Abstract
The TEL/AML1 fusion gene results from the most frequent t(12;21)(p13;q22) translocation in childhood acute lymphoblastic leukemia (ALL). Its contribution to transformation is largely unknown, in particular with respect to survival and apoptosis. We therefore silenced TEL/AML1 expression in leukemic REH cells by RNA inhibition, which eventually led to programmed cell death. Microarray and 2D gel electrophoresis data demonstrated a differential regulation of heat-shock proteins (HSPs), among them HSP90, as well as of its client, survivin. Consistent with these findings, ectopic expression of TEL/AML1 in Ba/F3 cells increased protein levels of HSP90 and survivin and conferred resistance to apoptotic stimuli. Our data suggest that TEL/AML1 not only contributes to leukemogenesis by affecting an antiapoptotic network but also seems to be indispensable for maintaining the malignant phenotype. The functional relationship between TEL/AML1, HSP90, and survivin provides the rational for targeted therapy, be it the fusion gene or the latter 2 proteins.
Introduction
The TEL/AML1 fusion gene is generated by t(12;21)(p13;q22), the most frequent chromosomal translocation in childhood acute lymphoblastic leukemia (ALL).1 There is convincing evidence that the gene fusion already takes place in utero in the majority of children with TEL/AML1-positive ALL, and this suggests, together with studies in twins, that it represents an early, or even initiating, event in leukemia development.2,3 This translocation, however, is not sufficient for the manifestation of a clinical disease, but seems rather to induce a protracted preleukemic state in which additional postnatal genetic events may accumulate, eventually leading to malignant transformation.4,5
The role of TEL/AML1 expression in the affected cell was recently addressed in several studies and indicates an interference with differentiation in the B lineage as well as enhanced self-renewal of B cells.6–8 Of importance, such mutations that impair hematopoietic differentiation are assumed to interfere also with apoptosis.9 The mechanisms by which the fusion protein breaches this crucial anticancer shield have, so far, not been identified.
The function of genes, in particular of fusion genes, has been studied in recent years by gene silencing using RNA interference (RNAi), a powerful strategy to specifically target the gene of interest.10 In this study, we investigated the functional contribution of TEL/AML1 to the malignant phenotype focusing on the evasion of programmed cell death by silencing the fusion gene in the t(12;21)-positive cell line REH.
Materials and methods
Cell culture
The B-cell precursor (BCP) leukemic cell line REH (having the TEL/AML1 fusion gene, the second TEL allele deleted and AML1 retained) and the mouse IL3-dependent BCP Ba/F3 cells were grown in 24-well plates (Nunc, Roskilde, Denmark) at 1 × 106 cells/mL.
Short-interfering RNA (siRNA) design and transfection of cells
siRNAs targeting the fusion region of TEL/AML1 were designed11 and synthesized by Dharmacon (Lafayette, CO). siRNA duplexes were handled essentially as described.12 The transfection of REH cells with siRNA duplexes at a concentration of 230 nM was performed using Lipofectamin 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions.
Assessment of apoptosis
Apoptotic cell death was evaluated using annexin V–FITC/PI staining (BD Biosciences Pharmingen, San Jose, CA) and fluorescence-activated cell sorting (FACS) analysis according to the manufacturer's recommendations.
Gene expression profiling
RNA from siRNA-treated and untreated REH cells was isolated using the RNeasy Mini Kit RNA isolation kit (QIAGEN, Valencia, CA). Further processing of RNA, hybridization to Affymetrix Human Genome U133A 2.0 chip gene arrays (Affymetrix, Santa Clara, CA), and data processing were performed by VBC Genomics (Vienna, Austria).
Protein analysis
Preparation of cell lysates, Western blot analysis, metabolic labeling, staining of cytoplasmic proteins, and separation by 2D gel electrophoresis were performed as described previously.13
TEL/AML1 vector and expression
The TEL/AML1 cDNA (kindly provided by O. Williams, London, United Kingdom8 ) was cloned into pCDNA 3.1/V5-His (Invitrogen) and transfected into Ba/F3 cells. The empty vector and a GFP-carrying pCDNA 3.1/V5-His vector were used as controls. Transfected cells were subcloned and stably expressing clones established.
Statistical analysis
Significant differences between values obtained in cell lines cultured under different experimental conditions were determined using the Student t test.
Results and discussion
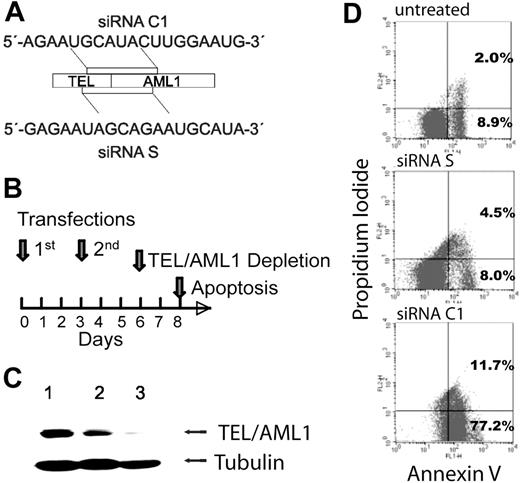
Several siRNAs targeting the fusion site of the TEL/AML1 mRNA were tested (Figures 1A and S1S1). Based on their influence on the fusion gene expression, C1 was chosen as the functional and S as the control siRNA and used in all further experiments. To obtain efficient silencing of TEL/AML1 in REH cells, siRNAs were transfected twice according to the time course depicted in Figure 1B, and protein depletion was confirmed at day 6 of the experiment (Figure 1C). At this time, cell viability was largely unaffected. However, 2 days later, cultures that were devoid of the fusion protein had a reduction of the cell count by about 60%, while those treated with the control siRNA S were reduced by only 20% compared with untreated REH cells. Moreover, assessment of early and late apoptosis indicated that the majority of TEL/AML1-silenced cells had started the apoptotic program, whereas controls contained only 10% to 15% mostly early apoptotic cells (Figure 1D). These data suggest that TEL/AML1 interferes with the delicate balance between life and death probably by engaging a potent antiapoptotic mechanism.
siRNA-mediated silencing of TEL/AML1 induces apoptosis in REH cells. (A) Schematic representation of the siRNA sequences (C1, functional; S, control) and their localization within the TEL/AML1 translocation breakpoint mRNA. (C1, position at bp 15-33, and S, at bp 6-24, accession no. S78496). (B) Time course of siRNA transfection, TEL/AML1 depletion, and induction of apoptosis. (C) Depletion of TEL/AML1 after 2 transfections with siRNA C1. Western blot analysis was performed from REH cell lysates treated with the control siRNA S (lane 2) or functional siRNA C1 (lane 3) or untreated (lane 1) at day 6 of the experiment. TEL/AML1 protein was detected using an anti-TEL antibody (kindly provided by P. Marynen, University of Leuven, Leuven, Belgium) and an antitubulin antibody (Oncogene/EMD Biosciences, San Diego, CA) for loading control. (D) Apoptosis was assessed by FACS analysis of annexin V–FITC/PI staining of untreated, siRNA S–treated (control), and siRNA C1–treated (functional) cells at day 8 of the experiment. Data are shown from 1 of 4 representative experiments and indicate early (annexin V single-positive cells) and late (annexin V and PI–positive cells) apoptosis. The difference in annexin V positivity of REH cells treated with the siRNA C1 compared with either control was significant (P < .03 and P < .01), while the 2 controls did not differ significantly between each other (P = .07).
siRNA-mediated silencing of TEL/AML1 induces apoptosis in REH cells. (A) Schematic representation of the siRNA sequences (C1, functional; S, control) and their localization within the TEL/AML1 translocation breakpoint mRNA. (C1, position at bp 15-33, and S, at bp 6-24, accession no. S78496). (B) Time course of siRNA transfection, TEL/AML1 depletion, and induction of apoptosis. (C) Depletion of TEL/AML1 after 2 transfections with siRNA C1. Western blot analysis was performed from REH cell lysates treated with the control siRNA S (lane 2) or functional siRNA C1 (lane 3) or untreated (lane 1) at day 6 of the experiment. TEL/AML1 protein was detected using an anti-TEL antibody (kindly provided by P. Marynen, University of Leuven, Leuven, Belgium) and an antitubulin antibody (Oncogene/EMD Biosciences, San Diego, CA) for loading control. (D) Apoptosis was assessed by FACS analysis of annexin V–FITC/PI staining of untreated, siRNA S–treated (control), and siRNA C1–treated (functional) cells at day 8 of the experiment. Data are shown from 1 of 4 representative experiments and indicate early (annexin V single-positive cells) and late (annexin V and PI–positive cells) apoptosis. The difference in annexin V positivity of REH cells treated with the siRNA C1 compared with either control was significant (P < .03 and P < .01), while the 2 controls did not differ significantly between each other (P = .07).
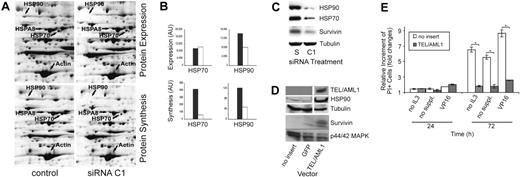
Having demonstrated the impact of TEL/AML1 protein depletion on cell survival, we set out to unravel the corresponding molecular mechanism. We used gene expression analysis in TEL/AML1-silenced cells and searched for affected genes that are involved in the control of cell survival and apoptosis (Table S1Table S1). Of interest, HSP90 and HSP70 were consistently found to be down-regulated after TEL/AML1 depletion in 2 independent experiments. The nonfunctional siRNA S was used as a control to prove that the results were obtained from specific silencing of the fusion gene. For this purpose, the expression of interferon-responsive genes10 was evaluated and found not to differ between these 2 populations (data not shown). To further confirm our findings on the protein level, we used high-resolution 2D gel electrophoresis of cytoplasmic proteins that were either metabolically labeled or stained, allowing quantification of synthesis rate and the amount of individual proteins, respectively. In TEL/AML1-silenced cells (day 6 of the experiment, Figure 1B), the synthesis rate and quantity of HSP90 had decreased, whereas HSP70 showed only a reduced synthesis rate (Figure 2A-B). Strikingly, both proteins were reduced 2 days later (Figure 2C). Further scrutiny of our gene expression data revealed a differential regulation of survivin upon silencing, a finding that was substantiated by reduced amounts of survivin protein 2 days later (Figure 2C) and correlated well with the high rate of apoptosis in these cells. To further corroborate the relationship between the fusion protein and HSP90 expression, TEL/AML1 was ectopically expressed in Ba/F3 cells, which was followed by up-regulation of HSP90 and survivin (Figure 2D). The suggested antiapoptotic effect of TEL/AML1 was supported in this model system since Ba/F3 cells expressing the fusion gene were resistant against growth factor withdrawal as well as VP16 treatment (Figure 2E). Further studies need to clarify the mode by which TEL/AML1 influences HSP expression and in particular its possible interference with heat-shock factors, dominant regulators of HSP.14
TEL/AML1 modulates genes with antiapoptotic functions. (A) Gel sections of 2D gels displaying HSP expression and synthesis of siRNA C1–treated (functional) and control REH cells on day 6 of the experiment, the time at which REH cells were depleted of TEL/AML1 (Figure 1B-C). Cells were metabolically labeled with 35S Protein Labeling Mix (MP Biomedicals, Irvine, CA). Cytoplasmic proteins were separated by 2D gel electrophoresis and proteins stained with a 400-nM solution of ruthenium II tris. Fluorography scanning was performed with the FluorImager 595 (GE Healthcare, Fairfield, CT) and autoradiography scanning, with the Phosphorimager SI (GE Healthcare), both at a resolution of 100 μm. Shown is 1 of 2 independent experiments. HSP90 expression was decreased in TEL/AML1-silenced REH cells (top right panel) compared with the control (bottom left panel). Protein synthesis of HSP70 (HSP70 and HSP8A) and HSP90 was decreased in TEL/AML1-silenced REH cells (bottom right panel) compared with control REH cells (bottom left panel), while the expression and synthesis of actin were unchanged. (B) The differences of protein amounts and protein synthesis of HSP90 and HSP70 from the gel presented in panel A were quantified and depicted as arbitrary units (AU). □ represent siRNA C1–treated cells; ▪, the control cells. (C) Immunoblots were performed using cell lysates from siRNA S– or C1–treated REH cells on day 8 of the experiment, and HSP90, HSP70, and survivin expression was detected by anti-HSP90, anti-HSP70 (Santa Cruz Biotechnologies, Santa Cruz, CA), and rabbit antisurvivin (Cell Signaling Technology, Danvers, MA) antibodies, respectively. The same lysate and amount of protein were used for tubulin as loading control. (D) Western blot analysis of cell lysates from TEL/AML1 stably expressing Ba/F3 cells was performed to assess HSP90 and survivin regulation by the chimeric protein. Ba/F3 cells that were transfected with either the empty vector or the GFP vector, as indicated at the bottom of the blot, served as controls. TEL/AML1 was detected by an anti–V5-HRP antibody (Invitrogen). For the detection of HSP90 and survivin, the same antibodies were used as in panel C. Loading control was performed with an anti-p44/p42 MAPK antibody (Cell Signaling Technology). The film was exposed only very shortly in order to visualize the increase in HSP90 and survivin. Upon longer exposure, the difference becomes less distinct due to the large amounts of these highly abundant proteins in all cells (data not shown). (E) TEL/AML1 confers resistance to apoptosis-inducing conditions. Stably TEL/AML1-expressing Ba/F3 cells (a fresh mixture of 3 clones with a fusion gene mRNA expression within the range of primary ALL cells) and controls (vector without insert) were grown at 0.5 × 106/mL in 4 replicates and subjected to either IL3 (WEHI-conditioned medium) or serum plus IL3 starvation (no suppl), or to VP16 (Bristol-Myers Pharmaceuticals, Hounslow, United Kingdom) treatment (1 mg/mL). Apoptosis was assessed by PI staining after 24 and 72 hours. The relative increment of PI-positive cells under apoptosis-inducing conditions was calculated against that of untreated cells. Data in the graph represent mean ± SD of 3 separate experiments. *Statistically significant changes (P < .001).
TEL/AML1 modulates genes with antiapoptotic functions. (A) Gel sections of 2D gels displaying HSP expression and synthesis of siRNA C1–treated (functional) and control REH cells on day 6 of the experiment, the time at which REH cells were depleted of TEL/AML1 (Figure 1B-C). Cells were metabolically labeled with 35S Protein Labeling Mix (MP Biomedicals, Irvine, CA). Cytoplasmic proteins were separated by 2D gel electrophoresis and proteins stained with a 400-nM solution of ruthenium II tris. Fluorography scanning was performed with the FluorImager 595 (GE Healthcare, Fairfield, CT) and autoradiography scanning, with the Phosphorimager SI (GE Healthcare), both at a resolution of 100 μm. Shown is 1 of 2 independent experiments. HSP90 expression was decreased in TEL/AML1-silenced REH cells (top right panel) compared with the control (bottom left panel). Protein synthesis of HSP70 (HSP70 and HSP8A) and HSP90 was decreased in TEL/AML1-silenced REH cells (bottom right panel) compared with control REH cells (bottom left panel), while the expression and synthesis of actin were unchanged. (B) The differences of protein amounts and protein synthesis of HSP90 and HSP70 from the gel presented in panel A were quantified and depicted as arbitrary units (AU). □ represent siRNA C1–treated cells; ▪, the control cells. (C) Immunoblots were performed using cell lysates from siRNA S– or C1–treated REH cells on day 8 of the experiment, and HSP90, HSP70, and survivin expression was detected by anti-HSP90, anti-HSP70 (Santa Cruz Biotechnologies, Santa Cruz, CA), and rabbit antisurvivin (Cell Signaling Technology, Danvers, MA) antibodies, respectively. The same lysate and amount of protein were used for tubulin as loading control. (D) Western blot analysis of cell lysates from TEL/AML1 stably expressing Ba/F3 cells was performed to assess HSP90 and survivin regulation by the chimeric protein. Ba/F3 cells that were transfected with either the empty vector or the GFP vector, as indicated at the bottom of the blot, served as controls. TEL/AML1 was detected by an anti–V5-HRP antibody (Invitrogen). For the detection of HSP90 and survivin, the same antibodies were used as in panel C. Loading control was performed with an anti-p44/p42 MAPK antibody (Cell Signaling Technology). The film was exposed only very shortly in order to visualize the increase in HSP90 and survivin. Upon longer exposure, the difference becomes less distinct due to the large amounts of these highly abundant proteins in all cells (data not shown). (E) TEL/AML1 confers resistance to apoptosis-inducing conditions. Stably TEL/AML1-expressing Ba/F3 cells (a fresh mixture of 3 clones with a fusion gene mRNA expression within the range of primary ALL cells) and controls (vector without insert) were grown at 0.5 × 106/mL in 4 replicates and subjected to either IL3 (WEHI-conditioned medium) or serum plus IL3 starvation (no suppl), or to VP16 (Bristol-Myers Pharmaceuticals, Hounslow, United Kingdom) treatment (1 mg/mL). Apoptosis was assessed by PI staining after 24 and 72 hours. The relative increment of PI-positive cells under apoptosis-inducing conditions was calculated against that of untreated cells. Data in the graph represent mean ± SD of 3 separate experiments. *Statistically significant changes (P < .001).
In this study, we present in 2 model systems a clear functional relationship between TEL/AML1 and the apoptotic network with a major impact on the members of the heat-shock protein family HSP90 and HSP70 as well as on survivin, a potent member of the inhibitor of the apoptosis IAP family.15–17 The occurrence of TEL/AML1 and its interference with the apoptosis control enables the affected cell to survive, thereby providing the opportunity to acquire further mutations and eventually turn into a fully malignant phenotype. The propensity of leukemic REH cells to undergo apoptosis after TEL/AML1 depletion provides further evidence that this survival advantage is also indispensable for maintaining the leukemic phenotype, a feature that is shared by other leukemia-associated fusion genes.18–20 And finally, our data (1) provide the rational for the potential clinical use of inhibitors against HSP90 or survivin that are frequently up-regulated during cancer development15,17 and currently undergoing phase 1 clinical trials also for pediatric patients with recurrent or refractory ALL,21,22 and (2) support a future siRNA-based therapy23 in this subtype of leukemia. Such targeted treatment approaches would ideally affect all the fusion gene–carrying cells, be it the chemotherapy-sensitive bulk of leukemic cells, or, more importantly, also the leukemic stem cell as well as the preleukemic clone, the definite and proposed sources of relapses, respectively.24–26
Authorship
Contribution: All authors have substantially contributed to the content of the paper and have agreed to the submission in its current version; C.D. designed and performed research, analyzed data, and wrote the first draft of the paper; C.G. performed and analyzed the 2D PAGE data; G.K., A.I., C.L., J.B., and A.M.D. performed and analyzed experiments and contributed to the experimental design; E.R.P.-G. is responsible for the design of the study, the integrity of the data, and writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: E. Renate Panzer-Grümayer, CCRI and St Anna Kinderspital, Kinderspitalgasse 6, A-1090 Vienna, Austria; e-mail: renate.panzer@ccri.at.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported in part by a grant from the Jubiläumsfonds der ÖNB 10720, the FWF P17551-B14, and the GENAU-CHILD GZ 200.136/1-VI/1/2005 to E.R.P.-G.
We would like to thank Idriss M. Bennani-Baiti for reading the paper and stimulating discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal