Abstract
The proteasome is a proteolytic complex for intracellular degradation of ubiquitinated proteins which are involved in cell-cycle regulation and apoptosis. A constitutively increased proteasome activity has been found in myeloma cells. We studied circulating proteasome levels and their prognostic significance in sera of 50 control subjects, 20 persons with monoclonal gammopathies of undetermined significance (MGUS), and 141 previously untreated patients with multiple myeloma (MM) by an anti-20S proteasome enzyme-linked immunoabsorbent assay (ELISA). Serum proteasome concentrations were significantly elevated in MM compared with controls (P < .001), in MM versus MGUS (P = .03), and in active (n = 101) versus smoldering (n = 40) MM (P < .001). In patients with active MM, there was a significant (P < .001) decrease from pretreatment to post-treatment proteasome concentrations in responders to chemotherapy, but not in nonresponders. Circulating proteasome levels were identified as a prognostic factor for overall survival in the univariate (P < .001 log-rank test) and in the multivariate (hazard ratio, 4.38) survival analysis in patients with active MM. We demonstrate for the first time that increased serum proteasome concentrations correlate with advanced disease and are an independent prognostic factor in MM.
Introduction
Multiple myeloma (MM) is a malignant B-cell neoplasia which is characterized by an uncontrolled proliferation of aberrant plasma cells in the bone marrow. Because this disease is presently still incurable by conventional chemotherapy, novel therapeutic targets are urgently needed. Recent studies have shown that the inhibition of the ubiquitin-proteasome system can be successfully used as a targeted therapy in MM.1–5
Proteasomes are major nonlysosomal proteolytic complexes which are localized in the cytoplasm and in the nucleus of eukaryotic cells.6,7 The 26S proteasome structure is composed of a 20S catalytic core complex which forms a cylindrical structure which is capable of adenosine triphosphate (ATP)–independent proteolysis. The 20S core structure is associated with a 19S regulatory complex that recognizes ubiquitinylated proteins and has an ATP-ubiquitin–dependent proteolytic activity. The proteasome system is the major nonlysosomal system for the degradation of short half-life proteins. This includes proteins and peptides which are involved in basic cellular processes, such as cell-cycle regulation and apoptosis,8 transcriptional regulation,9 or antigen processing.10 Thus, the protein degradation by the ubiquitin-proteasome pathway has a major regulatory function for proliferation activity and survival of both normal and malignant cells.11 In cancer cells, proteasomes are often overexpressed. Abnormally high expression of proteasomes was found in human leukemia cells,12 renal cancer cells,13 or in breast cancer cell lines.14 In previous in vitro studies we and others could demonstrate antiproliferative and proapoptotic effects of different proteasome inhibitors in leukemia cell lines15 and in myeloma cells.16–19 Furthermore, an inhibition of osteoclast development and activation of osteoblasts by proteasome inhibitors was recently demonstrated.20,21 Recent data also show that proteasome inhibitors induce antiangiogenesis in multiple myeloma via direct and indirect effects on bone marrow endothelial cells (BMECs)22 which gives a rationale for targeting the proteasome in the bone marrow microenvironment. Clinical trials could already demonstrate the efficacy of the proteasome inhibitor bortezomib in a substantial portion of patients with relapsed or refractory MM.23–25
Circulating proteasome levels (cProteasome) can be measured in serum or plasma samples by enzyme-linked immunoabsorbent assay (ELISA) techniques. Preliminary data suggest that proteasome concentrations in peripheral blood are elevated in patients with certain types of malignant diseases.26–29 Furthermore, in small patient cohorts it has been shown that cProteasome correlates with tumor burden.26,29 We developed an ELISA technique to detect circulating 20S proteasome components in serum or plasma samples.30 Previous studies with this assay have shown that cProteasome is elevated in patients with systemic autoimmune diseases and correlates with disease activity.30 Because there are only limited data on the circulating proteasome levels in multiple myeloma and no data on its prognostic value, we examined circulating proteasome levels in a large cohort of 161 patients with monoclonal gammopathies of undetermined significance (MGUS) or newly diagnosed multiple myeloma (MM). The aim of this study was to evaluate whether cProteasome is elevated in patients with MM compared with healthy donors or patients with MGUS and whether there are differences in proteasome concentrations between asymptomatic smoldering (SMM) and active myeloma (AMM).
In a longitudinal study we investigated treatment-related changes of proteasome serum concentrations in prechemotherapy versus postchemotherapy samples in patients with active MM who received conventional-dose therapy (CT) or high-dose therapy (HDT). Furthermore, the prognostic value of cProteasome for overall survival was evaluated in patients with symptomatic MM in a univariate analysis and in a multivariate model including established prognostic parameters.
Patients, materials, and methods
Patients
A total of 211 peripheral blood samples from 50 healthy blood donors, 20 patients with MGUS, and 141 consecutive patients with newly diagnosed multiple myeloma were collected at 2 study centers at the university hospitals in Berlin and Ulm between 1999 and 2005. Patients with symptomatic MM, who received previous chemotherapy before presentation at study center or patients in whom cytogenetic samples were not available, were not included. Patients were included after informed consent was given. Approval was obtained from the Charité Institutional Review Board for this study. Sample asservation and recording of clinical data were approved by the local institutional review boards. Peripheral blood was processed immediately by centrifugation at 1500g for 10 minutes, and serum samples were stored at −76 °C. Twenty patients were classified as MGUS, 40 as asymptomatic, smoldering MM (SMM), and 101 patients as symptomatic, active MM (AMM), according to the International Myeloma Working Group criteria.31
One hundred one patients with symptomatic myeloma had an indication for treatment. Forty-eight patients received standard-dose chemotherapy (CT). Standard treatment regimens were melphalan/prednisone (n = 29), doxorubicin/dexamethasone (n = 11), oral idarubicin/dexamethasone (n = 3), bendamustine/prednisone (n = 2), dexamethasone monotherapy (n = 2), or thalidomide/dexamethasone (n = 1). Fifty-three patients younger than 70 years received high-dose therapy (HDT) with melphalan 200 mg/m2 (n = 44) or melphalan 140 mg/m2 (n = 9). From the total of 101 treated patients with symptomatic MM, 12 patients did not complete the scheduled therapy course because of treatment-related toxicity (n = 9) or noncompliance (n = 3). In 50 patients with active MM who received CT (n = 29) or HDT (n = 21), pretreatment and posttreatment blood samples were available and taken just before and at the end of the first-line chemotherapy. All 50 patients who were evaluated for pretreatment versus post-treatment serum proteasome levels completed the scheduled treatment course. Treatment characteristics are shown in Table 3.
Proteasome ELISA
Circulating serum proteasome levels were detected by a self-developed sandwich ELISA as described previously.30 Briefly, 20S proteasomes were enriched from human serum and red blood cells by ion exchange chromatography and fractionated ammonium sulfate precipitation. Monoclonal anti-proteasome antibodies were generated by immunization of mice with 50 μg of the purified human proteasome protein emulsified in complete adjuvant. Spleen cells were fused with SP2/0-Ag14 myeloma cells, and antibody activity of hybrid colonies was detected in solid-phase binding assays. A polyclonal antibody was generated by immunization of a rabbit with a purified red cell proteasome preparation. The assay was constructed by coating microtiter plates overnight with the polyclonal anti-proteasome antibody K42 in a 1:1000 dilution. Remaining binding sites were blocked with 0.5% fetal calf serum in phosphate-buffered saline. Serum samples were incubated in a 1:10 dilution for 1 hour at room temperature. Standard curves for quantification were established, using purified proteasomes in a concentration range from 0 to 10 000 ng/mL. The biotin-labeled monoclonal anti-proteasome antibody HP903 was used for detection in a 1:1000 dilution and incubated for 1 hour at room temperature. Bound antibodies were detected after labeling with streptavidin-peroxidase (POD). Microtiter plates were read at 450 nm after 30 minutes of incubation with tetramethylbenzidine as POD substrate. Intra-assay and interassay variations detected in serum samples of known high or low proteasome concentrations were lower than 10%. The recovery of cProteasome was between 95% and 105% when purified human immunoglobulins were spiked in different concentrations to serum samples of known high or low proteasome concentrations, indicating that serum proteasome detections in patients with MM are not influenced by serum immunoglobulin levels.
The upper reference limit for normal serum or plasma proteasome levels was determined at 380 ng/mL (mean + 2 SD), based on the analysis of the sera from 50 healthy donors. Storage of serum samples at room temperature from 2 hours up to 24 hours before freezing did not lead to variations greater than 10%.
Prognostic factors
In 101 patients with active MM a univariate and multivariate survival analysis was performed. Forty patients with smoldering MM were not included into the survival analysis. In addition to demographic data and disease characteristics (Table 1), previously identified prognostic factors such as albumin, β2-microglobulin, CRP,32 HDT,33 and chromosomal deletion 13q1434 were included in the survival analysis. Chromosomal deletion 13q14 was detected by interphase fluorescence in situ hybridization analysis (FISH) using Vysis LSI 13 (RB-1) probe (Vysis, Des Plaines, IL), as described previously.35,36
Statistical analysis
Comparisons between the independent groups of healthy donors, patients with MGUS, and patients with MM or between smoldering versus active MM were calculated by the Mann-Whitney U test. For the longitudinal comparison of pretreatment versus posttreatment samples the Wilcoxon test was applied. Survival data were analyzed in 101 patients with active MM. The univariate survival analysis was performed by the Kaplan-Meier method combined with the log-rank test. Variables found to be statistically significant at the P less than .05 level were entered into a Cox proportional hazards regression analysis that used a backward-stepping procedure to identify the most significant model. The calculations were performed with SPSS software version 11.0 (SPSS Science, Chicago, IL). For all tests, P values less than .05 were considered as statistically significant.
Results
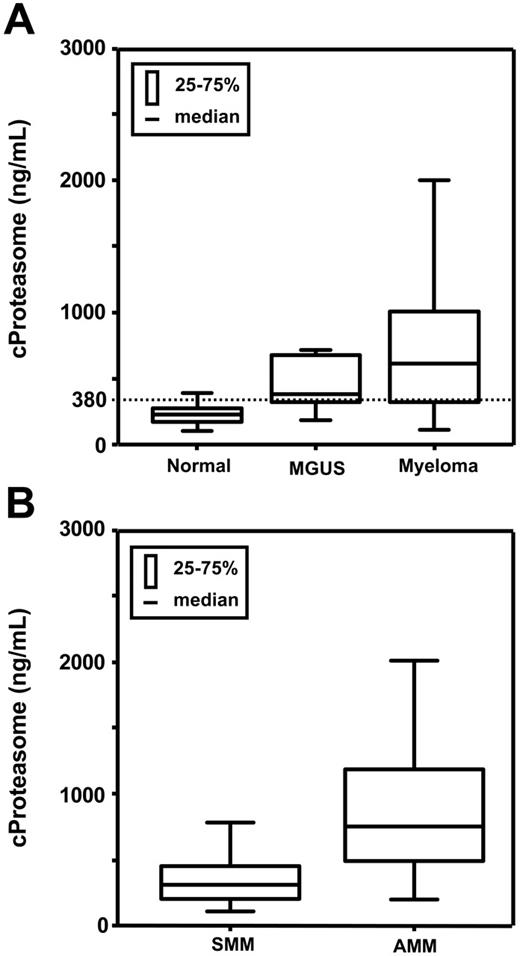
Circulating proteasome concentrations were detectable in all 211 serum samples. Patient characteristics are summarized in Table 1. There was no significant difference in cProteasome between female and male persons, and there was also no correlation of serum proteasome concentrations with age, neither in the control group nor in patients with myeloma. The median serum proteasome values were 224.05 ng/mL (range, 102.5-391.70 ng/mL) in healthy persons, 378.10 ng/mL (range, 183.70-716.40 ng/mL) in patients with MGUS, and 599.60 ng/mL (range, 108.50-5181.60 ng/mL) in patients with myeloma (Table 2). As shown in Figure 1, the proteasome concentrations of patients with multiple myeloma were significantly elevated compared with healthy donors (P < .001) and to persons with MGUS (P = .033). Serum proteasome levels were also significantly higher in persons with MGUS versus healthy persons (P < .001), whereas the difference between MGUS and smoldering MM did not reach statistical significance. Circulating proteasome levels in 101 patients with active MM were significantly (P < .001) elevated (median, 744.30 ng/mL; range 193.40-5181.60 ng/mL) compared with patients with smoldering myeloma (median, 314.65 ng/mL; range, 108.50-1252.60 ng/mL). In 141 myeloma patients with smoldering (n = 40) or active (n = 101) MM, there was a significant correlation of cProteasome with creatinine (Spearman-Rho, P = .04), but the correlation coefficient was low (r = .243). In patients with MM no significant correlation was found between cProteasome and β2-microglobulin, CRP, or albumin.
Patient characteristics
| Characteristic . | Value . |
|---|---|
| Total no. of persons | 211 |
| Sex, n | |
| Male | 108 |
| Female | 103 |
| Diagnosis, n | |
| Healthy donors | 50 |
| MGUS | 20 |
| Multiple myeloma | 141 |
| Median age, y (range) | |
| Healthy donors | 40 (29-63) |
| MGUS | 62 (37-74) |
| Myeloma | 62 (28-87) |
| MM subtype, n | |
| IgA | 30 |
| IgG | 92 |
| Bence-Jones | 16 |
| Nonsecretory | 3 |
| MM disease activity, n | |
| Smoldering (SMM) | 40 |
| Active = symptomatic (AMM) | 101 |
| Characteristic . | Value . |
|---|---|
| Total no. of persons | 211 |
| Sex, n | |
| Male | 108 |
| Female | 103 |
| Diagnosis, n | |
| Healthy donors | 50 |
| MGUS | 20 |
| Multiple myeloma | 141 |
| Median age, y (range) | |
| Healthy donors | 40 (29-63) |
| MGUS | 62 (37-74) |
| Myeloma | 62 (28-87) |
| MM subtype, n | |
| IgA | 30 |
| IgG | 92 |
| Bence-Jones | 16 |
| Nonsecretory | 3 |
| MM disease activity, n | |
| Smoldering (SMM) | 40 |
| Active = symptomatic (AMM) | 101 |
Circulating serum proteasome levels in healthy donors and patients with MGUS, SMM, or active MM
| . | Healthy donors . | MGUS . | Total MM . | Smoldering MM . | Active MM . |
|---|---|---|---|---|---|
| N | 50 | 20 | 141 | 40 | 101 |
| cProteasome level | |||||
| Median, ng/mL | 224.05 | 378.10 | 599.60 | 314.65 | 744.30 |
| Range, ng/mL | 102.5-391.70 | 183.70-716.40 | 108.50-5181.60 | 108.50-1252.60 | 193.40-5181.60 |
| . | Healthy donors . | MGUS . | Total MM . | Smoldering MM . | Active MM . |
|---|---|---|---|---|---|
| N | 50 | 20 | 141 | 40 | 101 |
| cProteasome level | |||||
| Median, ng/mL | 224.05 | 378.10 | 599.60 | 314.65 | 744.30 |
| Range, ng/mL | 102.5-391.70 | 183.70-716.40 | 108.50-5181.60 | 108.50-1252.60 | 193.40-5181.60 |
P values between healthy donors and patients with MGUS, between healthy donors and total patients with MM, and between SMM and AMM were less than .001. The P value between patients with MGUS and the total patients with MM was .033.
Box plots of proteasome serum levels in healthy donors, patients with MGUS, and patients with MM. (A) Healthy donors, MGUS, MM. (B) Smoldering MM (SMM), active MM (AMM), P < .001. The dotted line indicates the upper limit evaluated for healthy persons (mean ± 2 SD, 380 ng/mL).
Box plots of proteasome serum levels in healthy donors, patients with MGUS, and patients with MM. (A) Healthy donors, MGUS, MM. (B) Smoldering MM (SMM), active MM (AMM), P < .001. The dotted line indicates the upper limit evaluated for healthy persons (mean ± 2 SD, 380 ng/mL).
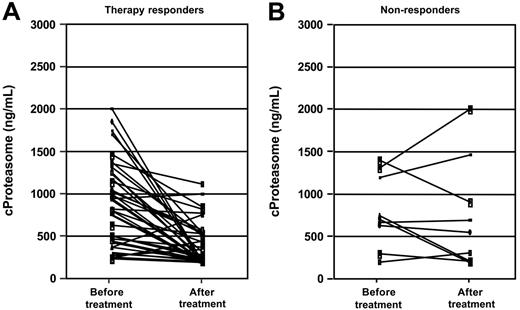
Sequential cProteasome measurements in 50 patients with active MM were performed before the start and at the end of first-line chemotherapy. All patients who were included in this analysis completed the scheduled therapy course. Forty-one patients achieved a partial or complete response, assessed according to the Blade criteria,37 and 9 patients did not achieve a remission after chemotherapy (Table 3). In those patients who had a partial or complete response to treatment, cProteasome decreased significantly from pretreatment to post-treatment values (P < .001), whereas there was no decrease in the patient group without treatment response (P = .68) (Figure 2). The decrease of cProteasome levels did not differ in patients receiving CT versus HDT or in patients achieving CR versus PR. Those patients who had post-therapy cProteasome levels higher than 380 ng/mL (upper reference limit) had a significantly shorter overall survival than patients with normalized post-treatment cProteasome concentrations (P = .03). Median survival after the end of treatment was 30.2 months in patients with elevated post-treatment proteasome levels versus not reached in patients with normalized levels.
Treatment characteristics of patients with symptomatic MM
| Characteristic . | N . |
|---|---|
| Total no. of patients | 101 |
| Treatment | |
| Standard-dose chemotherapy | 48 |
| High-dose chemotherapy | 53 |
| Treatment completion | |
| Completed therapy course | 89 |
| Premature discontinuation | 12 |
| Pretreatment and posttreatment sample available | 50 |
| Treatment | |
| Standard-dose chemotherapy | 29 |
| High-dose chemotherapy | 21 |
| Response according to EBMT criteria37 | |
| Complete response | 5 |
| Partial response | 36 |
| No change | 4 |
| Progressive disease | 5 |
| Characteristic . | N . |
|---|---|
| Total no. of patients | 101 |
| Treatment | |
| Standard-dose chemotherapy | 48 |
| High-dose chemotherapy | 53 |
| Treatment completion | |
| Completed therapy course | 89 |
| Premature discontinuation | 12 |
| Pretreatment and posttreatment sample available | 50 |
| Treatment | |
| Standard-dose chemotherapy | 29 |
| High-dose chemotherapy | 21 |
| Response according to EBMT criteria37 | |
| Complete response | 5 |
| Partial response | 36 |
| No change | 4 |
| Progressive disease | 5 |
Pretreatment and posttreatment circulating proteasome concentrations in patients with active MM who received chemotherapy. (A) Responders, P < .001 Wilcoxon-test. (B) Nonresponders, P = .68.
Pretreatment and posttreatment circulating proteasome concentrations in patients with active MM who received chemotherapy. (A) Responders, P < .001 Wilcoxon-test. (B) Nonresponders, P = .68.
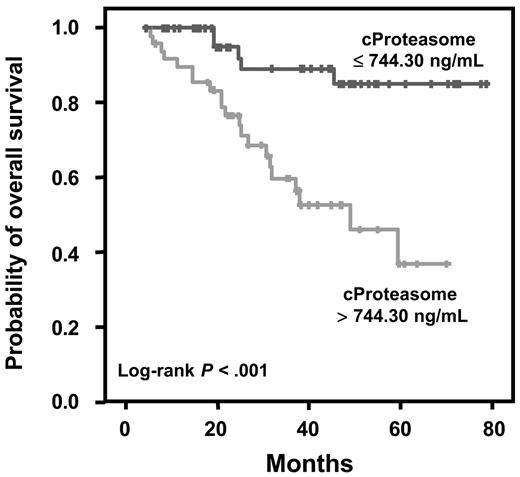
The prognostic relevance of cProteasome, albumin, β2-microglobulin, CRP, HDT, and deletion 13q14 was analyzed in 101 patients with active MM. As cut-off values we used the previously published values for CRP (< 0.6 versus > 0.6 mg/dL), albumin (< 35 versus > 35 g/L), and β2-microglobulin (< 5.5 versus > 5.5 mg/L).38 Interphase FISH analysis for deletion 13q14 could be performed in 98 of 101 patients, whereas 3 bone marrow samples were not evaluable. In the Kaplan-Meier analysis patients with active MM with serum proteasome concentrations lower than the median value of 744.30 ng/mL had a significantly longer survival compared with patients with cProteasome greater than the median value (median not reached versus 48.9 months, P < .001) (Figure 3). Furthermore, β2-microglobulin (P = .03), CRP (P = .001), HDT (P = .01), and deletion 13q14 (P = .01) were significant prognostic factors, whereas the parameters age, sex, immunoglobulin type, albumin, and creatinine did not reach statistical significance in the univariate analysis. Furthermore, in a multivariate Cox regression analysis in 101 patients with active MM, including those factors which had a prognostic significance for overall survival in the univariate analysis, cProteasome was the most powerful independent prognostic factor (hazard ratio, 4.38; 95% CI, 1.63-11.75). HDT and deletion 13q14 remained also as significant prognostic factors (Table 4)
Prognostic relevance of circulating proteasome levels in patients with active MM. Cut-off value: median, P < .001 log-rank test.
Prognostic relevance of circulating proteasome levels in patients with active MM. Cut-off value: median, P < .001 log-rank test.
Multivariate Cox regression analysis of variables with independent prognostic significance for overall survival in 101 patients with active MM
| Parameter . | N . | P . | Relative risk . | 95% CI . |
|---|---|---|---|---|
| cProteasome level | ||||
| 0 to median | 52 | 1.00 | NA | |
| Greater than median | 49 | .003 | 4.38 | 1.63-11.75 |
| del13q14 | ||||
| FISH negative | 59 | 1.00 | NA | |
| FISH positive | 39 | .006 | 3.82 | 1.71-8.55 |
| Chemotherapy | ||||
| Conventional dose | 48 | 1.00 | NA | |
| High dose | 53 | .011 | 0.33 | 0.14-0.78 |
| β2-Microglobulin level | ||||
| 0-5.5 mg/L | 76 | NA | NA | |
| Greater than 5.5 mg/L | 25 | NS | NA | NA |
| CRP level | ||||
| 0-0.6 mg/dL | 50 | NA | NA | |
| Greater than 0.6 mg/dL | 51 | NS | NA | NA |
| Parameter . | N . | P . | Relative risk . | 95% CI . |
|---|---|---|---|---|
| cProteasome level | ||||
| 0 to median | 52 | 1.00 | NA | |
| Greater than median | 49 | .003 | 4.38 | 1.63-11.75 |
| del13q14 | ||||
| FISH negative | 59 | 1.00 | NA | |
| FISH positive | 39 | .006 | 3.82 | 1.71-8.55 |
| Chemotherapy | ||||
| Conventional dose | 48 | 1.00 | NA | |
| High dose | 53 | .011 | 0.33 | 0.14-0.78 |
| β2-Microglobulin level | ||||
| 0-5.5 mg/L | 76 | NA | NA | |
| Greater than 5.5 mg/L | 25 | NS | NA | NA |
| CRP level | ||||
| 0-0.6 mg/dL | 50 | NA | NA | |
| Greater than 0.6 mg/dL | 51 | NS | NA | NA |
NA indicates not applicable; and NS, not significant.
Discussion
The ubiquitin-proteasome system plays a major role in the turnover of many regulatory proteins which govern a diverse array of cellular functions, including proliferation, apoptosis, and antigen presentation.10,39 Anomalies in the ubiquitin-proteasome pathway have been described in various pathologic conditions.40 In malignant diseases the dysregulation of the proteasome-dependent degradation of cell-cycle proteins, transcriptional factors, tumor suppressors, and proto-oncogenes leads to uncontrolled cell proliferation and tumor growth.41–43 Thus, the inhibition of the proteasome system recently became a novel therapeutic target in several malignant diseases and especially in multiple myeloma.44,45 It was previously demonstrated that circulating proteasome concentrations can be measured by ELISA techniques.26–29 Because the 20S complex is the central core of multiple proteasome subtypes,46–48 it could be expected that the detection of 20S proteasome components would reflect the level of the total proteasome expression.28 Thus, we developed an ELISA system based on the purification of the 20S proteasome component. Using this assay, the circulating proteasome concentration was found to correlate with disease activity in rheumatic diseases.30 In the present study we investigated for the first time a large cohort of previously untreated patients with MM and could demonstrate that serum proteasome levels are significantly elevated in patients with multiple myeloma compared with healthy donors. We found no correlation of cProteasomes with age in the healthy control subjects or in the MM group. The values we found in healthy donors were similar to those measured by the ELISA test developed by Wada et al.26 Our data from patients with MM are also in accordance with previously published data showing that serum proteasome levels are elevated in patients with solid tumors and with certain hematologic malignancies, including a small group of 12 patients with MM.26,27 Wada et al26 could show that cProteasome correlates with the number of blast cells in chronic myelogenous leukemia and demonstrated by immunohistochemical studies that proteasome components are strongly expressed in most of the corresponding malignant cells in certain hematologic malignancies, including MM.26 Data from patients with non-Hodgkin lymphoma showed significantly elevated circulating proteasome levels in Ann Arbor stages III and IV versus stages I and II.26 Thus, the investigators concluded that proteasome serum levels could mainly originate from the malignant cell population and reflect the tumor burden.
In the literature, the data on cProteasome in MM is limited. In addition to cProteasome measurements in 12 patients reported by Wada et al,26 plasma proteasome levels of a group of 27 patients with MM were measured by another proteasome ELISA, but these were all pretreated patients; thus, the reported cProteasome levels may be changed during therapy.27 In the present study we analyzed cProteasome levels in 141 patients with newly diagnosed MM, 20 patients with MGUS, and 50 control subjects. We found that cProteasome is significantly elevated in active versus smoldering MM. There was a weak correlation of cProteasomes with creatinine in patients with myeloma but not in control subjects or patients with MGUS, thus probably reflecting impaired renal function in advanced disease. In 101 previously untreated patients with active MM, cProteasome was the most powerful prognostic factor for overall survival among HDT, deletion 13q14, β2-microglobulin, and CRP in the multivariate analysis. This result suggests that cProteasome expression had a greater effect on overall survival than tumor mass.
Data on circulating proteasome levels of patients with untreated cancer which were evaluated before and after chemotherapy are also rare. In our longitudinal measurements we found that effective chemotherapy is accompanied by a significant decrease in cProteasome, irrespective of CT or HDT.
The clearance mechanisms of circulating proteasomes are unknown. In patients with sepsis a half-life of cProteasomes of 2 to 6 hours has been observed, whereby a rapid decrease of cProteasomes was seen parallel to the decrease of the inflammatory reaction (K.E., unpublished data). It is likely, that cProteasomes in patients with cancer are cleared after the elimination of malignant tumor cells. Even though cProteasomes are likely to reflect increased proteasome expression by tumor cells27,29 or cellular turnover, the origin of the increased circulating proteasome concentrations, which is detected by ELISA tests in serum or plasma samples, remains to be clarified. Wada et al48 reported that proteasome concentrations are elevated in the culture media of human leukemic cell lines, suggesting a proteasome secretion mechanism by cancer cells (T. Tanaka, unpublished data, 1993). In vitro data indicate an alteration of the subcellular distribution of certain proteasome components during differentiation of leukemic cell lines which show a decrease in nuclear and cytoplasmatic expression and an increase in cell membranes and cell culture supernatants.49 Furthermore, there is evidence that increased proteasomes in cancer can also originate from nonmalignant cells. In a study of patients with malignant melanoma a high level of proteasome expression could be shown by immunohistochemical staining in cancer cells but also in stromal cells of the tumor environment.29 Because of the important role of bone marrow microenvironment in the pathophysiology of MM,50–52 the inhibition of myeloma-stromal cell interactions by proteasome inhibitors is of particular interest. Recent data show that the proteasome inhibitors alter the function of bone marrow osteoclasts,20 osteoblasts,21 and endothelial cells in MM.22
The present study gives the first report on the prognostic value of circulating proteasomes in a malignant disorder. Our data support the clinical significance of circulating proteasome levels as a novel parameter which appear to reflect disease activity and may predict overall survival in MM, thus providing a potentially useful platform for targeted therapy, including proteasome inhibitors such as bortezomib. Larger multicenter studies are warranted to evaluate the clinical significance of circulating proteasomes under treatment with proteasome inhibitors.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Orhan Sezer, Department of Hematology and Oncology, Charité-Universitätsmedizin Berlin, 10117 Berlin, Germany; e-mail: sezer@charite.de.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal