Abstract
Multiple myeloma is characterized by increased osteoclast activity that results in bone destruction and lytic lesions. With the prolonged overall patient survival achieved by new treatment modalities, additional drugs are required to inhibit bone destruction. We focused on a novel and more potent structural analog of the nonsteroidal anti-inflammatory drug etodolac, known as SDX-308, and its effects on osteoclastogenesis and multiple myeloma cells. SDX-101 is another structural analog of etodolac that is already used in clinical trials for the treatment of B-cell chronic lymphocytic leukemia (B-CLL). Compared with SDX-101, a 10-fold lower concentration of SDX-308 induced potent (60%-80%) inhibition of osteoclast formation, and a 10- to 100-fold lower concentration inhibited multiple myeloma cell proliferation. Bone resorption was completely inhibited by SDX-308, as determined in dentin-based bone resorption assays. SDX-308 decreased constitutive and RANKL-stimulated NF-κB activation and osteoclast formation in an osteoclast cellular model, RAW 264.7. SDX-308 effectively suppressed TNF-α–induced IKK-γ and IκB-α phosphorylation and degradation and subsequent NF-κB activation in human multiple myeloma cells. These results indicate that SDX-308 effectively inhibits multiple myeloma cell proliferation and osteoclast activity, potentially by controlling NF-κB activation signaling. We propose that SDX-308 is a promising therapeutic candidate to inhibit multiple myeloma growth and osteoclast activity and that it should receive attention for further study.
Introduction
Despite the introduction of novel and more potent treatment regimens, multiple myeloma (MM) remains an incurable plasma cell malignancy. A major source of morbidity and possible mortality associated with MM is the formation of osteolytic lesions throughout the axial skeleton. Lytic bone lesions occur in 70% to 80% of these patients1 and are frequently associated with severe bone pain and pathological fractures. In addition to being sequelae of MM, osteolytic lesions also occur in connection with bone metastases of various solid tumors. Osteolytic lesions represent an extreme of a continuum of dysregulation of the normal bone remodeling process resulting from excessive bone resorption mediated by activated osteoclasts (OCLs). Therefore, treatments designed to target both the MM cells and the OCLs should improve the outcomes of patients with MM by controlling malignant cell proliferation and osteolytic lesions.
Etodolac (1,8-diethyl-1,3,4,9-tetrahydropyrano-[3,4-b] indole-1-acetic acid) is a nonsteroidal anti-inflammatory drug (NSAID) that inhibits both cyclooxygenase (COX)-1 and COX-2 and that is approved for the treatment of degenerative joint disease and rheumatoid arthritis.2,3 SDX-101 is an R-enantiomer of the COX inhibitor etodolac, which lacks significant COX-inhibitory activity.4 Therefore, SDX-101 has a more favorable safety profile than racemic etodolac, due to a lack of COX-1–dependent side effects such as gastrointestinal toxicity, or recently reported COX-2–dependent side effects, including myocardial infarction and acute cardiovascular death.4,5 R-etodolac (SDX-101) was recently demonstrated to induce cytotoxicity, overcome drug resistance, and enhance the activity of dexamethasone in MM.1,6 It is currently being tested in phase 2 clinical trials for the treatment of refractory chronic lymphocytic leukemia. A recently reported tetrahydropyrano-indole structural analog of etodolac, namely SDX-308, has been shown to display in vitro cytotoxic activity on tumor lines and antitumor efficacy in mice through oral administration without COX-inhibitory effects.
In this study we analyzed the effect of SDX-308 on OCL formation and on MM cell growth. Our results show that SDX-308 inhibits OCL formation and MM cell growth. We propose that the inhibition of OCL formation may be a result of SDX-308–mediated down-regulation of NF-κB activity. Our study provides a preclinical rationale for initiating clinical trials of these novel agents to improve outcome in MM and, potentially, for other diseases, such as osteoporosis, that are associated with high OCL activity.
Materials and methods
Chemicals
SDX-101 and SDX-308 (Figure 1) were kindly provided by Salmedix (San Diego, CA; now Cephalon, Frazer, PA). Both drugs were freshly prepared in dimethyl sulfoxide (DMSO) for each experiment. Recombinant human receptor activator of NF-κB ligand (RANKL) was purchased from Roche (Branchburg, NJ), and human macrophage–colony-stimulating factor (M-CSF) was obtained from R&D Systems (Minneapolis, MN). α-Minimal essential medium (α-MEM), fetal calf serum (FCS), L-glutamine, and other cell culture reagents were purchased from Invitrogen (Carlsbad, CA). Horse serum was purchased from Hyclone (Logan, UT). DEAE-dextran was obtained from Amersham Biotech (Piscataway, NJ), and Ficoll-Hypaque and all other chemicals were from Sigma-Aldrich Chemicals (St Louis, MO).
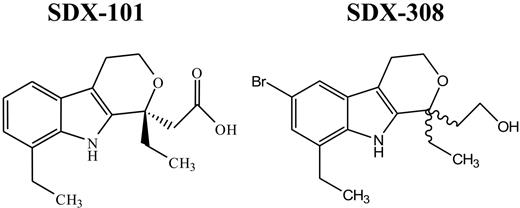
Chemical structure of SDX-101 and SDX-308. Wavy lines indicate the chiral center.
Chemical structure of SDX-101 and SDX-308. Wavy lines indicate the chiral center.
Cell lines and culture conditions
These studies conformed to guidelines of the Institutional Review Board of the University of Pittsburgh, and all subjects signed approved consent forms. Bone marrow cells were obtained from healthy volunteers or untreated multiple myeloma patients. Bone marrow mononuclear cells were isolated by separation on Ficoll-Hypaque gradients as described previously.7
CD34+ cells were isolated from leukaphereses products obtained from patients who were scheduled for autologous transplantation. Leukaphereses products were subjected to positive selection for CD34+ cells with the Isolex 300 device. Flow cytometry subset analyses showed that CD34+ selected cells had predominantly mature phenotype (mean ± SD: CD34+/CD38+, 95.5% ± 5.3%; CD34+CD33+, 79.9% ± 20.1%; CD34+DR+, 98.9% ± 1.2%). Isolated cells were stored in liquid nitrogen with 10% DMSO until use.
Murine monocytic RAW 264.7 cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA) and were cultured in α-MEM supplemented with 10% FCS, 2 mM L-glutamine, and 100 U/mL penicillin–streptomycin. This cell line expresses RANK and differentiates into tartrate-resistant acid phosphatase (TRAP)–positive OCLs in the presence of bone slices or RANKL.8 MM cell lines RPMI-8226 and OPM2 were purchased from ATCC. Dr Steve Rosen kindly provided the Dex-sensitive (MM.1S) human MM cell line (Northwestern University, Chicago, IL). MM.1S, RPMI-8226, and OPM2 cells were cultured in RPMI-1640 medium with 10% FCS and 100 U/mL penicillin–streptomycin.
Proliferation assay
MM.1S cells (6 × 104/well), RPMI-8226 cells (3 × 104/well), or OPM2 cells (3 × 104/well) were incubated in 96-well culture plates (Costar, Cambridge, MA) in the presence of RPMI-1640 medium containing 10% FCS and SDX-101 (10 μM, 100 μM, or 1 mM) or SDX-308 (1 μM, 10 μM, or 100 μM) for 48 hours at 37°C/5% CO2. DNA synthesis was measured by 3H-thymidine incorporation (3H-TdR; NEN Products, Zaventem, Belgium). Cells were pulsed with 3H-thymidine (1 μCi/well [0.037 MBq]) during the last 14 hours of culture, harvested onto glass-fiber filter mates (Wallac, Gaithersburg, MD) using an automatic cell harvester (Tomtec Harvester 96; MachIII, Hamden, CT), and counted using a Wallac TriLux Beta plate scintillation counter. All experiments were performed in triplicate.
Osteoclast formation assay.
Nonadherent mononuclear cells (105 cells/well) from either healthy donors or MM patients were seeded in 96-well multiplates at 100 μL/well in α-MEM containing 20% horse serum, 10 ng/mL M-CSF, and 50 ng/mL RANKL. SDX-101 (30 μM, 50 μM, 75 μM, or 100 μM) or SDX-308 (3 μM, 5 μM, 7.5 μM, or 10 μM) was added to the appropriate wells for different periods of time (1, 2, or 3 weeks). Half-media changes were carried out twice a week using drug-containing media where appropriate. The culture was incubated for a total of 3 weeks at 37°C with 5% CO2 and 95% humidity. Differentiation into OCLs was assessed by staining with monoclonal antibody 23c6 using a Vectastatin-ABC-AP kit (Vector Laboratories, Burlingame, CA). The antibody 23c6, which recognizes CD51/61 dimer constituting the OCL vitronectin receptor, was generously provided by Michael Horton (Rayne Institute, Bone and Mineral Center, London, UK). The 23c6+ multinucleated OCLs containing 3 or more nuclei per OCL were scored using an inverted microscope.
For RAW 264.7 cells, OCL differentiation assay was carried out in 96-well plates. The cells (4 × 103/well) were treated with 50 ng/mL RANKL for 5 days, in the absence or presence of SDX-101 (75 μM) or SDX-308 (7.5 μM). Half-media change was performed every 2 to 3 days by replacement with fresh medium containing RANKL with or without tested drugs. Cells were fixed and stained with a TRAP-staining kit (Sigma-Aldrich) according to the manufacturer's instructions. TRAP-positive multinuclear cells (> 3 nuclei) were counted under the inverted microscope. Each OCL formation assay was performed at least 3 times with cells from different donors.
Osteoclastic bone resorption on dentin slices
Bone marrow cells from healthy donors (2 × 105 cells/well) were seeded on whale dentin slices in 96-well plates in 100 μL/well α-MEM containing 20% horse serum, 10 ng/mL M-CSF, and 50 ng/mL RANKL. Half-media changes were performed twice a week. SDX-101 (75 μM) and SDX-308 (7.5 μM) were added into the appropriate wells whenever the medium was changed. Cultures were incubated for 3 weeks at 37°C in 5% CO2 and 95% humidity. After 3 weeks, dentin slices were stained for TRAP positivity to confirm OCL formation. Then bone resorption lacunae were stained with hematoxylin. Fixed small representative areas on dentin slices were selected for evaluation, and the mean resorption areas were determined. Pit area was quantified with the public domain National Institutes of Health (NIH) ImageJ program. Images were obtained with an Olympus IX70 microscope (Olympus, Melville, NY) and were acquired with MagnaFire software, version 4.1 (Optronics, Goleta, CA).
Colony assays
Briefly, colony assays were performed as described.9 CD34+-enriched leukapheresis cells were plated onto 35-mm plastic culture dishes (1500 cells/dish) in 1 mL methylcellulose medium (Methocult GF H4434; StemCell Technologies, Vancouver, BC, Canada) and were cultured in the presence of SDX-101 (75 μM), SDX-308 (7.5 μM), or DMSO (0.1%) as control. Methocult GF H4434 contained the following hematopoietic growth factors: 3 U/mL recombinant human (rh) erythropoietin, 50 ng/mL rhSCF, 10 ng/mL rhGM-CSF, and 10 ng/mL rhIL-3. Cells were incubated at 37°C in 5% CO2 with 95% humidity for 14 to 16 days. Formation and relative distribution of erythroid burst-forming unit (BFU-E), granulocyte colony-forming unit (CFU-G), and granulocyte macrophage colony-forming unit (CFU-GM) colonies were evaluated by scoring under an inverted microscope. Each colony experiment was repeated 3 times.
Osteoblast differentiation assays
MC-42 cells, which contain a stably transfected 1.3-kb Og2 (mouse osteocalcin gene 2) promoter driving expression of a firefly luciferase gene, were plated in 35-mm plates and cultured in α-MEM–containing ascorbic acid (50 μg/mL) for 15 days and were treated with SDX-101 (75 μM) or SDX-308 (7.5 μM) for 24 hours. Cells were then harvested for luciferase and alkaline phosphatase (ALP) activity assay. Luciferase and ALP activity were normalized to total protein content of the sample.10
To determine the effect of SDX-101 and SDX-308 on the mineralization potential of cultures, MC-42 cells were grown as described in the previous paragraph for 15 days. Inorganic phosphate was then added to a final concentration of 5.0 mM in the presence or absence of previously stipulated concentrations of SDX-101 or SDX-308 for 48 hours. Samples were then stained using the von Kossa method.11 Images were obtained by direct scanning of the mineralization dish using the ScanMaker 9800 XL (Microtek International, Taiwan), and the randomly selected representative areas were shown.
DEAE dextran–mediated transfection of NF-κB luciferase reporter gene
To determine NF-κB activation in the mouse osteoclast cell line RAW 264.7, cells were transiently transfected with a NF-κB luciferase reporter gene. The 3kB-Luc-SV40 plasmid, which was kindly provided by Dr Xu (University of Western Australia, Perth), contains 3 NF-κB binding sites from the interferon gene upstream of the luciferase coding region.12 Plasmid (0.4 μg) was directed into RAW 264.7 cells (2 × 106) using the DEAE-dextranDextran method. Transfected cells were seeded in 24-well plates (105/well) in 10% FCS for 36 hours before treatment with SDX-101 and SDX-308 for 1 hour, followed by RANKL stimulation (150 ng/mL) for another 8 hours. After cell harvest, the cells were lysed and the luciferase activity was assayed with Promega Luciferase Assay System (Promega, Fitchburg Center, WI).
Western blot analysis
Western blotting was performed as previously described.13,14 Briefly, cells were harvested, washed 3 times with phosphate-buffered saline, and lysed with lysis buffer (1% NP-40, 0.5 mM DTT, 1 mM sodium orthovanadate, 1 μg/mL aprotinin 1 μM sodium fluoride 50 μM phenylmethylsulfonyl fluoride).
For detection of p65 translocation, cellular nuclear and cytoplasmic extracts were prepared with Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL). Cell lysates (20-40 μg) were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to PVDF membrane (Millipore, Danvers, MA). were probed with anti-p65, anti–phospho p65, anti–IκB-α, anti–phospho IκB-α, or anti–phospho IKK-γ (all from Cell Signaling, Beverly, MA), and β-actin antibody (Amersham, Piscataway, NJ). Immune complexes were detected using enhanced chemiluminescence (Amersham).
Statistical analyses
Each experiment was repeated at least 3 times, and all quantitative data are presented as mean ± SD. Statistical differences were determined by Student t test. Results were considered significantly different for P < .05.
Results
SDX-308 and SDX-101 inhibit OCL formation in a dose-dependent manner
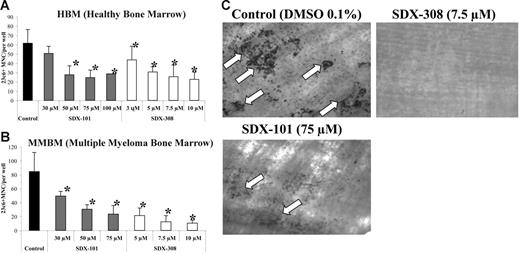
We tested the effect of SDX-101 and SDX-308 on OCL formation using human nonadherent mononuclear bone marrow cells from healthy donors and untreated MM patients. SDX-101 and SDX-308 induced a concentration-dependent inhibition of osteoclastogenesis in cultures from both healthy donors and MM patients. We tested concentrations ranging from 30 to 100 μM for SDX-101 and 3 to 10 μM for SDX-308. SDX-101 concentrations of 50 μM and higher elicited statistically significant inhibition of OCL formation from healthy donor bone marrow (Figure 2A). Surprisingly, SDX-308 showed a 10-fold more potent inhibitory effect than SDX-101 and inhibited OCL formation significantly at 3 μM and 5 μM, respectively (Figure 2A). The same effects were observed in OCL formation assays using MM mononuclear bone marrow cells (Figure 2B).
SDX-101 and SDX-308 inhibit OCL formation in a concentration-dependent manner and inhibit bone resorption. Nonadherent bone marrow cells from (A) healthy donor (1 × 105) and (B) MM patients were cultured in 100 μL α-MEM/20% horse serum, 50 ng/mL RANKL, and 10 ng/mL M-CSF for 3 weeks with either 30, 50, 75, and 100 μM SDX-101; 3, 5, 7.5, and 10 μM SDX-308; or vehicle. After 3 weeks, the cultures were stained with the 23c6 antibody. 23c6-positive OCLs containing 3 or more nuclei were scored microscopically. All experiments were performed in triplicate. Results are shown as mean ± SD. Asterisks indicate significant difference from control (P < .05). (C) Nonadherent bone marrow cells from healthy donor (1 × 105) were cultured in 100 μL α-MEM/20% horse serum, 50 ng/mL RANKL, and 10 ng/mL M-CSF for 3 weeks with 75 μM SDX-101, 7.5 μM SDX-308, or vehicle (DMSO 0.1%). After 3 weeks, dentin slices were stained with TRAP to confirm OCL formation on the slices. Resorption lacunae (arrows) were stained with hematoxylin and the images were obtained using an Olympus IX70 microscope equipped with a 20×/0.40 numeric aperture objective lens (Olympus). Images were acquired through Magnafire 4.1 software (Optronics). The pit area was quantified using the public-domain NIH Image program.
SDX-101 and SDX-308 inhibit OCL formation in a concentration-dependent manner and inhibit bone resorption. Nonadherent bone marrow cells from (A) healthy donor (1 × 105) and (B) MM patients were cultured in 100 μL α-MEM/20% horse serum, 50 ng/mL RANKL, and 10 ng/mL M-CSF for 3 weeks with either 30, 50, 75, and 100 μM SDX-101; 3, 5, 7.5, and 10 μM SDX-308; or vehicle. After 3 weeks, the cultures were stained with the 23c6 antibody. 23c6-positive OCLs containing 3 or more nuclei were scored microscopically. All experiments were performed in triplicate. Results are shown as mean ± SD. Asterisks indicate significant difference from control (P < .05). (C) Nonadherent bone marrow cells from healthy donor (1 × 105) were cultured in 100 μL α-MEM/20% horse serum, 50 ng/mL RANKL, and 10 ng/mL M-CSF for 3 weeks with 75 μM SDX-101, 7.5 μM SDX-308, or vehicle (DMSO 0.1%). After 3 weeks, dentin slices were stained with TRAP to confirm OCL formation on the slices. Resorption lacunae (arrows) were stained with hematoxylin and the images were obtained using an Olympus IX70 microscope equipped with a 20×/0.40 numeric aperture objective lens (Olympus). Images were acquired through Magnafire 4.1 software (Optronics). The pit area was quantified using the public-domain NIH Image program.
Since our results demonstrated that both compounds significantly inhibited OCL formation, we next performed bone resorption studies to determine whether the inhibition of OCL differentiation by SDX-101 and SDX-308 was accompanied by abrogation of bone resorption activity of mature OCLs. Consistent with a significant inhibition of OCL formation by SDX-101 (75 μM), bone resorption was also inhibited significantly (measured bone resorption area, 1.3 vs 8.6 mm2 for untreated control group). Treatment with SDX-308 (7.5 μM) resulted in complete inhibition of bone resorption (Figure 2C).
To investigate further whether SDX-101 and SDX-308 affect early or late OCL development, we conducted time-course experiments. Human bone marrow cultures from healthy donors and MM patients were treated with SDX-101 and SDX-308 for all 3 weeks, the first or last 2 weeks, and the first or last week of the 3-week culture period. We observed the strongest inhibition of OCL formation when the drug was added during the entire 3-week period (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). In contrast, when drug treatment was carried out for only the first or last 1 or 2 weeks of culture, the inhibitory effect in most experiments was less pronounced (Figure S1), suggesting that the compounds might not have acted preferentially on a particular stage of OCL development.
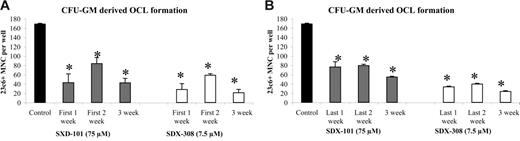
To determine whether these compounds might affect preosteoclast development, we repeated the experiments using already committed OCL precursors (CFU-GM) to set up an OCL formation assay. As seen in Figure 3A-B, treatment of committed OCL precursors with SDX-101 and SDX-308 in different time-course experiments resulted in the same inhibition of OCL formation as treatment of undifferentiated hematopoietic cells (Figure S1).
SDX-308 and SDX-101 do not affect preosteoclast development. Purified cells from CFU-GM colonies were cultured with 10 ng/mL M-CSF and 50 ng/mL RANKL for 21 days. Control (DMSO 0.1%), SDX-101 (75 μM), or SDX-308 (7.5 μM) was added to the culture twice a week. Drugs were added (A) for either for the first week only, the first 2 weeks, or all 3 weeks or (B) for either the last week only, the last 2 weeks, or all 3 weeks. Cells were then fixed and stained with 23c6 antibody to detect the multinucleated mature OCLs. Data shown are the mean ± SD of multinucleated cells (MNCs) per well of at least 8 wells. Asterisks indicate significant difference from control (P < .05). All experiments were performed independently 3 times.
SDX-308 and SDX-101 do not affect preosteoclast development. Purified cells from CFU-GM colonies were cultured with 10 ng/mL M-CSF and 50 ng/mL RANKL for 21 days. Control (DMSO 0.1%), SDX-101 (75 μM), or SDX-308 (7.5 μM) was added to the culture twice a week. Drugs were added (A) for either for the first week only, the first 2 weeks, or all 3 weeks or (B) for either the last week only, the last 2 weeks, or all 3 weeks. Cells were then fixed and stained with 23c6 antibody to detect the multinucleated mature OCLs. Data shown are the mean ± SD of multinucleated cells (MNCs) per well of at least 8 wells. Asterisks indicate significant difference from control (P < .05). All experiments were performed independently 3 times.
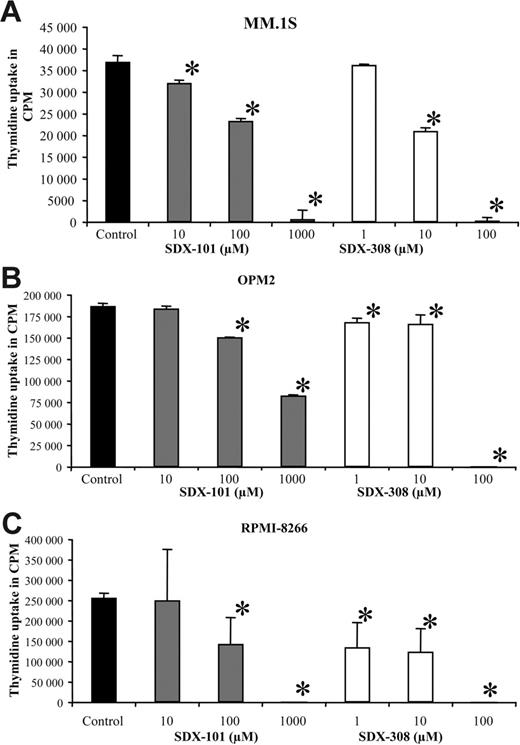
SDX-308 is 10-fold more potent than SDX-101 in inhibiting the growth of MM cells
SDX-101 and SDX-308 significantly inhibited the growth of different MM cell lines (MM1.S, OPM2, and RPMI-8226) in a concentration-dependent manner, as measured by 3H-thymidine uptake. SDX-101 significantly (P < .01) inhibited the growth of MM.1S, OPM2, and RPMI-8226 cell lines at concentrations between 10 and 100 μM compared with control. In contrast, SDX-308 showed 10- to 100-fold greater antiproliferative activity, significantly (P < .01) inhibiting MM growth at 1 μM in OPM2 and RPMI-8266 cells and at 10 μM in MM.1S cells compared with control treatment (Figure 4A-C).
SDX-101 and SDX-308 inhibit growth of MM cell lines. (A-C) MM cell lines MM.1S (6 × 104/well), OPM2 (3 × 104/well), and RPMI-8226 (3 × 104/well) were incubated in 96-well culture plates in the presence of RPMI-1640 medium containing 10% FCS and SDX-101 (10, 100 μM, and 1 mM), SDX-308 (1, 10, and 100 μM), or control (DMSO 0.1%) for 48 hours at 37°C with 5% CO2. DNA synthesis was measured by 3H-thymidine incorporation. All experiments were performed in triplicate. Results are shown as mean ± SD. Asterisks indicate significant difference from control (P < .05).
SDX-101 and SDX-308 inhibit growth of MM cell lines. (A-C) MM cell lines MM.1S (6 × 104/well), OPM2 (3 × 104/well), and RPMI-8226 (3 × 104/well) were incubated in 96-well culture plates in the presence of RPMI-1640 medium containing 10% FCS and SDX-101 (10, 100 μM, and 1 mM), SDX-308 (1, 10, and 100 μM), or control (DMSO 0.1%) for 48 hours at 37°C with 5% CO2. DNA synthesis was measured by 3H-thymidine incorporation. All experiments were performed in triplicate. Results are shown as mean ± SD. Asterisks indicate significant difference from control (P < .05).
SDX-101 and SDX-308 are not toxic to hematopoietic precursors
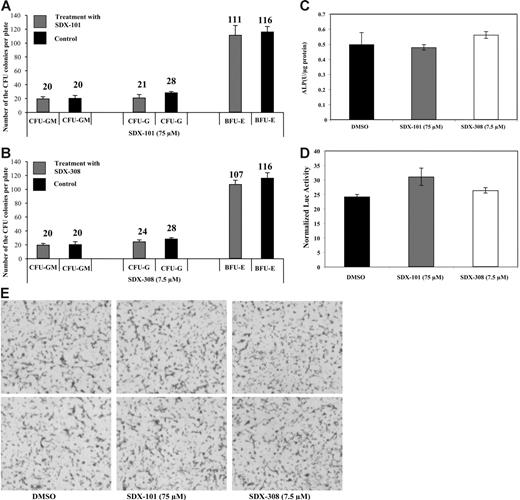
We then sought to determine whether SDX-101 and SDX-308 have toxic effects on hematopoietic progenitors by colony-forming assays. Purified human CD34+ cells were embedded in the methylcellulose culture, drugs were added (75 μM SDX-101, 7.5 μM SDX-308, or 0.1% DMSO as control), and the cells were incubated for 14 days. As shown in Figure 5A-B, the number of CFU-G, CFU-GM, and BFU-E colonies formed after exposure to either SDX-101 or SDX-308 was not different compared with control-treated cells, indicating that the drugs are not toxic to the hematopoietic progenitors under these experimental conditions.
Neither SDX-101 nor SDX-308 shows toxic effects on hematopoietic progenitors or affects osteoblast differentiation. (A-B) CD34+ cells were used for standard colony-formation assays using Methocult GF H4434 (Stem Cell Technologies, Vancouver, BC, Canada). The assay was performed in the presence of SDX-101 (75 μM) (⊡, top), SDX-308 (7.5 μM) (⊡, bottom), or DMSO (0.1%) (▪) as control. Numbers of CFU colonies formed were quantified under an inverted microscope after 14 days. Formation and relative distribution of BFU-E, CFU-M, and CFU-GM colonies were evaluated. Images were obtained using an Olympus microscope (numeric aperture 0.40, 10× magnification), and software (MagnaFire 4.1, Optronics). Data shown are the mean ± SD of triplicates of colony-formation assay. (C) ALP activity and (D) Og2 promoter activity: MC-42 cells were plated at a density of 5 × 104 cells/cm2 in 35-mm plates and were cultured in ascorbic acid (50 μg/mL) containing α-MEM for 15 days and treated with the indicated concentration of SDX-101, SDX-308, or volume-matched vehicle for 24 hours. Cells were then harvested for ALP assay and luciferase assay. ALP activity and luciferase activity were normalized into total protein. (E) Mineralization. MC-42 cells were grown as described in Figure 4C-D for 15 days. Inorganic phosphate was then added to a final concentration of 5.0 mM in the presence or absence of SDX-101, SDX-308, or vehicle for 48 hours. Samples were then stained using the von Kossa method. Images were obtained by direct scanning of the mineralization dish using the ScanMaker 9800Xl (Microtek International), and the randomly selected representative areas were shown.
Neither SDX-101 nor SDX-308 shows toxic effects on hematopoietic progenitors or affects osteoblast differentiation. (A-B) CD34+ cells were used for standard colony-formation assays using Methocult GF H4434 (Stem Cell Technologies, Vancouver, BC, Canada). The assay was performed in the presence of SDX-101 (75 μM) (⊡, top), SDX-308 (7.5 μM) (⊡, bottom), or DMSO (0.1%) (▪) as control. Numbers of CFU colonies formed were quantified under an inverted microscope after 14 days. Formation and relative distribution of BFU-E, CFU-M, and CFU-GM colonies were evaluated. Images were obtained using an Olympus microscope (numeric aperture 0.40, 10× magnification), and software (MagnaFire 4.1, Optronics). Data shown are the mean ± SD of triplicates of colony-formation assay. (C) ALP activity and (D) Og2 promoter activity: MC-42 cells were plated at a density of 5 × 104 cells/cm2 in 35-mm plates and were cultured in ascorbic acid (50 μg/mL) containing α-MEM for 15 days and treated with the indicated concentration of SDX-101, SDX-308, or volume-matched vehicle for 24 hours. Cells were then harvested for ALP assay and luciferase assay. ALP activity and luciferase activity were normalized into total protein. (E) Mineralization. MC-42 cells were grown as described in Figure 4C-D for 15 days. Inorganic phosphate was then added to a final concentration of 5.0 mM in the presence or absence of SDX-101, SDX-308, or vehicle for 48 hours. Samples were then stained using the von Kossa method. Images were obtained by direct scanning of the mineralization dish using the ScanMaker 9800Xl (Microtek International), and the randomly selected representative areas were shown.
SDX-101 and SDX-308 have no inhibitory effect on osteoblast differentiation
To determine whether SDX-101 and SDX-308 affect osteoblast differentiation, we conducted 3 experiments. First, we tested whether these 2 drugs could inhibit ALP activity, an osteoblast differentiation marker, in MC3T3-E1 preosteoblastic cells (MC-42). As shown in Figure 5C, treatment of MC-42 cells with the drugs at concentrations that significantly inhibit OCL differentiation (SDX-101 75 μM and SDX-308 7.5 μM) did not decrease ALP activity. Second, we determined whether the drugs could inhibit osteoblast-specific Og2 (mouse osteocalcin gene 2) promoter activation in differentiated MC-42 cells. MC-42 cells contain a stably transfected 1.3-kb Og2 promoter that drives expression of a firefly luciferase gene and luciferase expression, which closely follows levels of endogenous osteocalcin mRNA.10 Cells were treated with the compounds for 24 hours and then harvested for luciferase and protein assay. As shown in Figure 5D, mOG2 promoter activity was not inhibited by either SDX-101 or SDX-308. Third, as shown in Figure 5E, the drug treatment did not inhibit the mineralization of osteoblasts, as measured by von Kossa staining. Taken together, these data indicate that neither SDX-101 nor SDX-308 affects osteoblast differentiation at concentrations that inhibit OCL differentiation and function.
SDX-308 inhibits NF-κB activation in OCL precursors
The transcription factor NF-κB plays a central role in OCL formation, and blocking NF-κB activity is a potential strategy for inhibiting OCL formation resulting in decreased bone resorption.
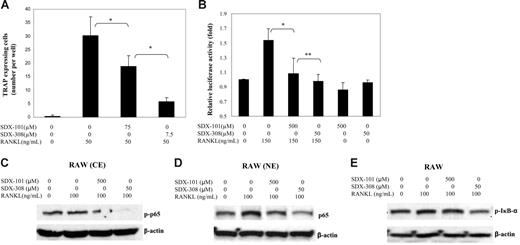
To investigate the effects of SDX-101 and SDX-308 on the RANKL-induced activation of NF-κB and OCL formation, we used an osteoclast cellular model with RAW 264.7 cells.8,15 The cells were incubated with SDX-101 or SDX-308 in the presence of RANKL and were allowed to differentiate into OCLs after 5 days of culture. As shown in Figure 6A, both SDX-101 and SDX-308 suppressed RANKL-induced OCL formation from RAW 264.7 cells. Again, SDX-308 at 7.5 μM induced significantly stronger inhibition than SDX-101 at 75 μM. Next we used RAW 264.7 cells transiently transfected with a NF-κB luciferase reporter gene and measured RANKL-stimulated luciferase activity under treatment with SDX-101 and SDX-308. As shown in Figure 6B, treatment with SDX-101 or SDX-308 significantly suppressed RANKL-induced NF-κB activation. Notably, a 10-fold lower concentration of SDX-308 produced a significantly stronger inhibition of the luciferase activity in RAW 264.7 than did SDX-101. SDX-101 and SDX-308 alone had no significant effects on the luciferase activity and cellular viability (data not shown).
SDX-101 and SDX-308 inhibit RANKL-induced osteoclast formation and NF-κB activation in RAW 264.7 cells. (A) RAW cells (1 × 104 per well) were cultured in the presence of RANKL (50 ng/mL), and drug vehicle, SDX-101, or SDX-308 was added to appropriate wells. After 5 days, the cells were fixed and stained for TRAP activity. TRAP+ multinucleated (> 3 nuclei) cells were recorded for each well. Data are the mean ± SD of at least 3 measurements. (B) RAW cells, transiently transfected with the 3kB-Luc-SV40 reporter gene, were treated with SDX-101, SDX-308, or vehicle in the presence or absence of RANKL. Luciferase activity was determined 8 hours after RANKL stimulation (150 ng/mL). *Significant difference from RNAKL-treated only (P < .05). (C-D) Raw cells were incubated with drug vehicle, SDX-101, or SDX-308 for 1 hour, treated with RANKL (100 ng/mL) for 30 minutes, and then lysed. Nuclear extracts (NE) and cytoplasmic extracts (CE) were prepared using a commercial kit (Pierce, Rockford, IL). Phospho-p65 in CE and p65 in NE were detected by Western blot assay. (E) Phospho-IκB-α was detected in whole cell lysates of RAW cells by Western blot analysis. (C-E) β-Actin served as loading control.
SDX-101 and SDX-308 inhibit RANKL-induced osteoclast formation and NF-κB activation in RAW 264.7 cells. (A) RAW cells (1 × 104 per well) were cultured in the presence of RANKL (50 ng/mL), and drug vehicle, SDX-101, or SDX-308 was added to appropriate wells. After 5 days, the cells were fixed and stained for TRAP activity. TRAP+ multinucleated (> 3 nuclei) cells were recorded for each well. Data are the mean ± SD of at least 3 measurements. (B) RAW cells, transiently transfected with the 3kB-Luc-SV40 reporter gene, were treated with SDX-101, SDX-308, or vehicle in the presence or absence of RANKL. Luciferase activity was determined 8 hours after RANKL stimulation (150 ng/mL). *Significant difference from RNAKL-treated only (P < .05). (C-D) Raw cells were incubated with drug vehicle, SDX-101, or SDX-308 for 1 hour, treated with RANKL (100 ng/mL) for 30 minutes, and then lysed. Nuclear extracts (NE) and cytoplasmic extracts (CE) were prepared using a commercial kit (Pierce, Rockford, IL). Phospho-p65 in CE and p65 in NE were detected by Western blot assay. (E) Phospho-IκB-α was detected in whole cell lysates of RAW cells by Western blot analysis. (C-E) β-Actin served as loading control.
The NF-κB/Rel transcription factors are present in the cytosol in an inactive state, complexed with the inhibitory IκB proteins. Activation occurs via phosphorylation of IκB-α, resulting in proteasome-dependent degradation of IκB-α and the release and nuclear translocation of active NF-κB. The key regulatory step in this pathway involves activation of the IκB kinase (IKK) complex, which consists of 3 tightly associated IKK subunits: IKK-α, IKK-β, and IKK-γ. IKK-γ serves as its regulatory subunit. Once IκB-α is activated, it leads to a phosphorylation of p65-p50 inducing a translocation into the nuclei, where it binds to the appropriate response element sequences in the promoter regions of target genes. Therefore, we next investigated whether SDX-101 and SDX-308 inhibit the phosphorylation and nuclear translocation of NF-κB p65. To achieve a significant stimulation for NF-κB signaling study, we used short-term exposure (30 minutes) of SDX-308 at the higher concentrations (50-100 μM). SDX-308 already displayed strong inhibition of constitutive and RANKL-induced p65 phosphorylation as well as NF-κB p65 nuclear translocation in OCL precursor RAW 264.7 cells (Figure 6C-D). SDX-101 had only minor effects.
Because NF-κB activation by most stimulators requires the phosphorylation and degradation of its inhibitory subunit, IκB-α, we next determined whether the inhibition of NF-κB activation by SDX-308 was caused by the suppression of IκB-α signaling. Our data showed that high levels of IκB-α phosphorylation were observed in the OCL cell line RAW 264.7. Treatment of SDX-308 decreased constitutive and RANKL-induced phosphorylation levels of IκB-α (Figure 6E). The densitometric analysis of the immunoblot data (3 experiments) showed that SDX-308, but not SDX-101, significantly inhibited IκB-α phosphorylation (Figure S2).
SDX-308 inhibits NF-κB activation in MM cells
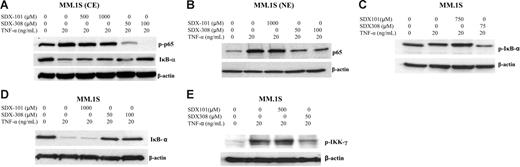
To confirm the inhibitory effects of SDX compounds on NF-κB activation in MM, we used human MM cell line MM.1S cells. TNF-α stimulation for 15 minutes resulted in the obvious phosphorylation and nuclear shift of NF-κB p65. However, pretreatment with SDX-308 significantly decreased TNF-α–induced p65 phosphorylation and nuclear translocation (Figures 7A-B, S3). Furthermore, as shown in Figure 7A, SDX-308 exposure also displayed strong inhibition of constitutive phosphorylation of p65. In addition, SDX-308 inhibited TNF-α–induced IκB-α phosphorylation and degradation, suggesting that SDX-308 might inhibit the release of p65 from IκB-α (Figure 7A [middle panel], C-D). In contrast, SDX-101 had little or no obvious effect on IκB-α phosphorylation and degradation.
SDX-308 inhibits NF-κB activation signaling in MM cell line MM.1S cells. MM.1S MM cells were incubated with drug vehicle, SDX-101, or SDX-308 for 1 hour, treated with TNF-α (20 ng/mL) for 15 minutes, and then lysed. NE and CE were prepared as described in Figure 6. (A-B) Phospho-p65 and IκB-α in CE and p65 in NE were detected by Western blot assay. (C-E) MM.1S cells were treated with drug vehicle, SDX-101, and SDX-308 for 1 hour and then stimulated by TNF-α in the presence of calyculin A (50 nM) for 15 minutes. Phospho-IκB-α, IκB-α, and phospho-IKK-γ in whole cell lysates were detected using the respective antibody. (A-E) β-Actin served as loading control.
SDX-308 inhibits NF-κB activation signaling in MM cell line MM.1S cells. MM.1S MM cells were incubated with drug vehicle, SDX-101, or SDX-308 for 1 hour, treated with TNF-α (20 ng/mL) for 15 minutes, and then lysed. NE and CE were prepared as described in Figure 6. (A-B) Phospho-p65 and IκB-α in CE and p65 in NE were detected by Western blot assay. (C-E) MM.1S cells were treated with drug vehicle, SDX-101, and SDX-308 for 1 hour and then stimulated by TNF-α in the presence of calyculin A (50 nM) for 15 minutes. Phospho-IκB-α, IκB-α, and phospho-IKK-γ in whole cell lysates were detected using the respective antibody. (A-E) β-Actin served as loading control.
As the regulatory subunit of the IKK complex, IKK-γ plays a central role in transporting IKK toward its activating kinase (eg, NIK). Activation of IKK is required for TNF-α–induced phosphorylation and degradation of IκB and subsequent p65 activation.16 Since SDX-308 inhibited the IκB-p65 pathway, we examined whether SDX-308 could attenuate the activation of IKK-γ. As shown in Figure 7E, SDX-308 treatment markedly decreased TNF-α–induced phosphorylation levels of IKK-γ in MM.1S cells, indicating the regulatory pathway of IKK-IκB was disrupted by SDX-308 treatment.
Discussion
Despite the development of novel treatment approaches and high-dose therapies, MM remains incurable. MM is frequently associated with the development of severe osteolytic lesions resulting in high morbidity. Therefore, novel rational treatment strategies that could target both MM cell viability and OCL function offer great promise for improving patient outcome.
To evaluate the effects of R-etodolac on the formation of OCL, we used a human bone marrow culture system from healthy donors and MM patients to generate OCL with in vitro RANKL/M-CSF stimulation. Although a minimum of 400 μM SDX-101 is required to inhibit the growth of MM cell lines, 75 μM SDX-101 was sufficient to significantly (50%) inhibit OCL formation. In contrast, just 7.5 μM SDX-308 strongly inhibits OCL formation (> 50%), a 10-fold higher potency than SDX-101. We also examined the effects of the compounds on OCL activity. OCL activity measured as bone resorption was significantly (by 75%) reduced by SDX-101, while it was completely (100%) inhibited by SDX-308; once again, SDX-308 displayed 10-fold greater potency than SDX-101.
OCLs develop from GM-CFU–derived cells, which are the committed granulocyte-macrophage progenitors17 formed from precursors of myelomonocytic origin.17–22 Regardless of the precise origin of OCL progenitors, it is clear that any disruption of hematopoiesis can profoundly affect OCL numbers. In previous studies, we have shown that inhibition of OCL formation can be induced by a shift of lineage commitment of hematopoietic precursors.23 Therefore, we tested whether the inhibition of OCL development is induced either by an early event in OCL precursor commitment or during OCL development and maturation. In time-course experiments, we found that drug treatment only for the first week of the 3-week OCL cultures was less effective at inhibiting OCL formation. In contrast, 3-week continuous treatment with SDX-101 or SDX-308 induced a stronger inhibition of OCL formation, suggesting that both drugs act not specifically on early stages of the OCL development but rather throughout OCL differentiation and maturation. In addition, treatment of already committed OCL precursors (CFU-GM) strongly inhibited OCL development, further supporting our findings that the drug does not act selectively on early OCL precursors. In accordance with these data are our findings that SDX-101 and SDX-308 did not affect transcription factors critical for OCL development, such as PU-1, c-fos, and NFATc1 (nuclear factor of activated T-cell c1) (data not shown). Research has shown that OCLs and MM have a strong interaction. Abe et al24 demonstrated that OCLs support the growth of myeloma cells. They also demonstrated that the adhesive interaction between myeloma cells and OCLs both increases the proliferation of myeloma cells and promotes the survival of OCLs. Therefore, inhibiting OCL cell growth and survival by SDX-308 might also enhance the antimyeloma effect.
In the present study, we found that SDX-101 inhibits the growth of MM cells with an IC50 of 0.1 to 1 mM. This is in accordance with the results of Yasui et al,1 who reported that SDX-101 inhibits the growth of different MM cell lines with an IC50 of 0.22 to 1.06 mM. In previous chronic lymphocytic leukemia (CLL) clinical trials, reduced average lymphocyte counts were observed in patients treated with SDX-101 at steady state blood levels of approximately 300 to 600 μM (L.L., oral communication, 2005). It has been shown that the cytotoxic potency of etodolac in primary CLL patient samples or in B-cell malignant cell lines does not depend on the COX-2–inhibitory properties of the drug. The inhibition of viability does not vary significantly between the potent COX-2 inhibitory S-enantiomer and the COX-2 inactive R-enantiomer (SDX-101) (L.L., oral communication, 2005). Interestingly, we found that SDX-308, a structural analog of SDX-101, induces inhibition of proliferation of MM cell lines at 10-to 100-fold lower concentrations than SDX-101, with an IC50 of 0.01-0.1 mM, although again SDX-308 remains a relatively impotent inhibitor of COX-2 when compared with R, S-etodolac (IC50 > 60μM versus 3.7 μM, respectively).26
It is known that osteoblast development and activity are inhibited in MM.26 Therefore, we were interested to find out whether SDX-101 and SDX-308 affect osteoblastogenesis or normal hematopoiesis. Importantly, neither SDX-101 nor SDX-308 treatment inhibited osteoblast differentiation and function, as demonstrated by ALP assay, Og2 promoter activation, and von Kossa staining (mineralization). In standard colony-formation assays using purified human CD34+ cells, we observed no effect upon hematopoietic precursor cytotoxicity by SDX-101 or SDX-308 at concentrations that significantly inhibited OCL formation (75 μM and 7.5 μM, respectively). These results show that neither compound had toxic or inhibitory effects on osteoblasts or hematopoietic precursors and further support the potential utility of these compounds, especially SDX-308, in the treatment of MM.
NF-κB plays a critical role in the regulation of the cell cycle, cell adhesion, cytokine production, apoptosis, and other important cellular processes in MM. OCL formation and activity are mediated by RANKL-induced NF-κB activation.27 Therefore, the inhibition of NF-κB activity might be an effective approach to target osteoclast activity and as well as growth of MM cells. In this study, we demonstrated that SDX-308 dose dependently inhibits OCL formation in human bone marrow mononuclear cells, as well as in the homogeneous murine OCL cell line RAW 264.7. SDX-308 inhibited constitutive and RANKL-induced NF-κB activation in RAW 264.7 cells by inhibiting the phosphorylation and nuclear translocation of p65, as well as phosphorylation of IκB-α. In accordance with the literature, MM.1S showed constitutive activation of NF-κB.28 SDX-308 also displayed a strong inhibition of constitutive and TNF-α–induced NF-κB p65 phosphorylation and nuclear translocation in MM MM.1S cells and suppressed TNF-α–induced phosphorylation and degradation of IκB-α. It has been documented that IKK-γ interacts preferentially with IKK-β and is essentially required for the activation of the IKK complex by facilitating the recruitment of IκB proteins into the IκB kinase complex.16,29 In addition, IKK-γ maintains a stoichiometric functional IKK complex and is required by upstream signals (eg, NIK) to activate IKK activity, and it serves as a regulatory function for IKK activation.30 Our data show that inhibition of the IκB-α–NF-κB pathway by SDX-308 might result from the suppression of the upstream IKK activation.
These data support the hypothesis that SDX-308 inhibits NF-κB activity in MM cells as well as in OCLs, resulting in inhibition of MM growth, OCL formation, and activity accompanied by decreased bone resorption. SDX-308 does not affect cells with lower NF-κB activity, such as hematopoietic precursors and osteoblasts, indicating a high selectivity toward MM cells and activated OCLs. Despite the fact that COX-2 has been implicated as an important regulator of OCL formation and bone destruction in bone metastasis,31 our data suggest that SDX-101 and SDX-308 exert their effects through COX-2–independent NF-κB inhibition. SDX-308 has a chiral center reminiscent of that of etodolac. As a racemic mixture, SDX-308 appears to be a relatively impotent inhibitor of COX-1 or COX-2, with in vitro IC50 values greater than 60 μM against both COX isoforms. By using dosing regimens of SDX-308 that display antitumor efficacy, preclinical studies conducted in tumor-bearing animals indicated that steady state blood levels were achieved in the range of 1 to 10 μM. This suggests that significant inhibition of COX-2 may not be observed at doses effective in the treatment of neoplastic disease (G.E. and L.L., unpublished results, 2005). Since COX is the key enzyme that catalyzes the synthesis of prostaglandin E-2 (PGE2), we analyzed the PGE2 levels under SDX-101 and SDX-308 treatment and found that neither SDX-101 nor SDX-308 inhibited PGE2 production of OCL (data not shown). In addition, analysis by Luminex 200 cytokine ELISA array of 33 cytokines secreted by OCL did not show significant inhibition of inflammatory cytokines such as IL-6 or IL-11 or other cytokines involved in osteoclast or MM activation (data not shown).
In summary, in this study we show for the first time that SDX-308 inhibits OCL formation and activity as well as MM growth by inhibition of the NF-κB pathway. Studies previously conducted in Daudi and HT-29 tumor-bearing animals suggest that SDX-308 has significant efficacy without demonstrable toxicity.4,5 Targeting both the myeloma cell and the activated OCL makes SDX-308 a very exciting potential drug in the treatment of MM as well as other diseases associated with high OCL activity that result in lytic bone lesions.
Authorship
Conflict of interest disclosure: Two authors, G.E. and L.L., previously worked for Salmedix, the company that developed SDX-308. Salmedix is now owned by Cephalon. The authors declare no competing financial interests.
Correspondence: Suzanne Lentzsch, Department of Medicine, Division of Hematology/Oncology, University of Pittsburgh Medical Center (UPMC) Cancer Pavilion, Fifth Floor, 5150 Centre Ave, Pittsburgh, PA 15232; e-mail: lentzschs@upmc.edu.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal