Abstract
Thrombotic microangiopathy and acute renal failure are cardinal features of postdiarrheal hemolytic uremic syndrome (HUS). These conditions are related to endothelial and epithelial cell damage induced by Shiga toxin (Stx) through the interaction with its globotriaosyl ceramide receptor. However, inflammatory processes contribute to the pathogenesis of HUS by sensitizing cells to Stx fractalkine (FKN), a CX3C transmembrane chemokine expressed on epithelial and endothelial cells upon activation, is involved in the selective migration and adhesion of specific leukocyte subsets to tissues. Here, we demonstrated a selective depletion of circulating mononuclear leukocytes expressing the receptor for FKN (CX3CR1) in patients with HUS. We found a unique phenotype in children with HUS distinct from that seen in healthy, uremic, or infected controls, in which monocytes lost CX3CR1, down-modulated CD62L, and increased CD16. In addition, the CD56dim natural killer (NK) subpopulation was decreased, leading to an altered peripheral CD56dim/CD56bright ratio from 10.0 to 4.5. It is noteworthy that a negative correlation existed between the percentage of circulating CX3CR1+ leukocytes and the severity of renal failure. Finally, CX3CR1+ leukocytes were observed in renal biopsies from patients with HUS. We suggest that the interaction of CX3CR1+ cells with FKN present on activated endothelial cells may contribute to renal injury in HUS.
Introduction
The postdiarrheal form of hemolytic uremic syndrome (HUS) is characterized by hemolytic anemia, thrombocytopenia, and acute renal failure,1,2 and has been associated with enterohemorrhagic infections caused by Shiga toxin (Stx)–producing Escherichia coli (STEC).3,4 Endothelial dysfunction induced by Stx is a central event to the development of thrombotic microangiopathy observed in children with HUS. However, clinical and experimental data suggest that the host's inflammatory response to other bacterial virulence factors plays a pivotal role in the pathogenesis of the disease. Lipopolysaccharide (LPS) O157 has been shown to activate platelets,5 and several other studies demonstrated activation of myeloid cells by Stx and LPS.6–8 Moreover, we have previously demonstrated that depletion of hepatic and splenic macrophages diminished Stx cytotoxicity in mice.9 We have also demonstrated that circulating levels of C-C chemokines for monocytes (Mo's) were associated with the severity of postdiarrheal HUS.8
The migration and interaction of leukocytes with blood vessels involve the elaboration of cytokines and chemokines by injured cells.10,11 Leukocyte attachment and rolling on endothelial cells is promoted by vasodilatation and expression of selectins, followed by integrin-mediated cellular adhesion.10,11 Chemokines can be divided broadly into inflammatory and homeostatic chemokines.12 However, the pattern of chemokine expression and function of vascular endothelium are completely modified once activated. Fractalkine, (FKN; CX3CL1) is the only member of a novel class of chemokines (CX3C). In contrast to all other known chemokines, FKN is a type 1 transmembrane protein, consisting of a chemokine head tethered to the cell surface by a mucin stalk and a short cytoplasmic tail.13 Metalloproteases lead to the proteolytic cleavage of FKN under constitutive or inflammatory conditions.14 FKN may thus function as an adhesion molecule (membrane-anchored form) as well as a potent soluble (s) chemoattractant.12
FKN has been detected on endothelial15,16 and epithelial cells,17,18 whereas the FKN receptor (CX3CR1) is expressed on Mo's, natural killer (NK) cells, and a subset of CD8+ T cells.19,20 CX3CR1 is absent on neutrophils and B cells,13,14,19 whereas both receptor and ligand have been noted on dendritic cells.21,22 Under conditions of physiologic flow, FKN mediates adhesion of monocytes and NK cells, independently of integrin15,19 and G-coupled protein receptors.23,24 In a murine model of glomerulonephritis, treatment with an anti-CX3CR1 antibody dramatically reduced leukocyte infiltration within glomeruli and improved renal function.25,26 Evidence of CX3CR1-expressing cells within human kidneys suggested a role for FKN in the pathogenesis of glomerulonephritis as well as in renal transplant rejection.27,28
Accordingly, we hypothesized that the interaction of CX3CR1-carrying cells with FKN present on activated endothelial cells could enhance renal damage during HUS. In the present study, we analyzed CX3CR1-expressing circulating leukocyte populations in children with HUS during the acute phase of the illness. We also evaluated serum concentrations of (s)FKN, and the extent of renal infiltration by CD68 or CX3CR1+ macrophages. We found a dramatic reduction of circulating CX3CR1+ monocytes and NK (CD56dim subset) and CD3+ NK cells (NKT) among children with HUS. We also noted a moderate degree of glomerular infiltration by CX3CR1+ macrophages. Posthoc analysis showed a negative correlation between the percentage of circulating CX3CR1+ leukocytes and the severity of renal failure. Collectively, our findings suggest that CX3CR1+ leukocytes may be involved in the pathogenesis of HUS, acting locally through cytotoxic mechanisms triggered after cell adhesion to vascular endothelium injured by Stx.
Patients, materials, and methods
Patients and samples
The study was approved by the Hospital Ethical Committees: the Comité de Bioética del Hospital Municipal del Niño de San Justo, San Justo, Buenos Aires, Argentina; the Comité de Bioética del Hospital Ricardo Gutiérrez, Capital Federal, Buenos Aires, Argentina; and that of Ste-Justine Hospital, Montreal, Canada. All patients were enrolled after informed consent was obtained from their parents. The Argentinean group included 31 children who were studied in the acute period of HUS. The diagnostic criteria for HUS were microangiopathic hemolytic anemia with schizocytes, thrombocytopenia (platelet count < 150 × 109/L), and acute renal failure (serum creatinine level > 106.0 μM). All patients developed HUS after a prodrome of gastroenteritis with bloody diarrhea. Half of the children showed evidence of Stx-producing E. coli O157 by stool culture and/or the presence of Stx antibody in serum. There were 18 girls and 13 boys in the study. Clinical and biochemical data of patients are presented in Table 1, and the absolute counts of peripheral leukocytes are summarized in Table 2. Blood samples (2 mL) were obtained by venopuncture into EDTA plastic tubes for biochemical and immunologic studies. Samples were collected before dialysis and/or transfusion at different days after the onset of diarrhea (Table 1). In some patients, a small amount of blood sample precluded measurements of all studied parameters, and the number of patients and controls analyzed for each determination is indicated in each figure. Two children with HUS died within 15 days of admission. Blood samples from 3 additional control groups, matched for age and sex, were collected and processed identically. These included (1) healthy children (HC) admitted for routine surgical procedures; (2) children with acute renal failure due to postinfectious glomerulonephritis evidenced by high levels of urea (7.1-21.5 mM) and creatinine (106.2-234.5 μM), low complement levels, hematuria, and proteinuria (“uremic children” [UC]). In some cases, neutrophilia was also noted. (3) Infected children (IC) with an acute bacterial urinary infection not related with HUS. In this group, platelet count, serum levels of urea and creatinine, and hematocrit were within the normal range.
Clinical and biochemical data of patients with HUS
| . | Severity of renal dysfunction . | ||
|---|---|---|---|
| Grade I . | Grade II . | Grade III . | |
| Total no. patients | 9 | 13 | 9 |
| General parameters | |||
| Age, mo | 31.0 ± 23.2 | 42.4 ± 36.3 | 40.3 ± 33.5 |
| Duration of diarrhea, d | 5.0 ± 3.6 | 1.8 ± 0.6 | 3.2 ± 0.9 |
| Blood and renal parameters | |||
| Platelets, × 109/L | 98.9 ± 123.5 | 67.6 ± 28.2 | 49.3 ± 21.1 |
| Hematocrit, proportion of 1 | 0.22 ± 0.03 | 0.21 ± 0.05 | 0.20 ± 0.06 |
| Urea, mM | 20.1 ± 14.0 | 28.6 ± 14.8 | 37.8 ± 27.5 |
| Creatinine, μM | 195 ± 167 | 292 ± 211 | 617 ± 297 |
| Time from the onset of diarrhea, d* | 5.5 ± 1.9 | 5.4 ± 4.4 | 7.0 ± 8.4 |
| . | Severity of renal dysfunction . | ||
|---|---|---|---|
| Grade I . | Grade II . | Grade III . | |
| Total no. patients | 9 | 13 | 9 |
| General parameters | |||
| Age, mo | 31.0 ± 23.2 | 42.4 ± 36.3 | 40.3 ± 33.5 |
| Duration of diarrhea, d | 5.0 ± 3.6 | 1.8 ± 0.6 | 3.2 ± 0.9 |
| Blood and renal parameters | |||
| Platelets, × 109/L | 98.9 ± 123.5 | 67.6 ± 28.2 | 49.3 ± 21.1 |
| Hematocrit, proportion of 1 | 0.22 ± 0.03 | 0.21 ± 0.05 | 0.20 ± 0.06 |
| Urea, mM | 20.1 ± 14.0 | 28.6 ± 14.8 | 37.8 ± 27.5 |
| Creatinine, μM | 195 ± 167 | 292 ± 211 | 617 ± 297 |
| Time from the onset of diarrhea, d* | 5.5 ± 1.9 | 5.4 ± 4.4 | 7.0 ± 8.4 |
According to Gianantonio et al's criteria,36 patients were classified as mild cases (grade I: no anuria), moderate cases (grade II: less than 7 days of anuria), or severe cases (grade III: more than 7 days of anuria). Data are presented as means ± SD.
The number of days between the time of blood sample collection and the onset of diarrhea.
Absolute numbers of circulating leukocytes in different clinical groups
| . | HC . | HUS . | UC . | IC . |
|---|---|---|---|---|
| No. patients | 21 | 31 | 20 | 17 |
| WBC count, × 109/L | 8.55 ± 2.71 | 17.06 ± 6.65* | 12.35 ± 5.42 | 20.56 ± 11.87* |
| Monocytes, % | 4.57 ± 1.63 | 7.30 ± 3.28* | 6.58 ± 3.16†‡ | 7.74 ± 2.71* |
| Monocytes, × 109/L | 0.40 ± 0.23 | 1.26 ± 0.77* | 0.70 ± 0.0.39§ | 1.50 ± 1.27* |
| Lymphocytes, × 109/L | 3.26 ± 1.22 | 5.14 ± 2.42§ | 3.20 ± 1.46‖ | 4.97 ± 5.00 |
| NK cells, × 109/L | 0.29 ± 0.16 | 0.20 ± 0.05† | 0.30 ± 0.06 | 0.64 ± 0.26 |
| NKdim, × 109/L | 0.18 ± 0.12 | 0.09 ± 0.08 | 0.22 ± 0.09 | 0.55 ± 0.60 |
| NKbright, × 109/L | 0.02 ± 0.02 | 0.04 ± 0.03 | 0.02 ± 0.01 | 0.04 ± 0.02 |
| . | HC . | HUS . | UC . | IC . |
|---|---|---|---|---|
| No. patients | 21 | 31 | 20 | 17 |
| WBC count, × 109/L | 8.55 ± 2.71 | 17.06 ± 6.65* | 12.35 ± 5.42 | 20.56 ± 11.87* |
| Monocytes, % | 4.57 ± 1.63 | 7.30 ± 3.28* | 6.58 ± 3.16†‡ | 7.74 ± 2.71* |
| Monocytes, × 109/L | 0.40 ± 0.23 | 1.26 ± 0.77* | 0.70 ± 0.0.39§ | 1.50 ± 1.27* |
| Lymphocytes, × 109/L | 3.26 ± 1.22 | 5.14 ± 2.42§ | 3.20 ± 1.46‖ | 4.97 ± 5.00 |
| NK cells, × 109/L | 0.29 ± 0.16 | 0.20 ± 0.05† | 0.30 ± 0.06 | 0.64 ± 0.26 |
| NKdim, × 109/L | 0.18 ± 0.12 | 0.09 ± 0.08 | 0.22 ± 0.09 | 0.55 ± 0.60 |
| NKbright, × 109/L | 0.02 ± 0.02 | 0.04 ± 0.03 | 0.02 ± 0.01 | 0.04 ± 0.02 |
Data are expressed as means ± SD. NK cell studies were performed on a lesser number of patients: HC (n = 18), HUS (n = 16), UC (n = 9), and IC (n = 8).
WBC indicates white blood cells.
P < .001 compared with HC.
P < .05 compared with HC.
P < .01 compared with HUS.
P < .005 compared with HC.
P < .005 compared with HUS.
Antibodies and studies
We used the following monoclonal antibodies (mAbs) to characterize Mo subpopulations by flow cytometry: CD14-PECy5 (mouse IgG2a, Immunotech, Marseille, France), CD16-PE (R-phycoerythrin, mouse IgG1; BD Biosciences, San Jose, CA), CX3CR1-FITC (fluorisotiocianated rat IgG2b; Medical & Biological Laboratories, Woburn, MA), and CD62L-PE (Caltag Laboratories, Burlingame, CA). To identify NK and NKT cells, we used CD3-PECy5 (mouse IgG1k; BD Biosciences), CD56-PE (mouse IgG1; Becton Dickinson, San Jose, CA), and CX3CR1.
Whole-blood samples (100 μL) were incubated with the specific conjugated mAb for 30 minutes at room temperature. Erythrocytes were then treated with FACS Lysing solution (BD Biosciences), washed, and resuspended in 0.2 μL of ISOFLOW (International Link SA, Buenos Aires, Argentina). In all cases, isotype-matched antibodies were assayed in parallel, and the fluorescence was measured on 100 000 events using the Cell Quest program (CellQuest, San Jose, CA) on a Becton Dickinson FACScan. Mo's were identified and gated according to their forward- and light-scattering (FSC/SSC) dotplot profiles and positive staining for CD14.
PBMC isolation
Blood was diluted 1:2 with saline, layered on a Ficoll-Hypaque cushion (Ficoll Pharmacia, Uppsala, Sweden; Hypaque, Wintthrop Products, Buenos Aires, Argentina) and centrifuged at 400g for 30 minutes as previously described.29 Peripheral blood mononuclear cells (PBMCs) were collected, washed twice, and resuspended in RPMI 10% heat-inactivated fetal calf serum (FCS) and antibiotics. Viability of PBMCs was more than 96% as determined by trypan blue exclusion test.
Influence of plasma or Stx on apoptosis of NK cells
PBMCs (1 × 106) were incubated for 18 hours in the presence or not of plasma from patients included in the different clinical groups (80%) at 37°C in 5% CO2, or in medium containing 10−6 to 10−8 M Stx type 2 (Stx2) (kindly provided by Dr Sugiyama Junichi, Denka Seiken Co Ltd, Nigata, Japan). After overnight incubation, cells were stained for surface CD3 and CD56 expression, and washed. Apoptosis was assessed based on the annexin V (AV)–FITC protein-binding assay according to manufacturer's instructions without the addition of propidium iodide (APO-AF kit; Sigma-Aldrich, St Louis, MO). Results were expressed as a percentage of AV-positive cells among CD3−CD56+ (NK) cells. CD56bright and CD56dim were distinguished by the level of CD56 expression.
Immunohistochemistry
We evaluated kidney biopsy specimens from children with epidemic HUS (D + HUS; n = 9), healthy control children of similar age (n = 4), children with atypical HUS due to factor H deficiency (n = 7), acute renal rejection (n = 1), systemic lupus class IV (International Society of Nephrology/Renal Pathology Society [ISN/RPS] 2003 classification; n = 2), or Henoch-Schölein purpura (n = 2). Tissue sections (3 μm) were cut on super-frost slides from alcohol formalin acid acetic– or Bouin fluid–fixed, paraffin-embedded blocks and immunostained with mAb anti-CD68 antigen (macrosialin; clone KP1, IgG1 k, 1:1500; DAKO SA, Glostrup, Denmark) and anti-CX3CR1 (rabbit polyclonal antibody, AB 1891, 1:20; Chemicon International, Temecula, CA). Sections were dewaxed in a microwave oven in citrate buffer (pH 6) at medium power. After microwave irradiation (2 times for 5 minutes for biopsy specimens fixed in alcohol formalin acid acetic, and 3 times for 5 minutes for specimens fixed in Bouin fluid), slides were allowed to cool at room temperature and were then incubated with the primary antibody, followed by an indirect streptavidin-biotin method and staining reaction with 3-3′ diaminobenzidine (DAB; DAKO ChemMate Detection Kit). Finally, sections were counterstained with hematoxylin. Images were captured from paraffin sections by a Leica DMLB 100 microscope with a Leica DC 300F camera (Leica, Heidelberg, Germany).
Statistic analysis
Results are expressed as means ± SEM. Comparative analyses were performed using a nonparametric, unpaired, 2-tailed Mann-Whitney U test. P values less than .05 were considered significant. Correlations between immunologic and clinical data were done with the nonparametric Spearman rank-correlation test.
Results
Definition of leukocyte populations by CX3CR1 expression
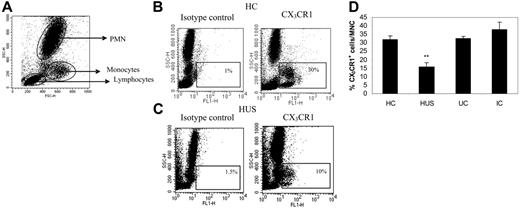
Circulating leukocytes were identified by their SSC-versus-FSC profile after whole-blood analysis by flow cytometry (Figure 1A). Therefore, comparative analysis of CX3CR1 expression on leukocytes was performed by multiparameter flow cytometry using the anti-CX3CR1–FITC mAb on whole blood to avoid variations in its cellular expression during isolation of leukocyte subpopulations. As shown in Figure 1B, several discrete CX3CR1+ mononuclear populations were identified in HC, whereas polymorphonuclear neutrophils (PMNs) were negative for CX3CR1, as previously described.13,14 In children with HUS the percentage of CX3CR1+ leukocytes was significantly reduced (Figure 1C-D), whereas all other groups had a similar expression (Figure 1D).
CX3CR1 expression in whole blood. Whole blood from HC (n = 10), patients with HUS (n = 19), IC (n = 10), and UC (n = 14) was stained with FITC-labeled anti-CX3CR1 and analyzed by flow cytometry as described in “Patients, materials, and methods.” (A) Representative dotplot of FSC versus SSC showing typical circulating leukocyte populations. Representative dotplots of FSC versus FITC fluorescence of (B) 1 healthy child and (C) 1 child with HUS, showing CX3CR1 expression in mononuclear populations. The corresponding isotype control is also presented. (D) Percentage of CX3CR1 cells among mononuclear cells was calculated according the gates indicated in panels B and C for the different clinical groups. Results are expressed as means ± SEM. **P < .001 compared with all clinical groups using the Mann-Whitney U test.
CX3CR1 expression in whole blood. Whole blood from HC (n = 10), patients with HUS (n = 19), IC (n = 10), and UC (n = 14) was stained with FITC-labeled anti-CX3CR1 and analyzed by flow cytometry as described in “Patients, materials, and methods.” (A) Representative dotplot of FSC versus SSC showing typical circulating leukocyte populations. Representative dotplots of FSC versus FITC fluorescence of (B) 1 healthy child and (C) 1 child with HUS, showing CX3CR1 expression in mononuclear populations. The corresponding isotype control is also presented. (D) Percentage of CX3CR1 cells among mononuclear cells was calculated according the gates indicated in panels B and C for the different clinical groups. Results are expressed as means ± SEM. **P < .001 compared with all clinical groups using the Mann-Whitney U test.
To investigate CX3CR1 expression on circulating mononuclear subpopulations from HC and children with HUS, we stained human peripheral blood cells with the corresponding phenotypic markers.
CX3CR1 expression on peripheral Mo's from patients with HUS
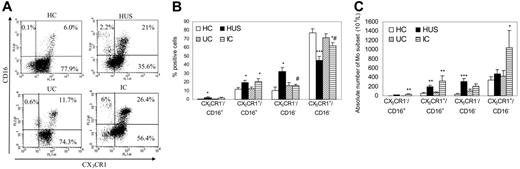
Mo heterogeneity has been recently demonstrated by flow cytometry according to the expression of CD14 and CD16 molecules.30–32 To investigate CX3CR1 expression on circulating mononuclear subpopulations, we stained human peripheral blood cells with antibodies directed against the corresponding phenotypic markers. As shown in Figure 2A, we could define 4 subsets of CD14+ Mo's: CX3CR1+/CD16−, CX3CR1+/CD16+ (double positive), CX3CR1−/CD16+, and CX3CR1−/CD16− (double negative). Most peripheral Mo's from HC corresponded to CX3CR1+/CD16− (approximately 78%), whereas the other subsets represented less than 15% of cells. Children with HUS showed a significant decrease in the percentages of the circulating CX3CR1+/CD16− “classical monocyte” subset (approximately 45%), with a simultaneous increase in all other subsets (Figure 2B).
CX3CR1 expression on peripheral monocytes from different clinical groups. Whole blood from HC (n = 10); patients with HUS (n = 19), UC (n = 14), and IC (n = 10) was stained with PECy5-labeled anti-CD14, PE-labeled anti-CD16, and FITC-labeled anti-CX3CR1 and analyzed by flow cytometry as described in “Patients, materials, and methods.” Mo subpopulations were defined according to their CD14 expression, and that of CX3CR1 and CD16 were depicted. (A) Representative dotplots from 1 child within each clinical group showing CX3CR1 and CD16 expression on CD14+ Mo's. Dot plot quadrants are defined as: CX3CR1+/CD16+, top right; CX3CR1−/CD16+, top right; and CX3CR1+/CD16−, bottom right. The percentages of the different subpopulations are shown within each quadrant. (B) According to CX3CR1 and CD16 membrane expression, 4 subpopulations were defined. Each bar represents the mean ± SEM of the percentage of cells for each subpopulation among HC (□); patients with HUS (▪); UC (▧), and IC (). (C) Each bar represents the mean ± SEM of the absolute number of cells for each Mo subset within the different clinical groups. *P < .05; **P < .01; ***P < .005 statistically different compared with HC and UC; #P < .05 compared with HUS.
CX3CR1 expression on peripheral monocytes from different clinical groups. Whole blood from HC (n = 10); patients with HUS (n = 19), UC (n = 14), and IC (n = 10) was stained with PECy5-labeled anti-CD14, PE-labeled anti-CD16, and FITC-labeled anti-CX3CR1 and analyzed by flow cytometry as described in “Patients, materials, and methods.” Mo subpopulations were defined according to their CD14 expression, and that of CX3CR1 and CD16 were depicted. (A) Representative dotplots from 1 child within each clinical group showing CX3CR1 and CD16 expression on CD14+ Mo's. Dot plot quadrants are defined as: CX3CR1+/CD16+, top right; CX3CR1−/CD16+, top right; and CX3CR1+/CD16−, bottom right. The percentages of the different subpopulations are shown within each quadrant. (B) According to CX3CR1 and CD16 membrane expression, 4 subpopulations were defined. Each bar represents the mean ± SEM of the percentage of cells for each subpopulation among HC (□); patients with HUS (▪); UC (▧), and IC (). (C) Each bar represents the mean ± SEM of the absolute number of cells for each Mo subset within the different clinical groups. *P < .05; **P < .01; ***P < .005 statistically different compared with HC and UC; #P < .05 compared with HUS.
Similarly, the percentage of CX3CR1+/CD16− Mo's was abnormally decreased in IC (approximately 62%), with a simultaneous increase in double-negative or double-positive cells, whereas the relative proportions of Mo subsets were similar in HC and UC. Nevertheless, abnormalities present in the IC group were less striking than among children with HUS, making them significantly different (Figure 2B). Interestingly, children with HUS presented a unique phenotype consisting of significantly reduced percentages of circulating Mo's expressing CX3CR1 (CX3CR1+ Mo: HC = 84.6% ± 2.7%, n = 22; HUS = 55.5% ± 4.0%, n = 31*; UC = 79.0% ± 4.3%, n = 20; and IC = 83.3% ± 1.9%, n = 17; *P < .001 compared with all other clinical groups).
Figure 2C shows that the distribution among the 4 subsets of circulating Mo's was also different in absolute numbers. IC and children with HUS had the total count of Mo's increased (Table 2). However, the number of regular Mo's (CX3R1+/CD16−) was significantly enhanced in IC, but was conserved in children with HUS, who showed, alternatively, an enhancement in double-positive and double-negative subsets (Figure 2C).
Finally, in agreement with previous reports among healthy adults, we noted that all groups showed a higher expression of CX3CR1 on CD16+ than CD16− Mo's (P < .001). However, Mo's from children with HUS showed an abnormally decreased CX3CR1 mean fluorescence intensity (MFI) on the CD16+ subset (CX3CR1 MFI: HC = 115.2 ± 11.1, n = 10; HUS = 71.3 ± 9.3, n = 19; P < .005), as well as on the CD16− subset (HC = 48.1 ± 5.2; HUS = 38.1 ± 3.9; P < .05).
CX3CR1/CD62L expression in peripheral Mo's from patients with HUS
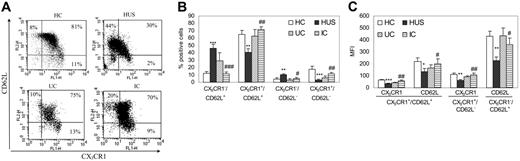
Since Mo subpopulations are distinguished also by the expression of the adhesion receptor CD62L,31 we further characterized Mo subpopulations by measuring CD62L together with CX3CR1 on CD14-gated leukocytes. Most circulating Mo's in HC (CX3CR1+/CD16− Mo's) also express CD62L selectin.31 Comparative analysis showed a significant reduction in the expression of the double-positive (CX3CR1+/CD62L+) population in children with HUS (Figure 3A-B). This was mainly due to the loss of CX3CR1, since the total percentage of CD62L+ cells was conserved (HC = 77.0% ± 5.0%, n = 11; HUS = 86.9% ± 1.6%, n = 13; nonsignificant), and even more, the CX3CR1−/CD62L+ subset was significantly increased (Figure 3B). In this case, the UC and IC groups showed a Mo subset distribution similar to that of HC, and the percentages of all Mo subsets in the IC group were statistically different from those found in children with HUS (Figure 3B).
CD62L/CX3CR1 expression on peripheral Mo's from different clinical groups. Whole blood from HC (n = 11), patients with HUS (n = 13), UC (n = 6), and IC (n = 11) was stained with PECy5-labeled anti-CD14, PE-labeled anti-CD62L, and FITC-labeled anti-CX3CR1, and analyzed by flow cytometry as described in “Patients, materials, and methods.” Mo subpopulations were defined according their CD14 expression, and those of CX3CR1 and CD62L were depicted. (A) Representative dotplots from 1 child within each clinical group showing CX3CR1 and CD62L expression on CD14+ Mo's. Dot plot quadrants are defined as: CX3CR1+/CD62L+, top right; CX3CR1−/CD62L+, top left; and CX3CR1+/CD62L−, bottom right. The percentages of the different subpopulations are shown within each quadrant. (B) According to CX3CR1 and CD62L membrane expression, 4 subpopulations were defined. Each bar represents the mean ± SEM of the percentage of cells for each subpopulation in HC (□), patients with HUS (▪), UC (▧), and IC () groups. (C) Each bar represents the mean ± SEM of the MFI of CX3CR1 and CD62L expression for each Mo subset defined in panel A for HC (□), patients with HUS (▪), UC (▨), and IC (). *P < .05; **P < .01; *** P < .001 statistically different compared with HC. #P < .05; ##P < .01; ###P < .001 compared with children with HUS.
CD62L/CX3CR1 expression on peripheral Mo's from different clinical groups. Whole blood from HC (n = 11), patients with HUS (n = 13), UC (n = 6), and IC (n = 11) was stained with PECy5-labeled anti-CD14, PE-labeled anti-CD62L, and FITC-labeled anti-CX3CR1, and analyzed by flow cytometry as described in “Patients, materials, and methods.” Mo subpopulations were defined according their CD14 expression, and those of CX3CR1 and CD62L were depicted. (A) Representative dotplots from 1 child within each clinical group showing CX3CR1 and CD62L expression on CD14+ Mo's. Dot plot quadrants are defined as: CX3CR1+/CD62L+, top right; CX3CR1−/CD62L+, top left; and CX3CR1+/CD62L−, bottom right. The percentages of the different subpopulations are shown within each quadrant. (B) According to CX3CR1 and CD62L membrane expression, 4 subpopulations were defined. Each bar represents the mean ± SEM of the percentage of cells for each subpopulation in HC (□), patients with HUS (▪), UC (▧), and IC () groups. (C) Each bar represents the mean ± SEM of the MFI of CX3CR1 and CD62L expression for each Mo subset defined in panel A for HC (□), patients with HUS (▪), UC (▨), and IC (). *P < .05; **P < .01; *** P < .001 statistically different compared with HC. #P < .05; ##P < .01; ###P < .001 compared with children with HUS.
The MFI of CD62L was abnormally reduced in HUS patients on double-positive (CX3CR1+/CD62L+) cells as well as on CX3CR1−/CD62L+ cells (Figure 3C), indicating that CD62L was either shed or downmodulated.
CX3CR1 expression on NK cells from patients with HUS
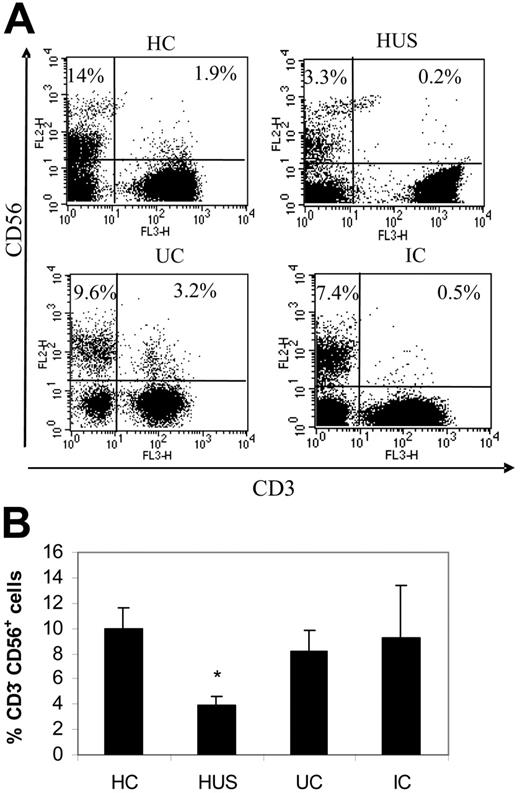
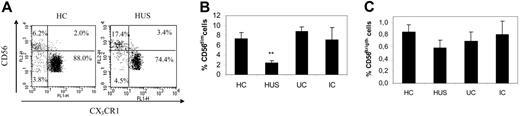
As the NK cell population (CD3−/CD56+) expresses CX3CR1,4 we determined by 3-color flow cytometry its percentage among lymphoid cells using anti-CD3–PECy5, anti-CD56–PE, and anti-CX3CR1–FITC mAbs. As shown in Figure 4, children with HUS presented a significant decrease in the percentage of NK cells compared with the other groups (Figure 4A-B). However, their absolute count of circulating NK cells was normal, but presented much fewer NK cells than did IC, the other clinical group who showed leukocytosis (Table 2). NK cells can be further divided into CD56dim and CD56bright subsets. The former is more abundant, express low levels of CD56, and is also positive for CD16 (CD56dimCD16+)33 and CX3CR1.34 Conversely, CD16 and CX3CR1 are absent on the CD56bright subset.34 Results in Figure 5 and Table 2 show that percentages and absolute counts of the more abundant CD56dim subset were significantly reduced in HUS compared with that of all other clinical groups, without variations in CD56bright cells. The selective decrease in the CD56dim subset could also be observed by analyzing the CD56dim/CD56bright ratio (HC = 10.0 ± 2.0, n = 14; HUS = 4.5 ± 1.3, n = 7; UC = 10.8 ± 2.5, n = 6; and IC = 11.0 ± 4.3, n = 6; P < .05, HUS compared with HC). Finally, a significant reduction in the NKT population was noted only in children with HUS, both in the relative (HC = 2.0% ± 0.4%, n = 14; HUS = 0.2% ± 0.8%, n = 9; P < .001) and in the absolute number (HC = 61.7 ± 8.7 × 106 cells/L, n = 8; HUS = 16.0 ± 5.7 × 106 cells/L, n = 8; P < .001). Moreover, the percentage of CX3CR1+ cells among NKT cells was also reduced in patients with HUS (HC = 57.0% ± 5.1%, n = 14; HUS = 33.6% ± 7.7%, n = 9; P < .05).
Quantification of NK cells in whole blood from different clinical groups. Whole blood from HC (n = 16), patients with HUS (n = 14), UC (n = 5), and IC (n = 6) was stained with PECy5-labeled anti-CD3 and PE-labeled anti-CD56 and analyzed by flow cytometry as described in “Patients, materials, and methods.” (A) Representative dotplots from 1 child within each clinical group showing NK (CD3−CD56+) and NKT (CD3+CD56+) subpopulations in the lymphocyte gate. Dot plot quadrants are defined as: CD3+/CD56+, top right; CD3−/CD56+, top left; and CD3+/CD16−, bottom right. The percentages of the different subpopulations are shown within each quadrant. (B) Each bar represents the mean ± SEM of the percentage of NK cells (CD3−CD56+) relative to lymphocytes. *P < .005 compared with MC.
Quantification of NK cells in whole blood from different clinical groups. Whole blood from HC (n = 16), patients with HUS (n = 14), UC (n = 5), and IC (n = 6) was stained with PECy5-labeled anti-CD3 and PE-labeled anti-CD56 and analyzed by flow cytometry as described in “Patients, materials, and methods.” (A) Representative dotplots from 1 child within each clinical group showing NK (CD3−CD56+) and NKT (CD3+CD56+) subpopulations in the lymphocyte gate. Dot plot quadrants are defined as: CD3+/CD56+, top right; CD3−/CD56+, top left; and CD3+/CD16−, bottom right. The percentages of the different subpopulations are shown within each quadrant. (B) Each bar represents the mean ± SEM of the percentage of NK cells (CD3−CD56+) relative to lymphocytes. *P < .005 compared with MC.
Quantification of NK subsets in different clinical groups. Whole blood from HC (n = 16), patients with HUS (n = 14), UC (n = 5), and IC (n = 6) was stained with PECy5-labeled anti-CD3, PE-labeled anti-CD56, and FITC-labeled anti-CX3CR1 and analyzed by flow cytometry as described in “Patients, materials, and methods.” (A) Representative dotplots from a healthy child and a child with HUS. By gating on CD3− lymphocytes, and according to CD56 and CX3CR1 membrane expression, NK cells are classified into CD56dim and CD56bright. The value in each quadrant represents the percentage relative to the total number of NK cells. (B) Each bar represents the percentage of CD56dim NK cells relative to the total number of lymphocytes, expressed as the mean ± SEM for each clinical group: HC (n = 16), patients with HUS (n = 14), UC (n = 5), and IC (n = 6). (C) Each bar represents the percentage of CD56bright NK cells relative to the total number of lymphocytes, expressed as the mean ± SEM, within each clinical group: HC (n = 18), patients with HUS (n = 16), UC (n = 9), and IC (n = 8). **P < .005 compared with control groups.
Quantification of NK subsets in different clinical groups. Whole blood from HC (n = 16), patients with HUS (n = 14), UC (n = 5), and IC (n = 6) was stained with PECy5-labeled anti-CD3, PE-labeled anti-CD56, and FITC-labeled anti-CX3CR1 and analyzed by flow cytometry as described in “Patients, materials, and methods.” (A) Representative dotplots from a healthy child and a child with HUS. By gating on CD3− lymphocytes, and according to CD56 and CX3CR1 membrane expression, NK cells are classified into CD56dim and CD56bright. The value in each quadrant represents the percentage relative to the total number of NK cells. (B) Each bar represents the percentage of CD56dim NK cells relative to the total number of lymphocytes, expressed as the mean ± SEM for each clinical group: HC (n = 16), patients with HUS (n = 14), UC (n = 5), and IC (n = 6). (C) Each bar represents the percentage of CD56bright NK cells relative to the total number of lymphocytes, expressed as the mean ± SEM, within each clinical group: HC (n = 18), patients with HUS (n = 16), UC (n = 9), and IC (n = 8). **P < .005 compared with control groups.
We then evaluated whether reduction in the NK CD56dim and NKT populations may have been due to specific cell sensitivity to some hypothetical death signals present within circulating blood of children with HUS. We isolated peripheral blood Mo's from healthy adult donors that were incubated with 80% plasma from HC or from patients with HUS. After overnight incubation, apoptosis of the specific subpopulations was assayed by flow cytometry using anti-CD56–PE and anti-CD3–PECy5 antibodies and AV-FITC, as previously described.35 We did not observe any significant enhancement in the apoptotic rate of either cell types after exposure to HUS-derived plasmas (data not shown). Similarly, no change in apoptosis of these cells was noted after exposure to 10−6 M or 10−8 M of Stx2 (data not shown).
Immunohistochemistry
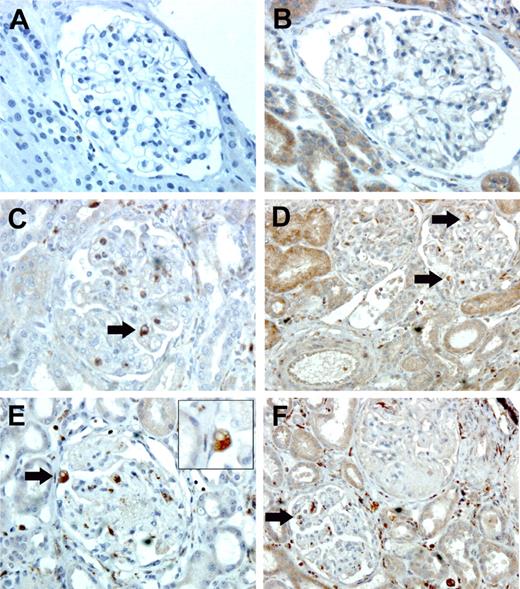
Single labeling for the macrophage marker macrosialin (CD68) and for CX3CR1 was performed on kidney sections from children with postdiarrheal and atypical HUS. We noted glomerular infiltration by CD68+ macrophages and CX3CR1+ cells in both forms of HUS (Figure 6). The presence of CD68− and CX3CR1− cells, probably corresponding to neutrophils within the glomeruli, and CX3CR1+ macrophages and/or lymphocytes within tubules of children with either form of HUS, were also observed. The number of infiltrating CD68+ and CX3CR1+ macrophages per glomerulus is shown in Table 3.
Immunohistochemistry on renal biopsies. Single labeling for CD68 (A,C,E) and for CX3CR1 (B,D,F). Immunohistochemistry was performed on kidney sections from HC (A, B), children with postdiarrheal HUS (C, D), and children with atypical HUS due to factor H deficiency (E, F). The figure shows (arrows) CD68+ and CX3CR1+ cells within glomeruli of children with either form of HUS. Original magnification, × 400 (A-C, E); × 200 (D, F); and × 1000, panel E inset (which shows magnified CD68+ macrophages). Images were acquired using a Leica microscope equipped with a 40 ×/0.65 NA (A-C, E) or 20 ×/0.50 NA (D, F) objective, or a 100 ×/1.30 NA oil-immersion objective lens (E inset). The camera and software (IM 50, V4.0 R11) were also from Leica (Heidelberg, Germany).
Immunohistochemistry on renal biopsies. Single labeling for CD68 (A,C,E) and for CX3CR1 (B,D,F). Immunohistochemistry was performed on kidney sections from HC (A, B), children with postdiarrheal HUS (C, D), and children with atypical HUS due to factor H deficiency (E, F). The figure shows (arrows) CD68+ and CX3CR1+ cells within glomeruli of children with either form of HUS. Original magnification, × 400 (A-C, E); × 200 (D, F); and × 1000, panel E inset (which shows magnified CD68+ macrophages). Images were acquired using a Leica microscope equipped with a 40 ×/0.65 NA (A-C, E) or 20 ×/0.50 NA (D, F) objective, or a 100 ×/1.30 NA oil-immersion objective lens (E inset). The camera and software (IM 50, V4.0 R11) were also from Leica (Heidelberg, Germany).
Immunohistochemistry from renal biopsies
| . | Age, y . | Change in time,* d . | No. cells/glomerulus . | |
|---|---|---|---|---|
| CD68+ . | CX3CR1+ . | |||
| Normal kidney | ||||
| TBD | 5 | — | 0 | 0 |
| TBD | 8 | — | 0 | 0 |
| TBD | 20 | — | 0 | 0 |
| TBD | 25 | — | 0 | 0 |
| Postdiarrheal HUS | ||||
| TBD | 0.75 | 30 | 1 | 3 |
| TBD | 2 | < 7† | 3 | 2 |
| TBD | 2.5 | 300 | 3 | 2 |
| TBD | 3 | 21 | 1 | 4 |
| TBD | 3 | 7 | 8 | 3 |
| TBD | 4 | 21 | 2 | 2 |
| TBD | 6 | NA | 3 | 4 |
| TBD | 6 | 30 | 2 | 4 |
| TBD | 6 | 21† | 14 | 5 |
| Atypical HUS | ||||
| TBD | 1 | NA | 2 | 10 |
| TBD | 6 | NA | 2 | 2 |
| TBD | 7 | Chronic | 2 | 5 |
| TBD | 8 | Chronic | NA | 8 |
| TBD | 8 | < 7 | 5 | 15 |
| TBD | 8 | < 7 | 3 | 8 |
| TBD | 16 | Chronic‡ | 1 | 0 |
| Acute rejection | ||||
| TBD | 3 | — | 7 | 10 |
| Systemic lupus | ||||
| TBD | 12 | — | 7 | 10 |
| TBD | 16 | — | 10 | 8 |
| Henoch—Schönlein purpura | ||||
| TBD | 6 | — | 26 | 36 |
| TBD | 8 | — | 4 | 2 |
| . | Age, y . | Change in time,* d . | No. cells/glomerulus . | |
|---|---|---|---|---|
| CD68+ . | CX3CR1+ . | |||
| Normal kidney | ||||
| TBD | 5 | — | 0 | 0 |
| TBD | 8 | — | 0 | 0 |
| TBD | 20 | — | 0 | 0 |
| TBD | 25 | — | 0 | 0 |
| Postdiarrheal HUS | ||||
| TBD | 0.75 | 30 | 1 | 3 |
| TBD | 2 | < 7† | 3 | 2 |
| TBD | 2.5 | 300 | 3 | 2 |
| TBD | 3 | 21 | 1 | 4 |
| TBD | 3 | 7 | 8 | 3 |
| TBD | 4 | 21 | 2 | 2 |
| TBD | 6 | NA | 3 | 4 |
| TBD | 6 | 30 | 2 | 4 |
| TBD | 6 | 21† | 14 | 5 |
| Atypical HUS | ||||
| TBD | 1 | NA | 2 | 10 |
| TBD | 6 | NA | 2 | 2 |
| TBD | 7 | Chronic | 2 | 5 |
| TBD | 8 | Chronic | NA | 8 |
| TBD | 8 | < 7 | 5 | 15 |
| TBD | 8 | < 7 | 3 | 8 |
| TBD | 16 | Chronic‡ | 1 | 0 |
| Acute rejection | ||||
| TBD | 3 | — | 7 | 10 |
| Systemic lupus | ||||
| TBD | 12 | — | 7 | 10 |
| TBD | 16 | — | 10 | 8 |
| Henoch—Schönlein purpura | ||||
| TBD | 6 | — | 26 | 36 |
| TBD | 8 | — | 4 | 2 |
Each row beneath the boldfaced headings indicates a different child within that category.
NA indicates not available; and —, not applicable.
Refers to the number of days elapsed between the onset of HUS and renal biopsy.
Cortical necrosis.
Recurrence after transplantation.
Correlations
Retrospectively, we analyzed the existence of correlations between the immunologic alterations found in patients with HUS and the severity of HUS, classified according to Gianantonio et al's criteria.36 We found a weak negative correlation between severity and the percentage of total circulating CX3CR1+ leukocytes. Among Mo subsets, the severity of HUS was positively correlated with CX3CR1−/CD62L+ cells (r = .60, P = .027, n = 13), and negatively correlated with CX3CR1+/CD62L+ (r = −.67, P < .001, n = 13).
Moreover, the expression of CD62L on both CX3CR1+ and CX3CR1− monocytes negatively correlated with severity (r = −.62, P = .022, n = 13; and r = −.56, P = .045, n = 13, respectively). In addition, there was no correlation between severity and CX3CR1+/CD56+ cells.
Discussion
The main finding of this study was the dramatic decrease of circulating CX3CR1-carrying monocytes and NK cells in children with HUS. This observation could be related to a unique phenomenon that involves all leukocyte populations or could be due to different effector and/or regulatory mechanisms for each individual cell type. Two general processes may explain the decrease of a specific leukocyte subset defined by a particular membrane receptor (CX3CR1): (1) regulatory mechanisms at the cellular level, which imply down-regulation of its membrane exposure or synthesis; or (2) disappearance from the circulation by specific interaction with its ligand (FKN, CX3CL1). Different mechanisms could underlie both processes. Modulatory cell pathways include enzymatic cleavage and/or impairment of the biosynthetic machinery via enhancement of the degradation rate, and/or enhanced internalization. On the other hand, specific recruitment of CX3CR1+ cells by soluble or membrane-bound FKN in tissues may explain a specific cell disappearance from circulation. Although further experimental models or in vitro studies must be designed to ascertain precisely whether one or more of these mechanisms are operating during HUS, valuable information can be obtained from the present study.
The remarkable changes observed in Mo's from patients with HUS can be summarized as a significant reduction in CX3CR1+ Mo's and a down-modulation of CD62L together with an enhanced expression of the CD16 molecule. However, the most distinctive feature of Mo's from HUS is the loss of the CX3CR1. In fact, Mo subset classification according to CX3CR1 and CD62L expression clearly showed that children with HUS present a unique Mo phenotype distinctive from all other clinical groups. We have demonstrated that children with HUS showed leukocytosis based on increased absolute counts of neutrophils and monocytes (Table 2)37 and, furthermore, that the increase of total Mo's results from the enhancement in the relative and absolute number of CX3CR1− Mo subsets. This altered distribution within Mo subsets observed in patients with HUS could be due to differentiation or modulatory changes of “classical Mo's” (CX3CR1+/CD16−/CD62L+)38 through the acquisition of CD16 and the loss of CD62L and CX3CR1. In this regard, enhancement in the percentage of CD16+ Mo's has been demonstrated after in vitro stimulation of human peripheral blood Mo's with IL-10,39 GM-CSF plus IL-4,38 and plasma from patients with HUS.37 The selectin CD62L (L-selectin), which mediates the initial attachment of leukocytes to endothelial cells,40,41 is shed following cell activation,42,43 and its down-regulation has been proposed to participate in the occurrence of leukocytosis.41 Down-modulation of CX3CR1 in monocytes has not been previously reported.
Alternatively, CX3CR1+ Mo's could be retained in inflamed tissues that express high levels of the CX3CR1 ligand, FKN, or CX3CL1. In this regard, immunohistochemistry detection of CD68+ macrophages and CX3CR1+ leukocytes in kidney sections from patients with HUS supports the concept that these cells could be locally retained. Bazan et al have also reported that FKN induces the migration of Mo's,13 and recently, Chapman et al44 and Ancuta et al45 reported that CX3CR1+ Mo's undergo both transendothelial migration in response to (s)FKN and efficient binding to FKN-expressing cells.
As a component of the innate immune system in circulation, the other CX3CR1-carrying population, NK cells, may be also attracted to areas of inflammation.34 In particular, CD56dim NK cells are strongly attracted by IL-8 and soluble CX3CL1, since they express high levels of CXCR1 and CX3CR1.34 On the contrary, CD56bright NK cells show little or no response to these chemokines, supporting a preferential modulatory role for this NK subset during the early stages of an immune response.33 Besides, circulating NKT cells are a more heterogeneous population, and nearly 50% of them express CX3CR1.34
The reduction observed specifically in the percentage of CD56dim NK cells and NKT cells in children with HUS, without a specific induction of the apoptotic rate of these populations, suggests that these cells are being retained on the endothelium or within tissues. The contribution of this phenomenon to endothelial and/or epithelial damage is still uncertain. However, systemic toxicity in patients treated with IL-2 has been related to the selective disappearance of circulating NK and NKT cells upon massive adherence on activated endothelium.46–48 Moreover, adult HUS has been reported as secondary to IL-2 treatment of metastatic melanoma.49 Although autologous endothelial cells are considered to be protected from NK cell–mediated lytic attack by the expression of MHC class I molecules, viruses, bacteria, or IL-2 administration may directly or indirectly activate endothelial cells, rendering them more susceptible to NK lysis.50 During STEC infections, the direct action of Stx and/or inflammatory mediators released by Mo's and NK cells activates endothelial cells.51,52 In this scenario, the expression of FKN could collaborate with the adherence of CX3CR1-expressing cells, Mo's, and NK cells, which in turn would produce cytotoxic factors, enhancing endothelial damage. Moreover, FKN expression is significantly enhanced in response to TNF, IL-1, and IFN-γ,13–15,53,54 and circulating levels of many cytokines are abnormally increased in patients with HUS.55,56
Finally, we could not rule out that soluble mediators may be inducing a downmodulation or shedding of CX3CR1 from the membrane of Mo's and NK cells in patients with HUS, as shown for NK cells upon activation by CD16 engagement14,57 or IL-15.58 We have measured (s)FKN in the plasma of children with HUS enrolled in a previous study8 (data not shown). Since there were large interindividual variations among healthy children or adults (range, 0-7498 pM), we could not obtain any meaningful interpretation.
Typical HUS occurs after bacterial enteritis; however, distinctive changes occurred in these patients compared with the group of IC. Although IC showed an expanded CD16+ Mo subset, the percentage of CX3CR1+ leukocytes and Mo's, NK and NKT populations, and the mean expression of CD62L were within the normal range. On the other hand, since UC presented Mo and NK phenotypes similar to that of HC, it is unlikely that the changes noted in HUS resulted from the azotemia and metabolic perturbations per se associated with acute renal failure.
Most important, correlation studies indicated that a lower percentage of circulating CX3CR1+ leukocytes or CX3CR1+ Mo's was associated with a longer duration of anuria and a poorer prognosis. Similarly, a lowered expression of CX3CR1+ NK cells has been associated with an increased disease activity in patients with multiple sclerosis.59 Based on the background discussed, we speculate that early Stx-mediated endothelial injury activates leukocytes with a high cytotoxic potential, and leads to a close leukocyte/endothelial cell interaction through the FKN pathway. This process will determine the specific inflammatory phenotype observed in patients with HUS, and the exacerbation of endothelial damage by cells of the innate immune system.
Authorship
Author contributions: M.V.R. and G.C.F. performed phenotypic studies on circulating leukocytes from patients and control groups; R.E., I.G., C.E-C., and G.V. selected patients and control children, obtained the informed consent from their parents and the approval from ethics committee, collected blood samples, and followed clinical and renal evolution; N.P. collected renal samples and performed immunohistochemistry on renal tissues; P.S. and M.C.S. collaborated in phenotypic studies on natural killer cells; H.T. collected urine samples for soluble fractalkine measurements as P.I. of the Synsorb-Pk clinical trial; C.C. performed ELISA for measurements of soluble fractalkine; F.P. codesigned this study with M.S.P., obtained approval from ethics committee, and collected samples for measurements of soluble fractalkine in plasma; M.S.P. designed and conducted immunologic and phenotypic studies and wrote the manuscript.
Conflict-of-interest statement: The authors declare no competing financial interests.
Correspondence: Marina S. Palermo, Academia Nacional de Medicina, Pacheco de Melo 3081, (C1425AUM), Buenos Aires, Argentina; e-mail: mspalermo@hematologia.anm.edu.ar.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marta Felippo, Nora Galassi, and Norma Riera for their excellent technical assistance. The authors also thank Fundación de la Hemofilia and Academia Nacional de Medicina for the use of the FACScan flow cytometer, and the Department of Hemotherapy of Centro de Educación Médica e Investigaciones Clínicas “Norberto Quirno” (CEMIC) for normal blood samples. The authors thank Anna Proietti, research nurse, for her assistance. The authors also want to thank le Comité de Recherché Cliniques, Ste-Justine Hospital Research Centre.
Supported by grants from Fundación Alberto J. Roemmers, and Agencia Nacional de Promoción Científica y Tecnológica, Argentina (M.S.P.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal