Infusion of endothelial cells may assist the recovery of hematopoiesis after bone marrow damage induced by cytotoxic therapies such as those used in cancer patients.
The interactions between endothelial cells (ECs) and hematopoietic cells have long fascinated developmental biologists, hematologists, and vascular biologists alike. These cells reside in close proximity in embryonic and adult tissues. The cells derive from a common embryonic precursor (the hemangioblast), and many groups proposed the existence of hemangioblasts in adults. Functionally, angiogenesis and hematopoiesis are intertwined at multiple levels. Vascular endothelium is a major conduit for hematopoietic cells in the body, whereas hematopoietic cells—at various stages of their differentiation—can modulate new vessel formation in development and disease.1–3 In this issue of Blood, Chute and colleagues uncover a new facet of this intricate functional relationship. Remarkably, the authors show that infusion of ex vivo–expanded primary murine brain–derived ECs (MBECs)—without hematopoietic stem cells—can protect hematopoietic precursors and accelerate the recovery of hematopoiesis after whole-body exposure to high doses of ionizing radiation.
Exposure to radiation or chemotherapeutics during cancer therapy severely impairs the bone marrow's capacity to sustain normal hematopoiesis. This effect is due to direct killing of hematopoietic precursors, as well as of mesenchymal and endothelial cells within bone marrow stroma. Cytokine therapy (to increase hematopoietic cell survival/mobilization) and cell-based therapies (mesenchymal bone marrow cells) have been proposed and are being clinically used or tested. Chute et al's results indicate that ECs, and in particular MBECs, may be a potentially viable strategy for cell-based therapy to accelerate hematologic recovery after bone marrow injury (see figure).
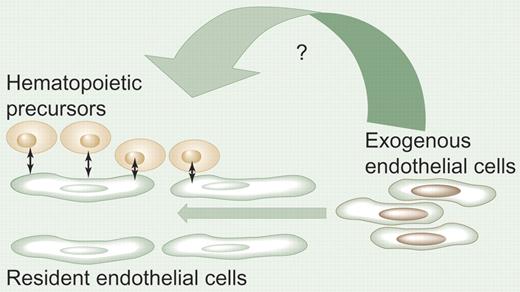
Promotion of hematopoiesis by endothelial cell infusion. In hematopoietic organs, the vascular endothelium interacts with the hematopoietic stem/progenitor cells either directly or indirectly via multiple soluble factor pathways. Following bone marrow damage, infusion of endothelial cells may help restore normal hematopoiesis by yet-unrecognized mechanisms. The effects may be exerted directly on hematopoietic stem cells (in the osteoblastic niche) or indirectly on bone marrow endothelium. Alternatively, infused endothelial cell–derived factors may effect survival, proliferation, or differentiation of more differentiated hematopoietic precursors. Illustration by Paulette Dennis.
Promotion of hematopoiesis by endothelial cell infusion. In hematopoietic organs, the vascular endothelium interacts with the hematopoietic stem/progenitor cells either directly or indirectly via multiple soluble factor pathways. Following bone marrow damage, infusion of endothelial cells may help restore normal hematopoiesis by yet-unrecognized mechanisms. The effects may be exerted directly on hematopoietic stem cells (in the osteoblastic niche) or indirectly on bone marrow endothelium. Alternatively, infused endothelial cell–derived factors may effect survival, proliferation, or differentiation of more differentiated hematopoietic precursors. Illustration by Paulette Dennis.
These exciting findings open new avenues for research and clinical translation but also raise important questions. How is MBEC infusion exerting these effects? It is tempting to speculate that MBECs restore damaged bone marrow endothelium and release growth and survival factors for hematopoietic cells. But the authors show that the infused MBECs cannot be found within the bone marrow or blood, and their indirect impact on bone marrow vasculature is unclear. Moreover, infusion of VEGF, PDGF, SDF-1, and IL-6 (all expressed by MBECs) could not rescue hematopoiesis in Chute et al's model. Where are the MBECs lodging after infusion? The entrapment of MBECs in microvasculature (eg, in lungs or liver) may be critical for the expression of the yet-unidentified survival/growth factor(s), which, in turn, may be responsible for the effects seen. Alternatively, ex vivo manipulation of the normally quiescent MBECs may change their growth/survival factor expression repertoire. It has been recognized for many decades that human blood contains circulating ECs (CECs), but their functions remain unclear. Is hematopoiesis one of them? Could autologous CECs, or ECs from another source (eg, bone marrow), be expanded and used as efficiently as the MBECs to assist hematopoietic recovery? Could the ECs be genetically modified to enhance their activity? The initial data of Chute et al on the use of fetal blood ECs are promising and warrant further investigation. Finally, infused ECs can home to sites of active angiogenesis.4 Thus, when we identify the appropriate EC type and/or EC-derived soluble factor(s), will their infusion have any impact on tumor angiogenesis/relapse in patients receiving cytotoxic therapies?
While we await the answers to these questions, the findings of Chute et al provide hope that these novel approaches will enhance hematopoietic recovery following bone marrow damage.
The author declares no competing financial interests. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal