Abstract
The prognosis for higher risk childhood B-cell non-Hodgkin lymphoma has improved over the past 20 years but the optimal intensity of treatment has yet to be determined. Children 21 years old or younger with newly diagnosed B-cell non-Hodgkin lymphoma/B-cell acute lymphoblastic leukemia (B-NHL/B-ALL) with higher risk factors (bone marrow [BM] with or without CNS involvement) were randomized to standard intensity French-American-British/Lymphoma Malignancy B (FAB/LMB) therapy or reduced intensity (reduced cytarabine plus etoposide and deletion of 3 maintenance courses M2, M3, M4). All patients with CNS disease had additional high-dose methotrexate (8 g/m2) plus extra intrathecal therapy. Fifty-one percent had BM involvement, 20% had CNS involvement, and 29% had BM and CNS involvement. One hundred ninety patients were randomized. The probabilities of 4-year event-free survival (EFS) and survival (S) were 79% ± 2.7% and 82% ± 2.6%, respectively. In patients in remission after 3 cycles who were randomized to standard versus reduced-intensity therapy, the 4-year EFS after randomization was 90% ± 3.1% versus 80% ± 4.2% (one-sided P = .064) and S was 93% ± 2.7% versus 83% ± 4.0% (one-sided P = .032). Patients with either combined BM/CNS disease at diagnosis or poor response to cyclophosphamide, Oncovin [vincristine], prednisone (COP) reduction therapy had a significantly inferior EFS and S (P < .001). Standard-intensity FAB/LMB therapy is recommended for children with high-risk B-NHL (B-ALL with or without CNS involvement).
Introduction
The outcome in children with advanced (bone marrow [BM] with or without CNS involvement) newly diagnosed B-cell non-Hodgkin lymphoma (B-NHL) has improved dramatically over the last 20 years, although the optimal and safest treatment has yet to be determined.1-8 About 90% of histologic subtypes of childhood de novo high-risk (BM with or without CNS) B-NHL are of the aggressive subtype, including mature B-cell acute lymphoblastic leukemia (B-ALL), Burkitt lymphoma (BL) and Burkitt-like (BLL) histology and to a lesser extent, an intermediate subtype, diffuse large B-cell lymphoma (DLBCL).9-11 Despite an improvement in overall survival (S) with multiagent intensive chemotherapy, there is a significant incidence of serious morbidity, including grade III/IV mucositis (40%-70%), systemic infection (60%-80%), myelosuppression (80%-100%), prolonged hospitalization (median 10-14 days during each induction cycle), and a toxic death rate of 1%-2% in remission with potential long-term cardiac and gonadal toxicities and secondary malignancies.4,5,7,8
Reduction therapy with cyclophosphamide, Oncovin (vincristine) and prednisone (COP), induction therapy with cyclophosphamide, Oncovin (vincristine), prednisone, Adriamycin (doxorubicin), and methotrexate (COPADM), and intensification with cytarabine (high dose and continuous infusion) plus etoposide (CYVE12 ) (Lymphoma Malignancy B [LMB] 86 and 89 group C therapy) was originally introduced by Patte et al and the Société Française d'Oncologie Pédiatrique (SFOP) for advanced (BM with or without CNS) childhood B-NHL and subsequently investigated by Cairo et al in the Children's Cancer Group (CCG) and by Atra et al for the United Kingdom Children's Cancer Study Group (UKCCSG).4,13,14 However, in view of the high morbidity of this therapy, the exact intensity that is required for optimal results is still unclear. The incidence of advanced (BM with or without CNS) de novo childhood B-NHL, however, is extremely low (< 75 cases/y in CCG, SFOP, and UKCCSG combined). Therefore, to determine whether standard LMB89 group C intensity is required for optimal event-free survival (EFS), we developed an international study group of 3 pediatric cooperative groups (CCG, SFOP, UKCCSG) to investigate standard versus reduced-intensity FAB/LMB96 (French-American-British/Lymphoma Malignancy B) treatment in children with advanced de novo B-ALL and CNS B-NHL. The reduced-intensity arm was designed to reduce grade III/IV infections, grade III/IV mucositis, decrease hospitalization, or potentially improve dose intensity over time compared to the standard CYVE intensity arm. This was the first international collaboration of 3 pediatric cooperative groups of its kind. We report the results of the highest-risk patients, those with bone marrow involvement (≥ 25 L3 blasts), CNS disease, or both (group C) The results of patients with intermediate-risk disease (group B) are reported in the accompanying paper.
Patients, materials, and methods
General
The protocol was approved by all of the local institutional review boards and written informed consent was obtained from all patients or their parent or legal guardian, in accordance with the Declaration of Helsinki. This study opened in May 1996 and closed to patient accrual in June 2001.
Eligibility
Newly diagnosed B-lineage non-Hodgkin lymphoma with either B-ALL, DLBCL, BL, or BLL according to the Revised European-American Lymphoma (REAL) classification (II 9, 10, 11) were eligible.9 Age range was 6 months or older and younger than 18 years (UKCCSG and SFOP) and younger than 21 years (CCG). Staging was performed as described by Murphy et al.15 Risk classification was defined as low risk (group A) with resected stage I and abdominal completely resected stage II; high risk (group C) with bone marrow disease (≥ 25% L3 blasts) or CNS disease defined by any one or more of the following: any L3 CSF blast, cranial nerve palsy, clinical spinal cord compression, isolated intracerebral mass, or cranial or spinal parameningeal extension; and intermediate risk (group B), all others. Exclusions to study enrollment included any one of the following: immunodeficiency, HIV positivity, prior solid organ transplantation, previous malignancy, or prior chemotherapy. Confirmation of B-cell lineage by national central immunophenotyping required 50% or greater neoplastic cells to express either CD20 or CD79a or both. An international central pathology review included at least 2 review pathologists from each of the 3 pediatric cooperative groups.
Treatment and randomization
Reduction phase.
Intravenous hydration, urine alkalinization, and allopurinol were recommended to reduce the incidence of tumor lysis syndrome during the reduction phase of cyclophosphamide, Oncovin (vincristine), and prednisone (COP) (Table 1; Figure 1A).4,13 Uricozyme, nonrecombinant urate oxidase, was available for patients treated in France and the United Kingdom.16 Rasburicase, recombinant urate oxidase, was available during the last few years of the study either on a compassionate basis17 or in a randomized trial with allopurinol18 in the United States. Evaluation of tumor response was performed on day 7 of COP. Response to COP was designated at complete response (CR), incomplete response (IR; 21%-99% tumor reduction), and nonresponse (NR; < 20% tumor reduction). COP nonresponse in itself was not considered a failure of the treatment strategy. Patients with a nonresponse to COP were nonrandomly assigned to the standard arm C1 (see “Intensification and randomization”). Patients in critical condition (renal failure, sepsis, grade III/IV organ toxicity) were allowed to receive a second course of COP prior to proceeding to induction (COPADM1).
Treatment therapy dose/schedule
| . | Full-intensity therapy dose/schedule . | Reduced-intensity therapy dose/schedule . |
|---|---|---|
| COP | Cyclophosphamide 300 mg/m2 day 1 | Same |
| Vincristine 1.0 mg/m2 day 1 | ||
| Prednisone 60 mg/m2 (divided doses) days 1-7 | ||
| IT MTX 8-15 mg/m2 day 1,3,5 | ||
| IT hydrocortisone 8-15 mg/m2 days 1,3,5 | ||
| IT cytarabine 15-30 mg/m2 days 1,3,5 | ||
| COPADM 1 | Vincristine 2.0 mg/m2 day 1 (maximum: 2.0 mg) | Same |
| Prednisone 60 mg/m2 (divided doses) days 1-5 then reduce over 3 d to 0 | ||
| HDMTX 8000 mg/m2 day 1 in 4-h infusion + rescue | ||
| Cyclophosphamide 250 mg/m2 every 12 h days 2-4 | ||
| Doxorubicin 60 mg/m2 day 2 | ||
| IT MTX 8-15 mg/m2 days 1,3,5 | ||
| IT hydrocortisone 8-15 mg/m2 days 2,4,6 | ||
| IT cytarabine 15-30 mg/m2 days 2,4,6 | ||
| COPADM 2 | Vincristine 2.0 mg/m2 d 1 (maximum: 2.0 mg) | Same |
| Prednisone 60 mg/m2 (divided doses) d 1-5 then reduce over 3 d to 0 | ||
| HDMTX 8000 mg/m2 d 1 in 4-h infusion + rescue | ||
| Cyclophosphamide 500 mg/m2 every 12 h d 2-4 | ||
| Doxorubicin 60 mg/m2 day 2 | ||
| IT MTX 8-15 mg/m2 days 1,3,5 | ||
| IT hydrocortisone 8-15 mg/m2 days 2,4,6 | ||
| IT cytarabine 15-30 mg/m2 days 2,4,6 | ||
| CYVE 1 and 2 | Cytarabine 50 mg/m2 IV days 1-5 | Deleted |
| CNS− | HD Ara-C 3000 mg/m2 days 2-5 | |
| Etoposide 200 mg/m2 days 2-5 | ||
| CYVE 1 and 2 | Cytarabine 50 mg/m2 IV days 1-5 | Deleted |
| CNS+ | HD Ara-C 3000 mg/m2 days 2-5 | |
| Etoposide 200 mg/m2 days 2-5 | ||
| IT MTX and hydrocortisone 8-15 mg/m2 day 1 | ||
| HDMTX 8000 mg/m2 day 18* | ||
| IT MTX, hydrocortisone, Ara-C (dose same as COPADM 1) day 19* | ||
| Mini CYVE 1 and 2 | Not applicable | Cytarabine 50 mg/m2 IV days 1-5 |
| CNS− | Mini HD Ara-C 2000 mg/m2 days 2-5 | |
| Etoposide 100 mg/m2 days 2-5 | ||
| Mini CYVE 1 and 2 | Not applicable | Cytarabine 50 mg/m2 IV days 1-5 |
| CNS+ | Mini HD Ara-C 2000 mg/m2 days 2-5 | |
| Etoposide 100 mg/m2 days 2-5 | ||
| HDMTX 8000 mg/m2 day 18† in 4-h infusion + rescue | ||
| IT MTX, hydrocortisone, Ara-C (dose same as COPADM 1) day 19† | ||
| M1 | Vincristine 2.0 mg/m2 day 1 (maximum: 2.0 mg) | Same |
| Prednisone 60 mg/m2 (divided doses) days 1-5 then reduce over 3 d to 0 | ||
| Cyclophosphamide 500 mg/m2 every 12 h days 2,3 | ||
| HDMTX 8000 mg/m2 day 1 in 4-h infusion + rescue | ||
| Doxorubicin 60 mg/m2 day 2 | ||
| IT MTX 8-15 mg/m2 day 2 | ||
| IT hydrocortisone 8-15 mg/m2 day 2 | ||
| IT cytarabine 15-30 mg/m2 day 2 | ||
| M2 | Cytarabine 50 mg/m2 sq every 12 h days 1-5 | Deleted |
| Etoposide 150 mg/m2 days 1-3 | ||
| M3 | Vincristine 2.0 mg/m2 day 1 (maximum: 2.0 mg) | Deleted |
| Prednisone 60 mg/m2 (divided doses) days 1-5 then reduce over 3 d to 0 | ||
| Cyclophosphamide mg/m2 days 1-2 | ||
| Doxorubicin 60 mg/m2 day 1 | ||
| M4 | Cytarabine 50 mg/m2 sq every 12 h days 1-5 | Deleted |
| Etoposide 150 mg/m2 days 1-3 |
| . | Full-intensity therapy dose/schedule . | Reduced-intensity therapy dose/schedule . |
|---|---|---|
| COP | Cyclophosphamide 300 mg/m2 day 1 | Same |
| Vincristine 1.0 mg/m2 day 1 | ||
| Prednisone 60 mg/m2 (divided doses) days 1-7 | ||
| IT MTX 8-15 mg/m2 day 1,3,5 | ||
| IT hydrocortisone 8-15 mg/m2 days 1,3,5 | ||
| IT cytarabine 15-30 mg/m2 days 1,3,5 | ||
| COPADM 1 | Vincristine 2.0 mg/m2 day 1 (maximum: 2.0 mg) | Same |
| Prednisone 60 mg/m2 (divided doses) days 1-5 then reduce over 3 d to 0 | ||
| HDMTX 8000 mg/m2 day 1 in 4-h infusion + rescue | ||
| Cyclophosphamide 250 mg/m2 every 12 h days 2-4 | ||
| Doxorubicin 60 mg/m2 day 2 | ||
| IT MTX 8-15 mg/m2 days 1,3,5 | ||
| IT hydrocortisone 8-15 mg/m2 days 2,4,6 | ||
| IT cytarabine 15-30 mg/m2 days 2,4,6 | ||
| COPADM 2 | Vincristine 2.0 mg/m2 d 1 (maximum: 2.0 mg) | Same |
| Prednisone 60 mg/m2 (divided doses) d 1-5 then reduce over 3 d to 0 | ||
| HDMTX 8000 mg/m2 d 1 in 4-h infusion + rescue | ||
| Cyclophosphamide 500 mg/m2 every 12 h d 2-4 | ||
| Doxorubicin 60 mg/m2 day 2 | ||
| IT MTX 8-15 mg/m2 days 1,3,5 | ||
| IT hydrocortisone 8-15 mg/m2 days 2,4,6 | ||
| IT cytarabine 15-30 mg/m2 days 2,4,6 | ||
| CYVE 1 and 2 | Cytarabine 50 mg/m2 IV days 1-5 | Deleted |
| CNS− | HD Ara-C 3000 mg/m2 days 2-5 | |
| Etoposide 200 mg/m2 days 2-5 | ||
| CYVE 1 and 2 | Cytarabine 50 mg/m2 IV days 1-5 | Deleted |
| CNS+ | HD Ara-C 3000 mg/m2 days 2-5 | |
| Etoposide 200 mg/m2 days 2-5 | ||
| IT MTX and hydrocortisone 8-15 mg/m2 day 1 | ||
| HDMTX 8000 mg/m2 day 18* | ||
| IT MTX, hydrocortisone, Ara-C (dose same as COPADM 1) day 19* | ||
| Mini CYVE 1 and 2 | Not applicable | Cytarabine 50 mg/m2 IV days 1-5 |
| CNS− | Mini HD Ara-C 2000 mg/m2 days 2-5 | |
| Etoposide 100 mg/m2 days 2-5 | ||
| Mini CYVE 1 and 2 | Not applicable | Cytarabine 50 mg/m2 IV days 1-5 |
| CNS+ | Mini HD Ara-C 2000 mg/m2 days 2-5 | |
| Etoposide 100 mg/m2 days 2-5 | ||
| HDMTX 8000 mg/m2 day 18† in 4-h infusion + rescue | ||
| IT MTX, hydrocortisone, Ara-C (dose same as COPADM 1) day 19† | ||
| M1 | Vincristine 2.0 mg/m2 day 1 (maximum: 2.0 mg) | Same |
| Prednisone 60 mg/m2 (divided doses) days 1-5 then reduce over 3 d to 0 | ||
| Cyclophosphamide 500 mg/m2 every 12 h days 2,3 | ||
| HDMTX 8000 mg/m2 day 1 in 4-h infusion + rescue | ||
| Doxorubicin 60 mg/m2 day 2 | ||
| IT MTX 8-15 mg/m2 day 2 | ||
| IT hydrocortisone 8-15 mg/m2 day 2 | ||
| IT cytarabine 15-30 mg/m2 day 2 | ||
| M2 | Cytarabine 50 mg/m2 sq every 12 h days 1-5 | Deleted |
| Etoposide 150 mg/m2 days 1-3 | ||
| M3 | Vincristine 2.0 mg/m2 day 1 (maximum: 2.0 mg) | Deleted |
| Prednisone 60 mg/m2 (divided doses) days 1-5 then reduce over 3 d to 0 | ||
| Cyclophosphamide mg/m2 days 1-2 | ||
| Doxorubicin 60 mg/m2 day 1 | ||
| M4 | Cytarabine 50 mg/m2 sq every 12 h days 1-5 | Deleted |
| Etoposide 150 mg/m2 days 1-3 |
IT doses are age adjusted below age 3 years. IV indicates intravenously; sq, subcutaneously.
Given after CYVE 1 only for CNS+ patients.
Given after mini CYVE 1 only for CNS+ patients.
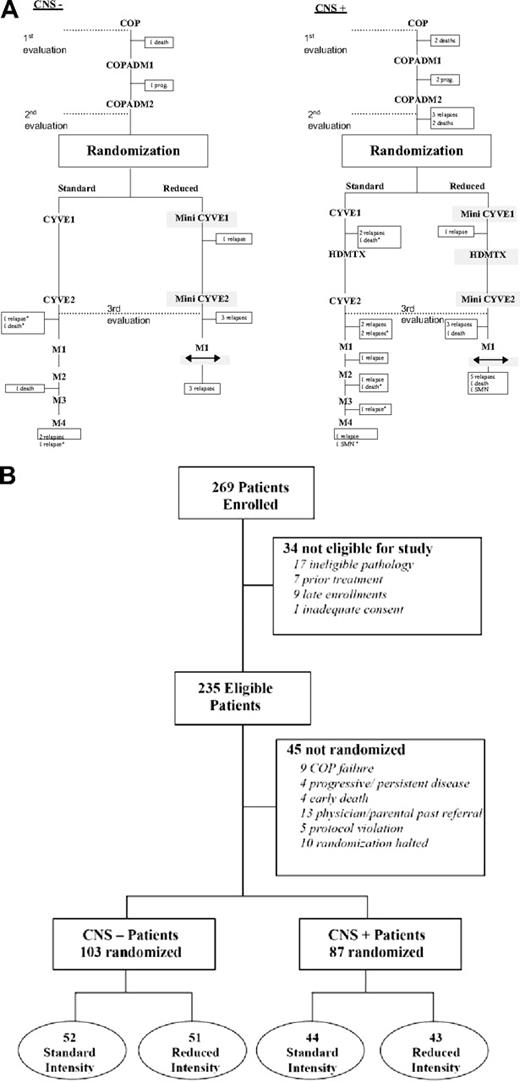
Treatment and follow-up. Treatment and design schema (A) and patient follow-up diagram (B). Reduction phase COP: cyclophosphamide, Oncovin (vincristine), and prednisone.4 Induction phase COPADM1 and COPADM2: cyclophosphamide, Oncovin (vincristine), prednisone, Adriamycin (doxorubicin), and methotrexate.4 Intensification phase CYVE or mini CYVE: cytosine arabinosine 3 g/m2 versus 2 g/m2 and etoposide 200 mg/m2 versus 100 mg/m2.4 Maintenance phase: maintenance 1, 2, 3 and 4.4 Rectangles indicate approximate times of treatment failure events; SMN, secondary malignant neoplasm.
Treatment and follow-up. Treatment and design schema (A) and patient follow-up diagram (B). Reduction phase COP: cyclophosphamide, Oncovin (vincristine), and prednisone.4 Induction phase COPADM1 and COPADM2: cyclophosphamide, Oncovin (vincristine), prednisone, Adriamycin (doxorubicin), and methotrexate.4 Intensification phase CYVE or mini CYVE: cytosine arabinosine 3 g/m2 versus 2 g/m2 and etoposide 200 mg/m2 versus 100 mg/m2.4 Maintenance phase: maintenance 1, 2, 3 and 4.4 Rectangles indicate approximate times of treatment failure events; SMN, secondary malignant neoplasm.
Induction phase.
On day 8 of COP or after a second course of COP, cyclophosphamide, Oncovin (vincristine), prednisone, Adriamycin (doxorubicin), methotrexate (COPADM1) was administered as previously described (high-dose methotrexate [HDMTX] 8 g/m2 over 4 hours followed by leucovorin rescue starting at hour 24 and given every 6 hours until serum methotrexate level is below < 1 × 10−7 M and with triple intrathecal [IT]chemotherapy with age-adjusted doses; Table 1; Figure 1A).4,13 Following recovery from COPADM1 and absolute neutrophil count (ANC) 1000/mm3 or higher and platelet count 100 000/mm3 or higher, COPADM2 was administered as previously described (Table 1; Figure 1A).4,13 In early 1997, because of the high incidence of severe mucositis, the infusion time of doxorubicin was changed from 48 to 6 hours in both COPADM1 and COPADM2. Disease assessment was determined after recovery of COPADM2.
Intensification and randomization.
Patients with no response to COP were nonrandomly assigned to standard arm C1. Patients without prior CNS disease were randomized to 2 standard courses of cytarabine and etoposide (CYVE) consolidation, as previously described4,13 (C1 arm) or 2 courses of reduced doses of cytarabine (3 g versus 2 g/m2/dose × 4 days) and etoposide (200 versus 100 mg/m2/dose × 4 days; experimental arm—mini CYVE; C2 arm) in combination with Ara-C continuous infusion (Table 1; Figure 1A). Patients with CNS disease were similarly randomized to C1 (CYVE × 2) versus C2 (mini CYVE × 2) but received additionally on both arms double intrathecal therapy with age-adjusted hydrocortisone and methotrexate on day 1 of each consolidation course and in between consolidation courses when ANC was 500/mm3 or higher and platelet 50 000/mm3 or higher an additional HDMTX course with triple IT chemotherapy (Table 1; Figure 1B). Disease assessment was determined at the end of consolidation therapy and patients with histologically positive disease were considered a treatment failure and were off protocol therapy.
Maintenance.
Patients randomized to standard C1 arm received 4 maintenance courses: M1, COPADM3; M2, cytarabine plus etoposide; M3, cyclophosphamide, vincristine, prednisone, and doxorubicin; and M4, cytarabine plus etoposide as previously described (Table 1; Figure 1A).4,13 Patients randomized to experimental arm C2 only received one maintenance course, M1 (Figure 1A). In April 2001, 2 months prior to study closure, the randomization was discontinued following an interim analysis suggesting increased events in the experimental C2 arm, and the remaining patients were nonrandomized to standard C1 arm.
Toxicity.
A reporting of whether or not toxicities known to be associated with this treatment (hemorrhage, stomatitis, diarrhea, constipation, vincristine-related pain, infection, seizures, metabolic, and neurologic) occurred with grade 3 or higher was specifically required for each treatment course. Other grade 3 or higher nonhematologic toxicities were reported if they occurred according to the Children's Cancer Group toxicity grading scale.
Statistical methods
Patients were randomized within each national cooperative group to C1 (standard) or C2 (reduced) intensity treatment following course COPADM2 using stratified, blocked randomization with equal allocation, block size of 4, and strata defined by all combinations of national group (UKCCSG, SFOP, or CCG), histology (DLBCL or not), and CNS disease at diagnosis (present [+] or absent [−]). The primary end point for analysis was event-free survival (EFS), which was defined as the minimum time to death from any cause, relapse, progressive disease, second malignant neoplasm (SMN), or biopsy-positive residual disease following CYVE2 or mini CYVE2. EFS was measured from the beginning of chemotherapy for the analysis of all eligible patients and from the date of randomization for comparison of the 2 randomized groups. The secondary end point was survival (S), which was the time to death from any cause measured from the start of therapy or the date of randomization as appropriate. In the randomized comparison, the criterion for detecting a reduction in treatment efficacy was that the lower 80% profile-likelihood confidence bound of the ratio of hazard functions of the reduced versus standard treatment groups, as estimated by a stratified Cox proportional hazards model, exceeded 1. This is equivalent to the use of a one-sided stratified log-rank test19,20 with 20% type I error. Interim monitoring was based on the methods of Lan-Demets.21 This criterion provided 90% power against a 12% reduction in 4-year EFS probability from a hypothesized baseline of 88%. This choice of type I error rate and power was appropriate because it was more important to have a high probability of detecting a true reduction in efficacy than to minimize the false-positive error rate in this treatment reduction trial. All analyses followed the intent-to-treat philosophy, in that patients were censored only for reasons of follow-up, and were retained in the assigned treatment group regardless of treatment received. Interim results were reviewed annually by an international, independent Data and Safety Monitoring Committee (DSMC) comprising 3 pediatric oncologists and a statistician who were experts in their respective areas. Product-limit estimates of EFS and S probabilities are reported throughout along with Greenwood standard errors.19,20 All eligible patients were included in analyses. Those specifically comparing the 2 randomized regimen were restricted to randomized patients. Estimates of overall EFS and S for patients on standard-intensity treatment were estimated using a bootstrap implementation of the method of Lunceford et al.22
Results
Patient demographics and randomization
Two hundred thirty-five eligible patients were enrolled in this study (Table 2; Figure 1B). Thirty-four patients who were originally enrolled as group C patients have been excluded from the analysis due to ineligible pathology (17), prior treatment (7), late enrollment in study (9), or inadequate consent (1). Two CNS+ patients who were enrolled and treated mistakenly as group B patients are included in the overall analysis, but not in the randomized comparison.
Summary of patient characteristics
| . | All patients . | Reduced treatment* . | Standard treatment* . | |||
|---|---|---|---|---|---|---|
| Frequency . | Percent . | Frequency . | Percent . | Frequency . | Percent . | |
| National group | ||||||
| Children's Oncology Group | 101 | 43 | 40 | 43 | 44 | 46 |
| Société Française d'Oncologie Pédiatrique | 98 | 42 | 39 | 41 | 39 | 41 |
| United Kingdom Children's Cancer Study Group | 36 | 15 | 15 | 16 | 13 | 14 |
| Sex | ||||||
| Male | 185 | 79 | 75 | 80 | 77 | 80 |
| Female | 50 | 21 | 19 | 20 | 19 | 20 |
| Age, y | ||||||
| 0-4 | 47 | 20 | 15 | 16 | 22 | 23 |
| 5-9 | 97 | 42 | 39 | 41 | 44 | 46 |
| 10-14 | 64 | 27 | 31 | 33 | 20 | 21 |
| 15-19 | 27 | 12 | 9 | 10 | 10 | 10 |
| Histology | ||||||
| BL/BLL/B-ALL | 204 | 87 | 84 | 90 | 84 | 88 |
| Diffuse B-LC/TCRLCL | 21 | 9 | 6 | 6 | 7 | 7 |
| Other/NOS/pending | 10 | 4 | 4 | 3 | 5 | 5 |
| BM/CNS involvement | ||||||
| BM+/CNS− | 121 | 51 | 51 | 54 | 52 | 54 |
| BM−/CNS+ | 46 | 20 | 14 | 15 | 21 | 22 |
| BM+/CNS+ | 68 | 29 | 29 | 31 | 23 | 24 |
| LDH level | ||||||
| Unknown | 16 | — | 6 | — | 7 | — |
| Less than or equal to 2 times normal | 41 | 19 | 16 | 18 | 15 | 17 |
| More than 2 times normal | 178 | 81 | 72 | 82 | 74 | 83 |
| . | All patients . | Reduced treatment* . | Standard treatment* . | |||
|---|---|---|---|---|---|---|
| Frequency . | Percent . | Frequency . | Percent . | Frequency . | Percent . | |
| National group | ||||||
| Children's Oncology Group | 101 | 43 | 40 | 43 | 44 | 46 |
| Société Française d'Oncologie Pédiatrique | 98 | 42 | 39 | 41 | 39 | 41 |
| United Kingdom Children's Cancer Study Group | 36 | 15 | 15 | 16 | 13 | 14 |
| Sex | ||||||
| Male | 185 | 79 | 75 | 80 | 77 | 80 |
| Female | 50 | 21 | 19 | 20 | 19 | 20 |
| Age, y | ||||||
| 0-4 | 47 | 20 | 15 | 16 | 22 | 23 |
| 5-9 | 97 | 42 | 39 | 41 | 44 | 46 |
| 10-14 | 64 | 27 | 31 | 33 | 20 | 21 |
| 15-19 | 27 | 12 | 9 | 10 | 10 | 10 |
| Histology | ||||||
| BL/BLL/B-ALL | 204 | 87 | 84 | 90 | 84 | 88 |
| Diffuse B-LC/TCRLCL | 21 | 9 | 6 | 6 | 7 | 7 |
| Other/NOS/pending | 10 | 4 | 4 | 3 | 5 | 5 |
| BM/CNS involvement | ||||||
| BM+/CNS− | 121 | 51 | 51 | 54 | 52 | 54 |
| BM−/CNS+ | 46 | 20 | 14 | 15 | 21 | 22 |
| BM+/CNS+ | 68 | 29 | 29 | 31 | 23 | 24 |
| LDH level | ||||||
| Unknown | 16 | — | 6 | — | 7 | — |
| Less than or equal to 2 times normal | 41 | 19 | 16 | 18 | 15 | 17 |
| More than 2 times normal | 178 | 81 | 72 | 82 | 74 | 83 |
For all patients, N = 235; for reduced treatment, N = 94; and for standard treatment, N = 96.
BL indicates Burkitt lymphoma; BLL, Burkitt-like lymphoma; B-LC, B large cell; TCRLCL, T-cell–rich large-cell lymphoma; NOS, not otherwise specified; and –, not determined.
Forty-five patients were not randomized: nonresponse to COP (n = 9), progressive/persistent disease (n = 4), early death (n = 4), physician/parent refusal (n = 13), protocol violation (n = 5), and halted randomization (n = 10).
Response to COP
Two hundred seventeen patients were evaluated for response to COP therapy. Of the 18 who were not evaluable, 3 were reported as having no evaluable tumor, and for the remaining 15 the institution did not perform a response evaluation for reasons that were not specified. Thirty-three patients (15%) had a CR, 175 patients (81%) had an IR, and 9 patients (4%) had a NR. Of the 9 COP nonresponders, 5 progressed and one developed a second malignancy in a median of 15 weeks (range, 1-172 weeks) from start of treatment, and all died at a median of 27 weeks (range, 1-205 weeks). The remaining 3 patients are alive at 3.1, 2.9, and 5.6 years.
EFS and overall S
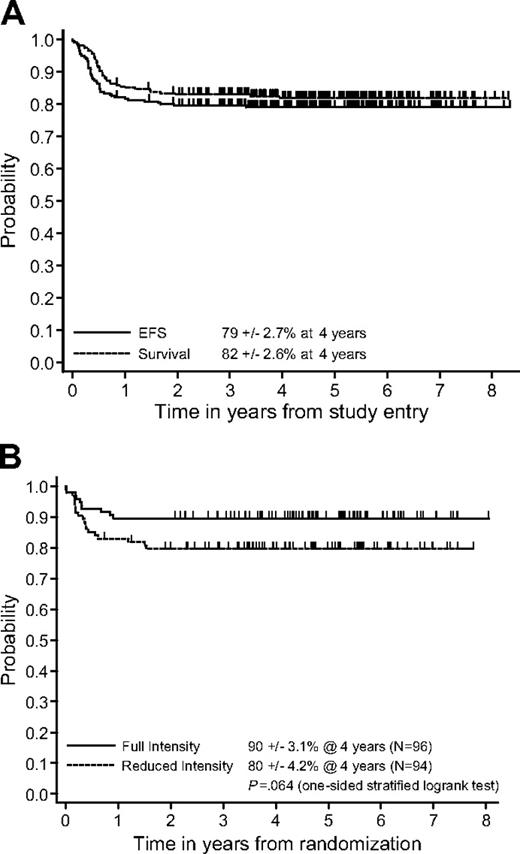
The probability of 4-year EFS and S for all patients entered on study was 79% ± 2.7% and 82% ± 2.6%, respectively (Figure 2A). In patients who responded following COPADM2 and who were randomized to standard treatment, the EFS 4 years after randomization was 90% ± 3.1% versus 80% ± 4.2% in those randomized to reduced-intensity treatment (Figure 2B; P = .064, one-sided stratified log-rank test; stratified Cox estimated hazard ratio 1.81; lower 80% profile likelihood confidence bound 1.30). S at 4 years in these 2 randomized groups was 93% ± 2.7% and 83% ± 4.0%, respectively (P = .032, one-sided stratified log-rank test). The difference in EFS met the interim monitoring criterion, and in April 2001 the DSMC halted the randomization to the reduced-treatment arm. At that time 5 patients who were still on therapy who had been randomized to the reduced arm but could benefit from a switch to the standard intensity arm were switched, and 3 were enrolled before April 2001 but reached the randomization point after that date and hence were not randomized. Seven additional patients were enrolled after April 2001 and were treated according to standard intensity until all strata were closed to accrual simultaneously in June 2001. This reduction in efficacy was evident in both CNS− patients (94 ± 3.2 versus 86 ± 4.8) and CNS+ patients (84 ± 5.5 versus 72 ± 6.9) The amount of efficacy diminution in the reduced treatment group may be slightly underestimated because 6 patients assigned to reduced intensity received components of standard therapy and 3 patients assigned to standard therapy received components of reduced therapy.
Probability of EFS and S of all patients and randomized patients. (A) Product-limit estimate of probability of EFS and S in all patients from study entry. EFS at 4 years, 79% ± 2.7%; S at 4 years, 82% ± 2.6%. (B) Product-limit estimate of probability of EFS from randomization of patients randomized to C1 versus C2 (mini CYVE and deletion M2, M3, M4 courses); EFS at 4 years 90% ± 3.1% versus 80% ± 4.2%, P = .064.
Probability of EFS and S of all patients and randomized patients. (A) Product-limit estimate of probability of EFS and S in all patients from study entry. EFS at 4 years, 79% ± 2.7%; S at 4 years, 82% ± 2.6%. (B) Product-limit estimate of probability of EFS from randomization of patients randomized to C1 versus C2 (mini CYVE and deletion M2, M3, M4 courses); EFS at 4 years 90% ± 3.1% versus 80% ± 4.2%, P = .064.
Subgroup analysis
The probability of 4-year EFS of all patients entered in the study, grouped by BM involvement only (BM+/CNS−), CNS involvement only (BM−/CNS+), and BM and CNS involvement (BM+/CNS+) was 88% ± 3.0%, 82% ± 5.6%, and 61% ± 6.0%, respectively (P < .001 overall and BM+/CNS+ versus others; Figure 3A). When corrected for reduced efficacy in those randomized to reduced-intensity therapy, the estimates are 91% ± 2.9%, 85% ± 5.6%, and 66% ± 6.5%, respectively. The 4-year EFS was 70% ±4.3% for all CNS+ patients. This was 75% ± 4.5% when corrected to reflect CNS+ patients receiving standard FAB/LMB therapy without cranial irradiation but with additional HDMTX and IT chemotherapy. This is not significantly different from the 4-year EFS of 79% ± 5.0% in historical controls with CNS disease treated on LMB89 with cranial irradiation.
Stratified probabilities. Probability of EFS and S stratified by BM, CNS, or BM/CNS (A) and response to COP reduction (B). (A) Product-limit estimate of probability of EFS in patients with BM disease only (BM+/CNS−), CNS disease only (BM−/CNS+), and combined BM and CNS disease (BM+/CNS+) (88% ± 3.0% versus 82 ± 5.6% versus 61% ± 6.0%, P < .001). (B) Product-limit estimate of probability of EFS in patients with complete response (CR; 100%), incomplete response (IR; 20%-99%) and nonresponse (NR; < 20%) after COP reduction therapy (94% ± 3.8% versus 78 ± 3.1% versus 30% ± 16%, P < .001).
Stratified probabilities. Probability of EFS and S stratified by BM, CNS, or BM/CNS (A) and response to COP reduction (B). (A) Product-limit estimate of probability of EFS in patients with BM disease only (BM+/CNS−), CNS disease only (BM−/CNS+), and combined BM and CNS disease (BM+/CNS+) (88% ± 3.0% versus 82 ± 5.6% versus 61% ± 6.0%, P < .001). (B) Product-limit estimate of probability of EFS in patients with complete response (CR; 100%), incomplete response (IR; 20%-99%) and nonresponse (NR; < 20%) after COP reduction therapy (94% ± 3.8% versus 78 ± 3.1% versus 30% ± 16%, P < .001).
The probability of 4-year EFS was 97% ± 3.0% among complete responders to day 7 COP reduction therapy, 78% ± 3.1% among incomplete responders, and only 30% ± 16% in patients among nonresponders (P < .001 overall, P = .017 CR versus IR; Figure 3B). Among the 33 complete responders, 16 received full-intensity treatment, 16 reduced-intensity treatment, and one died shortly after COP. Also, 25 complete responders had BM disease at diagnosis, 7 had BM and CNS involvement, and 1 had CNS involvement only.
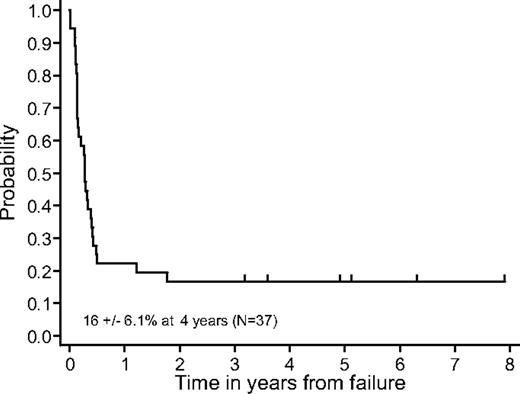
There was no significant difference in EFS due to diagnosis (DLBCL versus B-ALL), LDH (≤ 2 versus > 2 times normal upper limit), or age (5-year categories) in the entire cohort of patients. We additionally analyzed the survival of all patients on study who developed progressive or recurrent disease. The probability of 4-year survival of patients with persistent, progressive, or recurrent disease (excluding toxic deaths and secondary malignancy) was 16% ± 6.1% (Figure 4). There was no significant different in survival among these progressive/relapsed patients who were randomized to the standard versus reduced treatment (P = .24, log-rank test).
Survival probability after progression/recurrence. The probability of 4-year survival of patients with persistent, progressive, or recurrent disease, excluding those experiencing toxic deaths or secondary neoplasms, was 16% ± 6.1%.
Survival probability after progression/recurrence. The probability of 4-year survival of patients with persistent, progressive, or recurrent disease, excluding those experiencing toxic deaths or secondary neoplasms, was 16% ± 6.1%.
Treatment failure events
Forty-nine patients experienced an event and 42 have died (Figure 1A). One patient died prior to receiving chemotherapy. Death was unrelated to disease in another 10 patients (see “Toxicity and treatment-related deaths”). Of the remaining 38 patients, 14 patients had progressive or relapsed disease in the marrow with or without systemic (without CNS) sites, 13 in the CNS with or without systemic sites (without BM), 3 in the BM plus CNS with or without systemic sites, 4 in sites that did not include the BM or CNS, 2 with unknown site of progression/recurrence, and 2 developed a second malignancy (1 acute myeloid leukemia [AML], 1 ALL). All but 3 patients who had a CNS relapse had prior CNS disease. Thirty of the 36 patients who progressed or relapsed died, with median time to death of 95 days. The remaining 6 patients were alive a minimum of 3.1 years and a median of 5 years after progression or relapse.
Toxicity and treatment-related deaths
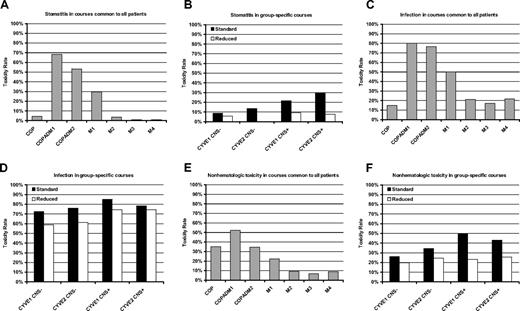
Stomatitis and infection were the most frequent single toxicities, occurring with grade III or IV at least once in 81% and 95% of patients, respectively. Grade III or IV stomatitis was reported most frequently during the first 2 COPADM courses, whereas infection was reported at high rates during most of therapy (Figure 5). Grade III/IV hemorrhage, transaminase, diarrhea, and electrolyte toxicities also occurred with cumulative rates exceeding 25%. There was a significant reduction, for patients treated with reduced therapy with mini CYVE, in grade III/IV stomatitis (OR = 0.27, P < .001), infection (OR = 0.56, P < .01, and other nonhematologic toxicity (OR = .70, P < .005).
Percentage of grade III/IV toxicities. Rates of grade III/IV stomatitis, infection, and other nonhematologic toxicities, within therapy courses. The left column shows the rates of these toxicities during COP, COPADM1, COPAMD2, and maintenance course that were common treatments received by all patients. The right column shows the comparative rates of these toxicities for the CYVE (standard) or mini CYVE (reduced) course received by patients with (CNS+) or without (CNS−) CNS disease at diagnosis. Reductions in stomatitis, infection, and other nonhematologic toxicities during these courses were statistically significant (P < .001, P < .01, and P < .005, respectively).
Percentage of grade III/IV toxicities. Rates of grade III/IV stomatitis, infection, and other nonhematologic toxicities, within therapy courses. The left column shows the rates of these toxicities during COP, COPADM1, COPAMD2, and maintenance course that were common treatments received by all patients. The right column shows the comparative rates of these toxicities for the CYVE (standard) or mini CYVE (reduced) course received by patients with (CNS+) or without (CNS−) CNS disease at diagnosis. Reductions in stomatitis, infection, and other nonhematologic toxicities during these courses were statistically significant (P < .001, P < .01, and P < .005, respectively).
The change from 48-hour to 6-hour doxorubicin infusion significantly reduced the incidence of stomatitis overall (P < .05). The cumulative rates and the rates for COPADM1 and COPADM2 were 79%, 67%, and 52%, respectively, with 6-hour infusion (n = 202) and 92%, 74%, and 63% with 48-hour infusion (n = 31).
The mean number of days of hospitalization was 8 (range, 1-50 days) during COP, 18 days (range, 0-49 days) during COPADM1, and 16 days (range, 5-66 days) during COPADM2. There was a significant reduction in average number of days of hospitalization in patients treated with CYVE versus mini CYVE (mean 4.6 days, P < .001). Forty-six patients had evidence of renal insufficiency during COP reduction, and 23 (10%) required assisted renal support (dialysis).
For 11 patients, death was not directly related to disease progression: 1 occurred prior to starting COP therapy, 2 occurred after COP (hemorrhage), 2 after COPADM2 (1 hemorrhage, 1 infection), 1 after CYVE (candidiasis pneumonia), 1 after mini CYVE (pneumonia), 2 during maintenance (1 basilar artery thrombosis, 1 infection), and 2 after intensification with autologous stem cell transplantation for nonhistologically documented partial remission. All but one of these patients received doxorubicin on the 6-hour infusion schedule.
The overall incidence of renal insufficiency during COP reduction phase was 46 of 235 (20%), tumor lysis syndrome 38 of 235 (16%), and use of dialysis 23 of 235 (10%). Patients treated in France (SFOP) used nonrecombinant urate oxidase but those patients in CCG did not have access to nonrecombinant urate oxidase. In comparing these 2 groups of patients, the incidence of renal insufficiency, tumor lysis syndrome, and dialysis was significantly less in SFOP versus CCG: renal insufficiency, 11 of 98 versus 27 of 101, P < .005; tumor lysis syndrome, 9 of 98 versus 26 of 101, P < .002; and dialysis, 3 of 98 versus 15 of 101, P < .004.
Discussion
This study is the largest randomized trial in children with high risk B-NHL (B-ALL with or without CNS involvement) and the only international multi-cooperative group randomized phase 3 trial conducted to date. Our results demonstrate that despite the significant benefits in the reduction of morbidity including grade III/IV mucositis, infection and days of hospitalization with reduced intensity FAB/LMB therapy, there was also a significant decrease in the EFS compared to standard FAB/LMB therapy. Children treated on the standard FAB/LMB therapy had excellent results, especially patients with only B-ALL and no CNS involvement (≥ 90% 4-year EFS) and these results compare favorably with other similar short-intensity regimens.3-5,8,23 These results also confirm the ability to reproduce excellent results from single pediatric cooperative group experiences across 3 international institutions in 8 different countries. Children with CNS disease, despite not receiving cranial irradiation but receiving an additional course of HDMTX (8 g/m2) and intrathecal chemotherapy, had a similar outcome to children with CNS disease treated with cranial irradiation on the previous LMB89 study.4 These results suggest that cranial irradiation could possibly be deleted from this subgroup of patients. These results are similar to the recent Berlin-Frankfurt-Munich (BFM) experience in children with CNS disease.5
Several factors were associated with a poor 4-year EFS. The subgroup of children with combined BM and CNS involvement at diagnosis had a significantly inferior outcome (61% ± 6%) versus children with BM or CNS only. These results are similar to the BFM experience in BFM/NHL 90 (56% ± 13%; personal oral communication, A. Reiter University of Giessen, Germany, September 2004). Secondly, similar to what was observed in the intermediate-risk group (group B), children with a poor response to COP reduction therapy (≤ 20% response) had a significantly inferior EFS compared to children with a CR (30% ± 16% versus 97% ± 3%, P < .001) and an IR (30% ± 16% versus 78%± 3%, P < .049).
These are the first results to demonstrate that a CR to initial COP reduction therapy in advanced (BM with or without CNS involvement) childhood mature B-NHL/B-ALL may be significantly important in overall prognosis similar to what has been demonstrated in precursor B-ALL.24,25 However, histology (BL versus BLL versus DLBCL) and LDH (normal versus ≥ 2 times normal) were not significant risk factors associated with 4-year EFS, although there were few patients with both DLBCL or normal LDL levels entered in the study. Despite the excellent overall survival results with FAB/LMB therapy for this high-risk group of patients, there is a significant amount of acute morbidity and potential long-term toxicity. The toxic death rate was 3%, the incidence of tumor lysis syndrome and renal failure was approximately 10% during COP reduction, and there is still a high incidence of grade III/IV mucositis, infection, myelosuppression, and prolonged hospitalization during the COPADM induction courses. Because a poor response to reduction therapy was associated with increased disease failure, alternative supportive care approaches should be considered to enhance the intensity of reduction therapy and reduce the incidence of tumor lysis syndrome. As shown in the previous SFOP LMB89 study in comparison with other concurrent protocols for advanced B-NHL/B-ALL, the SFOP patients of this study who had access to nonrecombinant urate oxidase had significantly less renal insufficiency, tumor lysis syndrome, and the use of dialysis compared to the CCG patients who did not have access to nonrecombinant urate oxidase. Goldman and Cairo et al recently demonstrated the significant and more rapid reduction of uric acid levels with recombinant urate oxidase versus allopurinol in children with hematologic malignancies at risk for tumor lysis syndrome.18 The current Children's Oncology Group study for de novo B-NHL with COP reduction therapy is now investigating the addition of recombinant urate oxidase to COP reduction therapy (COG ANHL01P1).
The survival of patients with recurrent or progressive disease is dismal (17% ± 6%). Sites of recurrence were mixed: 37% in BM with or without systemic involvement, 34% CNS with or without systemic involvement, and 8% BM+/CNS+. Newer approaches for retrieval therapy, including targeted monoclonal antibodies in combination with chemotherapy, radioconjugate antibody studies, and stem-cell transplantation approaches are needed in children with disease progression or recurrence.26-28
In summary, we have demonstrated in this randomized, prospective, international cooperative study that adjustment in FAB/LMB therapy in children with BM with or without CNS disease results in a significant reduction in acute morbidity (mucositis, infection, duration of hospitalization) but additionally, a significantly inferior 4-year EFS. Furthermore, compared to historical controls, deletion of cranial irradiation and replacement with HDMTX (8 g/m2) and IT chemotherapy appears to be associated with a similar overall survival in children with CNS disease. There appear to be subgroups of children who have a significantly inferior outcome, including those with a poor response to initial reduction therapy with COP and BM plus CNS disease. Future studies will be required to determine the optimal treatment for these subgroups of ultrahigh-risk children with B-NHL/ALL. Lastly, little is known about the biology and genetics of childhood B-NHL.29 Recent gene expression profiling in adults with DLBCL has identified genetic subgroups with different outcomes.30-32 Similar studies may be required in children to identify new novel targets and determine which subgroups may require alternative treatment strategies.
Authorship
Contribution: M.S.C. and C.P. designed and performed the research, analyzed data, and wrote the manuscript; M.G. designed and performed research and analyzed data; R.S. designed research, analyzed data, and wrote the manuscript; A.A. designed research, performed interim analyses, and analyzed data; C.R.P. designed and performed research; J.M. performed research; C.W. analyzed data; and S.L.P., M.R., and K.M. performed research, analyzed data, and wrote the manuscript.
Conflict-of-interest: The authors declare no competing financial interests.
A complete list of the participants in the FAB LMB 96 International Study Committee appears in the Supplemental Appendix (available on the Blood website; see the Supplemental Appendix link at the top of the online article).
Correspondence: Mitchell S. Cairo, Professor of Pediatrics, Medicine and Pathology, Chief, Division of Pediatric Hematology and BMT, Columbia University, 180 Fort Washington Ave, HP 506, New York, NY 10032; e-mail: mc1310@columbia.edu.
Presented in part at the American Society of Clinical Oncology (ASCO), June 2003, Chicago, IL, and the American Society of Hematology (ASH), December 2003, San Diego, CA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Department of Health and Human Services (COG); Cancer Research Campaign (UKCSSG); and Association pour la Recherche contre le Cancer, La Ligue Nationale Contre le Cancer, Institut Gustave Roussy (SFOP).
The authors would like to thank the members of the DSMC, Professors Michael Link, Alfred Reiter, David Harrington, and Robert Souhami for their continual and diligent oversight of this study. The authors would also like to thank Ms Linda Rahl, Ms Virginia Davenport, and Ms Donna Correia for their expert editorial assistance in the development of this manuscript as well as the other members on the international pathology review panel (Drs M. J. Terrier-Lacombe, C. Bayle, P. Felman, M. Lones, and A. Wotherspoon). The authors would finally like to thank all the members and investigators of the Children's Oncology Group, Société Française d'Oncologie Pédiatrique, and the United Kingdom Children's Cancer Study Group.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal