin is present. Thrombin-stimulated platelets in the second tube have formed a tight fibrin clot adherent to the stir bar at the bottom, with clear media containing few residual platelets above. By microscopy, the clot consisted of platelet-fibrin complexes (B). After pretreatment with rapamycin, thrombin-stimulated platelets in the third tube formed a loose, gelatinous clot that was only slightly retracted, revealing clear media just below the meniscus. Polymerized fibrin was present but was much more dispersed (C). Activated platelets treated with vehicle for rapamycin in the fourth tube formed a tightly retracted fibrin clot around the stir bar at the bottom with clear media above, equivalent to the response of control platelets. Microscopically, the clot consisted of aggregated platelets and tightly retracted polymerized fibrin (D), similar to the clot in the control incubations (B). This figure is representative of 4 separate experiments.
Rapamycin inhibits retraction of fibrin clots by activated human platelets. Washed human platelets were pretreated with buffer, rapamycin (10 nM), or the vehicle for rapamycin for 1 hour. Exogenous labeled fibrinogen was then added to the cells, and they were left unstimulated or activated with thrombin (1.0 U/mL) for 2 hours. Appearance of the platelet suspensions under each experimental condition is shown in the middle panel. The stir bar in each tube is indicated by an arrow. The panels labeled A to D are photomicrographs from tubes shown in the same order in the middle panel. Actin was stained in unactivated and activated platelets (green fluorescence). Red fluorescence indicates fibrin strands. The platelet suspension in the first tube (control) is opaque, indicating dispersed, single unactivated platelets as seen by microscopy (A). No polymerized fibrin is present. Thrombin-stimulated platelets in the second tube have formed a tight fibrin clot adherent to the stir bar at the bottom, with clear media containing few residual platelets above. By microscopy, the clot consisted of platelet-fibrin complexes (B). After pretreatment with rapamycin, thrombin-stimulated platelets in the third tube formed a loose, gelatinous clot that was only slightly retracted, revealing clear media just below the meniscus. Polymerized fibrin was present but was much more dispersed (C). Activated platelets treated with vehicle for rapamycin in the fourth tube formed a tightly retracted fibrin clot around the stir bar at the bottom with clear media above, equivalent to the response of control platelets. Microscopically, the clot consisted of aggregated platelets and tightly retracted polymerized fibrin (D), similar to the clot in the control incubations (B). This figure is representative of 4 separate experiments.
Overexpression of Bcl-3 protein in an integrin αIIbβ3–expressing cell line enhances the retraction of fibrin clots
To further explore the possibility that Bcl-3 influences fibrin retraction, we transfected Bcl-3 into a CHO cell line that stably expresses integrin αIIbβ3 (GPIIb/IIIa),36 which binds fibrinogen and facilitates retraction of fibrin clots23,33,48,49 (Figure 4A). CHO cells that express integrin αIIbβ3 underwent dramatic cellular spreading and formed polymerized actin networks throughout the cytoplasm when they adhered to immobilized fibrinogen, whereas wild-type CHO cells that do not express αIIbβ3 attached to fibrinogen much more slowly and exhibited neither extensive spreading nor intracellular actin reorganization. Both wild-type and integrin αIIbβ3–expressing CHO cells transfected with Bcl-3 had altered cellular spreading and patterns of intracellular polymerized actin (data not shown). This result is consistent with the possibility that Bcl-3 influences cytoskeletal interactions. Bcl-3 was detected in the cytoplasm and nuclei in both cell lines following transfection (data not shown). Mock-transfected integrin αIIbβ3–expressing CHO cells induced formation of loose, gelatinous clots when incubated with fibrinogen in suspension (Figure 4B, left tube). When this cell line overexpressed Bcl-3, the clots retracted at an accelerated rate and were much more condensed and compact (Figure 4B, right tube). Thus, overexpression of Bcl-3 in a surrogate cell type augmented retraction of fibrin clots, consistent with the studies of activated human platelets (Figure 3).
Overexpression of Bcl-3 enhances the retraction of fibrin clots. (A) Western analysis of Bcl-3 protein in lysates of CHO cells that express integrin αIIbβ3 (cell line PLA1) or wild-type CHO cells that are deficient in αIIbβ3 (cell line LK444) after transfection with cDNA for Bcl-3 or with empty vector. (B) Mock-transfected integrin αIIbβ3–expressing CHO cells (control) or integrin αIIbβ3–expressing CHO cells that overexpressed Bcl-3 (see panel A) were examined for the ability to retract fibrin clots. The cells were incubated with exogenous-labeled fibrinogen and stimulated with thrombin for 2 hours as in Figure 3. Mock-transfected cells formed a loose, gelatinous clot (the black arrow points to the edge of the cell-fibrin complex) with a rim of clear media at its edges. Bcl-3–overexpressing cells induced a compact retracted clot (black arrow) adherent to the stir bar at the bottom of the tube (white arrows) with clear media above, although the clots were not as tight as those formed by human control platelets activated by thrombin (Figure 3, tubes B,D). Panels in this figure are representative of 3 separate experiments. For a color figure of panel B that provides additional detail of the fibrin clots, see Figure S1 (available on the Blood website; see the Supplemental Figure link at the top of the online article).
Overexpression of Bcl-3 enhances the retraction of fibrin clots. (A) Western analysis of Bcl-3 protein in lysates of CHO cells that express integrin αIIbβ3 (cell line PLA1) or wild-type CHO cells that are deficient in αIIbβ3 (cell line LK444) after transfection with cDNA for Bcl-3 or with empty vector. (B) Mock-transfected integrin αIIbβ3–expressing CHO cells (control) or integrin αIIbβ3–expressing CHO cells that overexpressed Bcl-3 (see panel A) were examined for the ability to retract fibrin clots. The cells were incubated with exogenous-labeled fibrinogen and stimulated with thrombin for 2 hours as in Figure 3. Mock-transfected cells formed a loose, gelatinous clot (the black arrow points to the edge of the cell-fibrin complex) with a rim of clear media at its edges. Bcl-3–overexpressing cells induced a compact retracted clot (black arrow) adherent to the stir bar at the bottom of the tube (white arrows) with clear media above, although the clots were not as tight as those formed by human control platelets activated by thrombin (Figure 3, tubes B,D). Panels in this figure are representative of 3 separate experiments. For a color figure of panel B that provides additional detail of the fibrin clots, see Figure S1 (available on the Blood website; see the Supplemental Figure link at the top of the online article).
Targeted deletion of Bcl-3 results in less-efficient fibrin retraction and stabilization by murine platelets
We then examined fibrin retraction using platelets from genetically altered mice with targeted deletion of Bcl-3 (Bcl-3−/−) and wild-type (WT) animals. We found that little or no Bcl-3 is present in resting murine platelets but that it is expressed in WT platelets when they are activated with thrombin (Figure 5A), a pattern equivalent to that in human platelets.5–7 In a previous study, Bcl-3 protein was minimally detected in unstimulated WT platelets but was dramatically increased under some conditions.50 As expected, the protein was not present in activated platelets from Bcl-3−/− animals (Figure 5A). Further characterization of the cellular phenotype of Bcl-3−/− platelets demonstrated that they display the integrin αIIb subunit, aggregate in response to a variety of agonists, and translocate P-selectin to their surfaces following activation with thrombin (Figure 5B; data not shown). Tail clip bleeding times were similar in wild-type and Bcl-3−/−, indicating that Bcl-3–deficient platelets can acutely form hemostatic plugs (not shown). These findings indicate that targeted deletion of Bcl-3 does not interrupt expression of key gene products that control these activities at the megakaryocyte or mature platelet stages. In addition, platelet numbers and plasma fibrinogen levels were comparable in WT and Bcl-3−/−–deficient animals (not shown).
Retraction of platelet-fibrin clots is attenuated by deletion of Bcl-3 in murine platelets. (A) Platelets were isolated from Bcl-3−/− and WT mice as described in “Materials and methods.” The cells were left quiescent or were activated and were then probed for Bcl-3 by Western analysis. (B) Platelets from Bcl-3–deficient and WT mice were left quiescent or activated with thrombin (0.5 U/mL) for 5 minutes and then examined for surface P-selectin or integrin αIIbβ3 by flow cytometry as described in “Materials and methods.” Blue and red areas indicate staining by anti-αIIbβ3 or anti–P-selectin and as control immunoglobulin, respectively. In the right panel, thrombin (0.1 U/mL) was added to the PRP from WT or Bcl-3−/− animals, and aggregation was monitored as described in “Materials and methods.” Similar results were observed when ADP was used as an agonist (data not shown). (C) PRP was collected from Bcl-3−/− and WT mice and normalized for platelet counts. The PRP was then left quiescent or stimulated with thrombin. After 2 hours, samples of the platelet-fibrin clots were prepared for transmission electron microscopy (“Materials and methods”). (Left) Dense fibrin complexes formed around irregularly shaped, spiculated platelets in the PRP from control animals. The red arrows point to platelets surrounded by electron-dense fibrin complexes, with other platelets enmeshed in dense fibrin nearby. (Right) Dense fibrin polymers were infrequent in suspensions of Bcl-3–deficient platelets. The blue arrowhead points to a small area of dense fibrin mesh adjacent to a Bcl-3−/− platelet, with other platelets and a few scattered fibrin complexes nearby. The Bcl-3−/− platelets (3 are indicated by red arrows) were less spiculated and tended to retain discoid shape. Scale bar, 2 μm. (D) WT or Bcl-3−/− murine platelets were isolated, resuspended in media containing fluorescently labeled fibrinogen, and stimulated with thrombin. Platelet aggregation and the organization of labeled fibrin complexes were examined at the designated time points. WT platelets bound labeled fibrin on their surfaces, giving them a white/orange globular appearance, and retracted fluorescently labeled fibrin into dense, compact masses around the platelet aggregates. A few individual fibrin strands were visible at early time points (blue arrows) but had been retracted into the tight platelet-fibrin mesh by 60 minutes. Incubations with platelets from Bcl-3–deficient animals resulted in loose complexes of labeled fibers. Individual fibrin strands were easily visible at 15 minutes and 60 minutes (blue arrows, bottom panels), in contrast to their paucity the incubations with WT platelets (top panels). Data in this figure are representative of 3 independent experiments. Scale bar, 5 μm.
Retraction of platelet-fibrin clots is attenuated by deletion of Bcl-3 in murine platelets. (A) Platelets were isolated from Bcl-3−/− and WT mice as described in “Materials and methods.” The cells were left quiescent or were activated and were then probed for Bcl-3 by Western analysis. (B) Platelets from Bcl-3–deficient and WT mice were left quiescent or activated with thrombin (0.5 U/mL) for 5 minutes and then examined for surface P-selectin or integrin αIIbβ3 by flow cytometry as described in “Materials and methods.” Blue and red areas indicate staining by anti-αIIbβ3 or anti–P-selectin and as control immunoglobulin, respectively. In the right panel, thrombin (0.1 U/mL) was added to the PRP from WT or Bcl-3−/− animals, and aggregation was monitored as described in “Materials and methods.” Similar results were observed when ADP was used as an agonist (data not shown). (C) PRP was collected from Bcl-3−/− and WT mice and normalized for platelet counts. The PRP was then left quiescent or stimulated with thrombin. After 2 hours, samples of the platelet-fibrin clots were prepared for transmission electron microscopy (“Materials and methods”). (Left) Dense fibrin complexes formed around irregularly shaped, spiculated platelets in the PRP from control animals. The red arrows point to platelets surrounded by electron-dense fibrin complexes, with other platelets enmeshed in dense fibrin nearby. (Right) Dense fibrin polymers were infrequent in suspensions of Bcl-3–deficient platelets. The blue arrowhead points to a small area of dense fibrin mesh adjacent to a Bcl-3−/− platelet, with other platelets and a few scattered fibrin complexes nearby. The Bcl-3−/− platelets (3 are indicated by red arrows) were less spiculated and tended to retain discoid shape. Scale bar, 2 μm. (D) WT or Bcl-3−/− murine platelets were isolated, resuspended in media containing fluorescently labeled fibrinogen, and stimulated with thrombin. Platelet aggregation and the organization of labeled fibrin complexes were examined at the designated time points. WT platelets bound labeled fibrin on their surfaces, giving them a white/orange globular appearance, and retracted fluorescently labeled fibrin into dense, compact masses around the platelet aggregates. A few individual fibrin strands were visible at early time points (blue arrows) but had been retracted into the tight platelet-fibrin mesh by 60 minutes. Incubations with platelets from Bcl-3–deficient animals resulted in loose complexes of labeled fibers. Individual fibrin strands were easily visible at 15 minutes and 60 minutes (blue arrows, bottom panels), in contrast to their paucity the incubations with WT platelets (top panels). Data in this figure are representative of 3 independent experiments. Scale bar, 5 μm.
We then examined platelet-fibrin complex formation in vitro using cells from WT and Bcl-3–deficient animals. Aggregates formed in platelet-rich plasma from both wild-type and Bcl-3−/− mice in response to thrombin stimulation and the cellular clusters were stable over several hours (data not shown). Nevertheless, fibrin complexes that formed from exogenous fibrinogen in the presence of aggregated Bcl-3−/− platelets were loosely organized by visual inspection and were easily dispersed, whereas activated WT platelets induced formation of a tight cell-fibrin mass that resisted disruption when the clots were mechanically agitated. Transmission electron microscopy revealed a dense fibrin mesh surrounding activated WT platelets (Figure 5C, left panel); in contrast, the fibrin mesh associated with Bcl-3−/−–deficient platelets was dispersed and much less dense and compact compared with that in incubations with platelets from control animals (Figure 5C, right panel). Only infrequent dense fibrin complexes were seen in incubation with Bcl-3−/− platelets, whereas they were prominent in fibrin clots formed by WT platelets (Figure 5C). These differences in fibrin clot organization and density are strikingly similar to those described when fixed human platelets, which are unable to interact with and organize fibrin strands, were compared with living competent cells.51 Activated Bcl-3−/− platelets in fibrin complexes also appeared less dense and spicular in comparison to WT platelets (data not shown), suggesting that Bcl-3 modulates the intracellular contractile apparatus in these cells in parallel with its effect on retraction of the extracellular fibrin clot.
We also examined the configuration of fibrin complexes formed from fluorescently labeled fibrinogen in the presence of activated Bcl-3−/− and WT platelets. Both WT and Bcl-3–deficient platelets formed aggregates in response to thrombin stimulation, consistent with their behavior in other experiments (Figure 5B), and bound labeled fibrinogen on their surfaces (Figure 5D). WT platelets organized a tight cell-fibrin complex with few individual fluorescently labeled fibrin strands visible by 15 minutes and further tight retraction by 60 minutes (Figure 5D, upper panels). In contrast, the cell-fibrin mesh was much more loosely organized in the presence of Bcl-3−/− platelets. Individual fibrin strands were easily visible at the 15- and 60-minute time points in Bcl-3−/− incubations, whereas they were condensed into dense fibrin complexes in suspensions of activated WT platelets (Figure 5D, lower versus upper panels), consistent with the patterns by electron microscopy (Figure 5C).
Discussion
Activation-dependent synthesis of an induced protein that influences fibrin retraction represents a new biologic response for human and murine platelets. Condensation of fibrin is the central event in clot retraction and remodeling, a process that occurs over minutes to hours in vitro and is thought to contribute to thrombus remodeling, alter thrombolysis, and enhance wound healing.23,25,35,52 Retraction of fibrin clots requires activation of platelets25,51 and involves engagement of integrin αIIbβ3,32,33,49,53 although in surrogate cell lines it is also mediated by integrin αvβ3.35,54 Engagement of integrin αIIbβ3 on platelets, which initially occurs during aggregation, is followed by complex and incompletely defined contractile events that retract the extracellular fibrin network; additional receptors for polymerized fibrin may also contribute.23,32,33,49,53 Remodeling of intracellular cytoskeletal structures that form during later stages of platelet activation may retract extended filopodia and cause contraction of the clot.23 Using both loss-of-function (pharmacologic inhibition of mTOR by rapamycin in activated human platelets; platelets from Bcl-3−/− mice) and gain-of-function (Bcl-3–overexpressing cell lines) approaches, we found that Bcl-3 modulates fibrin retraction and that its expression by activated platelets is required for tight fibrin polymers to form (Figures 3–5). This establishes new physiologic activity for Bcl-3 and further demonstrates functional significance for signal-dependent protein synthesis by activated human platelets.5,9 Although roles for Bcl-3 in platelet function and clot remodeling have not been examined in vivo, Bcl-3–deficient mice have increased susceptibility to Streptococcus pneumoniae, Listeria monocytogenes,55 and Trypanosoma gondii.38 These microorganisms interact with platelets.56 One of the evolutionary advantages for clotting and clot retraction is capture and encapsulation of microorganisms as part of a complex and multifaceted response to wounding and injury.1,56–58 Thus, it is possible that a defect in fibrin retraction contributes to deficiencies in microbial containment and clearance in Bcl-3−/− animals.38,55
Bcl-3 is a member of the IκB family of intracellular proteins that control distribution of the transcriptional regulator nuclear factor κ B (NFκB) and, via this mechanism, expression of critical inflammatory proteins.21,38,55 Therefore, synthesis of Bcl-3 by activated platelets,5–7 a cell unable to transcribe nuclear mRNAs,9 and participation of Bcl-3 in fibrin polymer retraction (this study) were not predicted based on its known activities. Nevertheless, its domain organization, which includes ankyrin repeats and proline-rich N and C termini, suggested that Bcl-3 may be involved in protein-protein interactions in addition to those in NF-κB regulation.5 This was confirmed by its binding to the SH3 domain of Fyn in transfected cells and activated platelets.5 Similarly, Bcl-3 was recently found to also associate with Lck, a tyrosine kinase related to Fyn, via a target SH3 domain.59 Fyn regulates cytoskeletal functions.45–47 Furthermore, Bcl-3 and Fyn redistribute to the actin-rich cytoskeleton in aggregated platelets (A.S.W., unpublished data, 2001).47 Inhibition of tyrosine kinases in activated platelets interrupts attachments of integrin αIIbβ3 to the cytoskeleton and produces a defect in fibrin clot retraction,32 suggesting that modulation of the activity or distribution of Fyn by newly synthesized Bcl-3 may influence fibrin polymer retraction (Figures 3–5) by altering one or more steps in this sequence of events. Whether Bcl-3 regulates other postreceptor mechanisms required for clot reorganization23,35,54 remains to be determined.
Control of Bcl-3 synthesis by mTOR in activated platelets5 and inhibition of clot retraction when mTOR is blocked (Figure 3) identify new biologic roles for the mTOR signaling pathway. The findings clearly demonstrate that mTOR regulates functional responses in addition to those necessary for cellular proliferation and growth, which are its most intensely studied activities.18–20 In platelets, as in other cell types, mTOR regulates cellular functions in part by controlling the translation of a specific subset of mRNAs.5,9,20 Characterization of the ontogeny of mTOR and downstream effectors in model megakaryocytes (Figures 1–2) provided collateral insights into its activities in mature platelets in response to cellular activation (Figure 3). These studies demonstrated that mTOR is distributed in a developmentally regulated fashion and is present in the cytoplasm of proplatelet tips, the immediate precursors of platelets.4,41 It is also present in mature platelets isolated from circulating human blood and does not require new synthesis (Figure 1B-C). Similarly, the kinase S6K1 and its target, ribosomal S6 protein, are present in proplatelets and mature circulating platelets (Figures 1–2; data not shown).9 S6K1 and S6 are critical downstream components of the signaling cascade regulated by mTOR.18–20 We and others have previously demonstrated that key components of a second limb of the signaling pathway efferent to mTOR, consisting of 4E-BP1 and eukaryotic initiation factor 4E, are present in circulating human platelets and respond to cellular activation.5,7,60 This branch of the signaling cascade acts in parallel to S6K1.18,20 Thus, developmental expression of mTOR and its downstream effectors in precursor megakaryocytes and presence of these regulatory proteins in mature platelets support the observation that the mTOR pathway governs specialized translational control when these cells are activated by hemostatic and inflammatory signals.5 Additional studies indicate that platelets also have other signal-dependent mechanisms for post-transcriptional control of gene expression,4,7–9 providing evidence for previously unrecognized diversity in the functional repertoire of these cells.
In addition to modulating fibrin retraction, emerging evidence indicates that new synthesis of proteins by activated platelets alters other functional events relevant to thrombosis and inflammation. Examples of such functional changes include signaling of endothelial cells by interleukin-1β, which is synthesized by a novel mechanism,4,7,8 modulation of plasminogen activators by newly synthesized plasminogen activator inhibitor 161 and generation of a procoagulant surface on activated platelets and platelet microparticles by rapid synthesis of tissue factor.62 These observations suggest that synthesis of Bcl-3 and its influence on fibrin retraction may be part of a group of related responses that involve new expression of gene products and that are regulated by post-transcriptional pathways in activated platelets. In addition, synthesis of new proteins may be a determinant of the life span of these cells.63 Thus, signal-dependent translation of mRNAs by activated platelets may critically influence thrombosis and vascular remodeling. Genomic and proteomic analysis of human platelets also indicates previously unrecognized diversity of mechanisms that alter their phenotype, function and cellular interactions in response to activating stimuli.2,12,14,15,64,65
Pathways that regulate post-transcriptional gene expression in platelets may be targets for molecular therapy in thrombotic and inflammatory disorders, an issue of ongoing clinical investigation.24 As an example, rapamycin-impregnated coronary stents substantially reduce the rate of in-stent restenosis in comparison to other devices.29,66,67 Deposition of platelet-rich fibrin thrombi on stent surfaces and on adjacent vascular structures occurs rapidly in humans and experimental animals.68–70 The role of subsequent fibrin retraction and remodeling (Figures 3, 5), and the impact of additional platelet-mediated events, on ultimate endothelialization and neointima formation in the indwelling device are largely unexplored, but clearly may be critical.70 These events are modified by rapamycin (Figure 3). Previously unrecognized actions of mTOR and rapamycin that influence complex patterns of gene expression in deposited platelets (this study) and leukocytes,37,71 and the consequent functional responses that these proteins mediate, may therefore be key to the impact of this drug on the natural history of the stent-induced wound and in other conditions in which it is used as a therapeutic agent.20
Authorship
Contribution: A.S.W. performed, directed, and interpreted the experiments and wrote portions of the paper; M.M.D., H.S., N.D.T., J.F., and E.S. performed experiments; L.W.K. and T.M.M. analyzed data; K.H.A. performed experiments and analyzed data; and G.A.Z. codirected experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: the authors declare no competing financial interests.
Melvin M. Denis died on December 11, 2004.
Correspondence: Guy A. Zimmerman, Program in Human Molecular Biology & Genetics, Bldg 533, Rm 4220, University of Utah, Salt Lake City, UT 84112; Guy.zimmerman@hmbg.utah.edu.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ulrich Siebenlist for generously providing the Bcl-3–deficient mice used in this study and Paul Bray for integrin αIIbβ3–expressing CHO cell lines. We thank Margaret Vogel, Donnie Benson, and Jessica Phibbs for excellent technical assistance and the Labor and Delivery nurses and staff of Cottonwood and University of Utah hospitals for collecting umbilical cord blood for isolation of CD34+ hematopoietic stem cells. We also thank Chris Rodesch and Nancy Chandler and the University of Utah School of Medicine Research Microscopy Facilities for technical assistance. Adrienne Triplett and Diana Lim were invaluable in preparation of the manuscript and figures, respectively. We thank Josep Grinyo and Nuria Lloberas for useful discussions regarding the pharmacology of rapamycin and our colleagues in the Program in Human Molecular Biology and Genetics for helpful comments and critical review of the studies.
This work was supported by the National Institutes of Health (grant HL-66277, A.S.W.; grant HL-44525, G.A.Z.; grant HL-44513, T.M.M.; grant HL-75507, L.W.K.), the American Heart Association (Established Investigator Award, A.S.W.; Fellowship Award, H.S.), the American Diabetes Association (Physician-Scientist Training Award ADA no. PID2206052, M.M.D.), and Deutsche Forschungsgemeinschaft (DFG) (grant SCHW 1167/1-1, H.S.).




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal