Abstract
MS-275 is a benzamide derivative with potent histone deacetylase (HDAC) inhibitory and antitumor activity in preclinical models. We conducted a phase 1 trial of orally administered MS-275 in 38 adults with advanced acute leukemias. Cohorts of patients were treated with MS-275 initially once weekly × 2, repeated every 4 weeks from 4 to 8 mg/m2, and after 13 patients were treated, once weekly × 4, repeated every 6 weeks from 8 to 10 mg/m2. The maximum-tolerated dose was 8 mg/m2 weekly for 4 weeks every 6 weeks. Dose-limiting toxicities (DLTs) included infections and neurologic toxicity manifesting as unsteady gait and somnolence. Other frequent non-DLTs were fatigue, anorexia, nausea, vomiting, hypoalbuminemia, and hypocalcemia. Treatment with MS-275 induced increase in protein and histone H3/H4 acetylation, p21 expression, and caspase-3 activation in bone marrow mononuclear cells. No responses by classical criteria were seen. Our results show that MS-275 effectively inhibits HDAC in vivo in patients with advanced myeloid leukemias and should be further tested, preferably in patients with less-advanced disease.
Introduction
Treatment of relapsed and refractory acute leukemias remains a challenge. Approximately 30% of adults with newly diagnosed acute myeloid leukemia (AML) are primary refractory to chemotherapy, and at least 50% of those who achieve remission will relapse.1-4 Even when most aggressive therapeutic approaches, such as intensive chemotherapy and allogeneic stem cell transplantation (SCT), are used early, the cure rate remains at 35% to 40%.1,2,4-6 Certain subgroups, including older adults,7-9 patients with secondary AMLs,10-12 and patients with previous myelodysplasia (MDS) or other hematologic disorders,13 have extremely poor outcome with cure rate less than 10%. Thus, novel therapeutic approaches are urgently needed.
Histone deacetylases (HDACs) are critically important in the regulation of gene expression and compelling new targets for anticancer drug development.14-18 HDAC inhibitors (HDIs) have the ability to induce gene expression, differentiation, growth arrest, and apoptosis of transformed cells.14,16 The HDI MS-275 is a synthetic benzamide derivative that exhibits potent time- and dose-dependent growth inhibition and cytotoxicity against a panoply of human tumor cells in vitro,19-21 including leukemia cell lines and primary leukemia blasts,20,22-24 and produces tumor regression in xenograft models in vivo.19,20 The molecular effects behind MS-275 growth inhibitory and differentiating activity include transcriptional activation of antiproliferation genes such as p21 (independent of p53) and transforming growth factor-β type II receptor, and induction of maturation marker gelsolin.19,20,24,25
In preclinical toxicology studies, the maximum tolerated dose (MTD) for MS-275 administered orally for 28 days was 18 mg/m2 for rats and 6 mg/m2 for dogs.26 However, phase 1 study of MS-275 in adults with solid tumors demonstrated that MS-275 has a 30 to 50 times longer half-life (T1/2) than initially predicted from animal studies.27 After the initial 2 patients on that study experienced unexpected toxicities, the schedule of drug administration was changed from daily to every 14 days, and the MTD was achieved at 10 mg/m2. Dose-limiting toxicities (DLTs) were gastrointestinal (GI) symptoms and fatigue. Interestingly, MS-275 exerted a pharmacodynamic effect by increasing histone H3 acetylation in peripheral blood mononuclear cells (PBMCs) at all dose levels.27
Because gene silencing that occurs as a result of histone deacetylation and subsequent block in cell differentiation is an important mechanism in leukemogenesis,14,28-30 we wanted to test potential molecular, biologic, and clinical effects of MS-275 in adults with refractory and relapsed acute leukemias. As such, we conducted a phase 1 dose escalation trial to define MTD, DLT, and pharmacodynamic and pharmacokinetic profiles of MS-275 in this patient population, and results are presented in this report.
Patients, materials, and methods
Patient eligibility and selection
Adults, aged 18 years or older, with acute leukemia or high-risk MDS resistant to or relapsed after prior induction regimens; newly diagnosed acute leukemias in adults older than age 60 with poor-risk features (antecedent hematologic disorder, poor-risk/complex karyotype); AML arising from MDS or secondary AML; patients with acute promyelocytic leukemia who failed ATRA and arsenic trioxide; and chronic myelogenous leukemia (CML) in accelerated or blast crisis or interferon-refractory CML in chronic phase were eligible for study entry provided they met the following criteria: ECOG performance status 0 to 2, bilirubin 1.5 times normal or less, hepatic enzymes 2 times normal or less, serum creatinine 1.5 times normal or less, and left ventricular ejection fraction 45% or higher. Complete recovery from toxicities of previous treatment, an interval of 3 weeks or more from previous chemotherapy (hydroxyurea was allowed up to 24 hours prior to MS-275 administration), and an interval of 1 week or more from any growth factor therapy was required before beginning MS-275.
Patients were ineligible if they had peripheral blast count of 50 × 109/L or higher; disseminated intravascular coagulation; active central nervous system (CNS) leukemia; were eligible for SCT; received more than 3 prior courses of induction/reinduction therapy, concomitant radiotherapy, chemotherapy, or immunotherapy; or had coexisting medical or psychiatric conditions that could interfere with study procedures. Pregnant or lactating woman were ineligible. All patients provided written informed consent. This study was approved and conducted in accordance with the policies of Institutional Review Boards of the University of Maryland School of Medicine, Baltimore, and Johns Hopkins University, Baltimore.
Complete history and medical examination were performed within 7 days of study entry. The following laboratory parameters were obtained at 3 days or less before entry: complete blood count with differential; comprehensive electrolyte panel; coagulation profile; urinalysis; bone marrow (BM) aspirate/biopsy with histochemical, cytogenetic, and immunophenotypic analysis; chest X-ray; electrocardiogram; surveillance cultures of throat, stool, and urine; and pregnancy test. Additional studies were performed when clinically indicated.
Treatment schema
Patients received MS-275 tablets orally with food once a week for 2 or 4 consecutive weeks depending on the dose level (DL) and followed by a 2-week washout period. The drug was discontinued at any time for grade 3 or higher nonhematologic toxicity according to NCI CTC, Version 2, BMT criteria. The starting dose of 4 mg/m2 weekly was selected based on preliminary pharmacokinetic and safety data for the same dose of MS-275 administered biweekly in patients with solid tumors.27 Using a modified Fibonacci dose escalation scheme with a dose escalation increment of 2 mg/m2, the first patient cohort received MS-275 at 4 mg/m2 for 2 weeks in a row of 28-day cycle. Patients were entered on the study in cohorts of 3 to 6. The criteria for dose escalation were based on the safety data from the first treatment cycle. Dose was escalated to the next level if 0 of 3 patients experienced DLT. If 1 of 3 patients experienced DLT, the cohort was expanded to 6 patients. If 2 or more of 6 patients experienced DLT, no additional patients were entered at that dose. The occurrence of any DLT in 33% or more of a patient cohort defined the maximal administered dose (MAD). Once MAD was reached, an expanded cohort of patients (3-6 additional patients) was required to be treated at the highest prior well-tolerated dose level, MTD, and observed for DLT. DLT was defined as either grade 3 or higher drug-related (possibly, probably, definitely) nonhematologic toxicity or grade 4 myelosuppression lasting 28 days or more in the absence of residual leukemia (ie, following achievement of marrow tumor clearance). There was no limitation on the number of cycles administered as long as the patient did not have progressive disease or unacceptable toxicity. Initially, the dose escalation proceeded from 4 to 8 mg/m2 of MS-275 given weekly for 2 weeks in a row of a 28-day cycle (DL 1-3). Following completion of the first 3 dose levels with no MTD reached, and based on early clinical observations suggesting that prolonged exposure to HDIs or other differentiating agents may be required for their efficacy,27,31,32 instead of pursuing further dose escalation the schedule of drug administration was changed to once weekly for 4 weeks of a 42-day cycle and starting at 8 mg/m2.
Definition of response
To assess response to therapy, a BM aspiration was performed weekly for 4 weeks during the first cycle, at the end of each subsequent cycle, or at any time leukemia regrowth was suspected. Complete response (CR) required a normal BM aspirate with absence of identifiable leukemia, absolute neutrophil count (ANC) of 1 × 109/L or higher, platelet count of 100 × 109/L or higher, and absence of blasts in peripheral blood (PB). Partial response (PR) was defined as the presence of trilineage hematopoiesis in the marrow with recovery of ANC and platelets to the above-stated levels, but with 5% to 25% blasts in the marrow.33 Progressive disease (PD) was defined as greater than 50% increase in marrow or PB blasts from baseline or development of extramedullary leukemia.
Pharmacodynamic analysis
Serial marrow and/or peripheral blood cells were collected at weekly intervals to examine MS-275–induced changes in histone acetylation.
Immunocytochemical analysis of acetylated histone H3 was performed on bone marrow mononuclear cells (BMMCs) as described previously.27 The multiparameter flow cytometric analysis of protein acetylation versus caspase-3 activation was performed on serial BM aspirates using monoclonal antiacetylated lysine antibody (Cell Signaling Technology, Beverly, MA) followed by FITC-conjugated goat antimouse antibody (Caltag Laboratories, Burlingame, CA) and polyclonal PE-conjugated anti–caspase-3, active form antibody (BD Pharmingen, San Diego, CA) as described previously.34 The analysis of protein acetylation versus p21 expression or CD34 expression was performed using polyclonal antiacetylated lysine antibody (Cell Signaling Technology) versus monoclonal anti-p21Cip1 antibody (BD Transduction Laboratories, San Jose, CA) or monoclonal anti-CD34 antibody (BD Pharmingen). Primary antibodies were detected using PE-conjugated goat antimouse and FITC-conjugated goat antirabbit antibodies (Caltag Laboratories). For staining, BMMCs were isolated using Ficoll-Paque Plus (Amersham Biosciences, Piscataway, NJ), fixed in 0.4% paraformaldehyde, permeabilized with 0.4% Triton X-100, incubated with primary antibodies for 1 hour at room temperature, washed, incubated with secondary antibodies for 1 hour, washed, and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) using CellQuest software for acquisition and FloJo software for analysis as described previously.34
For Western blot analysis of histone acetylation, PBMCs and BMMCs were isolated using Ficoll-Paque Plus (Amersham Biosciences). Histones from the cells were prepared by a modification of a previously described method.35 Briefly, cells were washed in 2 mL HBSS and disrupted by 1 mL ice-cold lysis buffer A (10 mM Tris pH 7.6, 5 mM butyric acid, 1% Triton X-100, 1 mM MgCl2, and 1mM PMSF). Nuclei were collected by centrifugation at 8800g (14 000 rpm) for 15 minutes. The pellet was resuspended once with 250 μL ice-cold lysis buffer B (10 mM Tris pH 7.6, 0.25 M sucrose, 3 mM CaCl2, and 5 mM butyric acid). Sulfuric acid was added to a concentration of 0.4 N, and the tubes were incubated at 4°C overnight. Debris was pelleted by centrifugation, and the supernatant was collected. Histones were precipitated by addition of 10 vol of acetone and incubation at −20°C overnight. Pellets were collected by centrifugation, briefly dried under vacuum, and resuspended with dH2O. The total protein content was determined by a bicinchoninic acid assay kit (Pierce, Rockford, IL). The proteins 10 to 30 μg were separated by 15% SDS polyacrylamide gel electrophoresis (PAGE) and visualized using the antibodies for acetyl-histone H3, acetyl-histone H4, and histone-H2A, all from Upstate Biotechnologies (Lake Placid, NY). The immunoreactive proteins were detected using enhanced chemiluminescence (ECL) Western blotting analysis system (Amersham Biosciences). Radiographic films of histone acetylation were scanned and digitized (UN-SCAN-IT; Silk Scientific, Orem, UT).
Pharmacokinetic studies
Blood samples for pharmacokinetic studies (6 mL each) were collected in tubes containing sodium heparin at the following time points: immediately before drug administration, and at 0.5, 1, 2, 6, 12, 24, 48, 60, and 72 hours after the first dose of MS-275. All blood samples were kept on ice and centrifuged within 2 hours of collection at 3000g for 10 minutes at 4°C. Separated plasma was immediately frozen on dry ice and then stored at −70°C until analysis.
Concentrations of MS-275 in plasma were determined using a validated high-performance liquid chromatographic assay with mass-spectrometric detection, as described previously.36 The linear range of this assay is 1 to 100 ng/mL, with a lower limit of quantitation of 0.1 ng/mL. The values for precision and accuracy, determined during each analytical run by concurrent analysis of quality control samples, were within 12% relative error.
Estimates of pharmacokinetic parameters for MS-275 were derived from individual concentration-time data sets by noncompartmental analysis using the software package WinNonlin version 5.0 (Pharsight Corporation, Mountain View, CA), as described elsewhere.27 The pharmacokinetic parameters of interest included peak plasma concentration (Cmax), time to peak concentration (Tmax), area under the plasma concentration versus time curve extrapolated to infinity (AUC), apparent oral clearance (CL/F), and the terminal half-life (T1/2, z). Interindividual pharmacokinetic variability was assessed with the percentage of coefficient of variation (CV), expressed as the ratio of the standard deviation and the observed mean. The influence of the dose level on CL/F was evaluated using the Kruskal-Wallis one-way analysis of ranks test, followed by the Dunn multiple comparison test for identifying statistically significant group differences. These calculations were performed with the software package NCSS version 2001 (Number Cruncher Statistical Systems; J.L. Hintze, Kaysville, UT).
Results
Patient characteristics
A total of 39 patients with leukemia were entered into this phase 1 study of MS-275. One patient did not receive the drug because of elevation in liver function tests prior to drug administration. The remaining 38 patients were evaluable for toxicity and 34 for response. Their demographics and disease characteristics are detailed in Table 1. Median age was 65 (range, 25-86), and 66.7% (26 of 39) were men. Thirty-two (82%) of 39 patients had refractory disease, 18 patients (46.2%) were primary refractory, and 30 patients (76.9%) had abnormal karyotype. The median number of prior regimens was 2 (range, 0-3). Twenty-nine patients (74.4%) received ara-C in prior treatments, 3 patients had undergone prior autologous SCT, and 1 patient had undergone prior allogeneic SCT. Nine patients (23.1%) received tipifarnib (7 patients) or phenylbutyrate/5-azacitidine (2 patients) as their only prior therapies.
Patient characteristics
| MS-275 (dose level) . | Age, y . | Sex . | Diagnosis . | Stage of disease . | Cytogenetics . |
|---|---|---|---|---|---|
| 4 mg/m2 × 2/4 wk (DL 1) | |||||
| Patient 1 | 56 | F | AML, M3 | Refractory relapse 2 | 50XX, t(4;12), t(15;17), +4mar |
| Patient 2 | 69 | M | AML, M2 | Primary refractory | 47XY, 20q−, +11 |
| Patient 3 | 70 | M | AML, M1 | Refractory relapse 1 | 45X, −Y |
| Patient 4 | 72 | M | AML, M7 | Primary refractory | 46XY |
| Patient 5 | 49 | F | AML, M0 | Primary refractory | 45XX, der(2), der(3), −7, −11, −17, der(12), +2mar |
| 6 mg/m2 × 2/4 wk (DL 2) | |||||
| Patient 6 | 44 | F | AML, M4 | Primary refractory | 46X, t(X;7), inv(3) |
| Patient 7 | 85 | M | AML, M5 | Primary refractory | 46XY |
| Patient 8 | 49 | M | CML-BC | Relapse 1 | 46XY, t(9;22), t(15;17) |
| Patient 9 | 65 | F | AML, M5 | Relapse 1 | 46XX |
| Patient 10 | 54 | M | AML, M1 | Refractory relapse 1 | 42XY, −5, −7, +8, +9, inv(12), −16, −17, −17,der(18), −20, −20, −21, +2mar |
| 8 mg/m2 × 2/4 wk (DL 3) | |||||
| Patient 11 | 69 | F | AML, M2 | Refractory relapse 1 | 46XX, 7q- |
| Patient 12 | 50 | F | AML, M5 2° | Relapse 1 | 45X, t(9;11), −7, der(12)t(7;12) |
| Patient 13 | 74 | M | AML, M2 | Primary refractory | 45XY, −7 |
| 8 mg/m2 × 4/6 wk (DL 4) | |||||
| Patient 14 | 36 | F | AML, M4 | Relapse 2 | 47XX, t(4;7), inv(9), inv(16), i(17), +22 |
| Patient 15 | 72 | M | AML, M2 | Primary refractory | 47XY, +8/46XY, 20q- |
| Patient 16 | 86 | M | MDS/AML | Refractory relapse 1 | 46XY |
| Patient 17 | 76 | F | AML, M2 | Refractory relapse 1 | 47XX, 7q-, +11 |
| Patient 18 | 65 | F | AML, M7 | Refractory relapse 1 | 46XX |
| Patient 19 | 45 | M | AML, M4 | Refractory relapse 2 | 46XY, inv(16)/45XY, 7q−, add(8), der(12)t(12;17), der(17)t(12;17)del(17)(q11), −21 |
| Patient 20 | 65 | M | MDS/AML | Primary refractory | 46XY |
| Patient 21 | 66 | M | AML, M0 | Primary refractory | 47XY, +13 |
| Patient 22 | 25 | F | AML, M5 | Refractory relapse 2 | 50XX, t(3;19), +i(5),+8, +8, der(9)t(9;11)del(9), der(11)t(9;11), der(17)t(8;17),+19, +1mar |
| Patient 23 | 63 | F | AML, M5 2° | Refractory relapse 1 | 46XX, inv(3), t(11;22) |
| Patient 24 | 74 | F | MDS/AML | Primary refractory | 47XX, +8 |
| Patient 25 | 57 | M | AML, M4 | Refractory relapse 1 | 48XY, 5q−, +8, +13 |
| Patient 26 | 38 | M | AML, M1 | Primary refractory | 45XY, del(1), add(2), add(3), inv(8), add(9), add(12), der(12), add(13), add(14),der(16)t(16;17), −17, del(17), add(18), −19, der(20)t(9;20), +mar |
| Patient 27 | 73 | M | AUL | New diagnosis | 46X, −Y, t(11;21), +13 |
| Patient 28 | 65 | M | AML, M5 | Refractory relapse 1 | 46XY |
| Patient 29 | 67 | M | MDS/AML | Relapse 1 | 45XY, t(1;9), −7, 20q− |
| 10 mg/m2 × 4/6 wk (DL 5) | |||||
| Patient 30 | 55 | M | MDS/AML | Refractory relapse 1 | 47XY, inv(1), t(10:15), 13q−, +21 |
| Patient 31 | 73 | M | AML, M6 | Primary refractory | 42-51XY, 7q−, der(11), −12, −17, −18, add(19), t(12;20), +3-5mar |
| Patient 32 | 57 | M | AML, M4 | Primary refractory | 45XY, add(3), add(4), −5, 7q−, add(10), −12, add(13), del(16), −17, +2mar |
| Patient 33 | 70 | M | MDS/AML | Primary refractory | 46XY |
| Patient 34 | 72 | M | MDS/AML | Primary refractory | 48XY, +13, +21 |
| Patient 35 | 55 | M | AML, M4 | Refractory relapse 1 | 46XY |
| Patient 36 | 67 | M | AML, M5 | Relapse 1 | 48XY, +5, +7 |
| Patient 37 | 60 | M | AML, M7 | Primary refractory | 46XY, −1, add(1), del(2), add(3), add(5), −6, del(6), −7, del(8), del(10), del(12), add(15), add(16), add(17), del(18), +1-3mar |
| Patient 38 | 72 | M | MDS/AML | Primary refractory | 47XY, dup(4), dup(7), +8 |
| Patient 39 | 52 | F | MDS/AML | Primary refractory | 47XY, +8 |
| MS-275 (dose level) . | Age, y . | Sex . | Diagnosis . | Stage of disease . | Cytogenetics . |
|---|---|---|---|---|---|
| 4 mg/m2 × 2/4 wk (DL 1) | |||||
| Patient 1 | 56 | F | AML, M3 | Refractory relapse 2 | 50XX, t(4;12), t(15;17), +4mar |
| Patient 2 | 69 | M | AML, M2 | Primary refractory | 47XY, 20q−, +11 |
| Patient 3 | 70 | M | AML, M1 | Refractory relapse 1 | 45X, −Y |
| Patient 4 | 72 | M | AML, M7 | Primary refractory | 46XY |
| Patient 5 | 49 | F | AML, M0 | Primary refractory | 45XX, der(2), der(3), −7, −11, −17, der(12), +2mar |
| 6 mg/m2 × 2/4 wk (DL 2) | |||||
| Patient 6 | 44 | F | AML, M4 | Primary refractory | 46X, t(X;7), inv(3) |
| Patient 7 | 85 | M | AML, M5 | Primary refractory | 46XY |
| Patient 8 | 49 | M | CML-BC | Relapse 1 | 46XY, t(9;22), t(15;17) |
| Patient 9 | 65 | F | AML, M5 | Relapse 1 | 46XX |
| Patient 10 | 54 | M | AML, M1 | Refractory relapse 1 | 42XY, −5, −7, +8, +9, inv(12), −16, −17, −17,der(18), −20, −20, −21, +2mar |
| 8 mg/m2 × 2/4 wk (DL 3) | |||||
| Patient 11 | 69 | F | AML, M2 | Refractory relapse 1 | 46XX, 7q- |
| Patient 12 | 50 | F | AML, M5 2° | Relapse 1 | 45X, t(9;11), −7, der(12)t(7;12) |
| Patient 13 | 74 | M | AML, M2 | Primary refractory | 45XY, −7 |
| 8 mg/m2 × 4/6 wk (DL 4) | |||||
| Patient 14 | 36 | F | AML, M4 | Relapse 2 | 47XX, t(4;7), inv(9), inv(16), i(17), +22 |
| Patient 15 | 72 | M | AML, M2 | Primary refractory | 47XY, +8/46XY, 20q- |
| Patient 16 | 86 | M | MDS/AML | Refractory relapse 1 | 46XY |
| Patient 17 | 76 | F | AML, M2 | Refractory relapse 1 | 47XX, 7q-, +11 |
| Patient 18 | 65 | F | AML, M7 | Refractory relapse 1 | 46XX |
| Patient 19 | 45 | M | AML, M4 | Refractory relapse 2 | 46XY, inv(16)/45XY, 7q−, add(8), der(12)t(12;17), der(17)t(12;17)del(17)(q11), −21 |
| Patient 20 | 65 | M | MDS/AML | Primary refractory | 46XY |
| Patient 21 | 66 | M | AML, M0 | Primary refractory | 47XY, +13 |
| Patient 22 | 25 | F | AML, M5 | Refractory relapse 2 | 50XX, t(3;19), +i(5),+8, +8, der(9)t(9;11)del(9), der(11)t(9;11), der(17)t(8;17),+19, +1mar |
| Patient 23 | 63 | F | AML, M5 2° | Refractory relapse 1 | 46XX, inv(3), t(11;22) |
| Patient 24 | 74 | F | MDS/AML | Primary refractory | 47XX, +8 |
| Patient 25 | 57 | M | AML, M4 | Refractory relapse 1 | 48XY, 5q−, +8, +13 |
| Patient 26 | 38 | M | AML, M1 | Primary refractory | 45XY, del(1), add(2), add(3), inv(8), add(9), add(12), der(12), add(13), add(14),der(16)t(16;17), −17, del(17), add(18), −19, der(20)t(9;20), +mar |
| Patient 27 | 73 | M | AUL | New diagnosis | 46X, −Y, t(11;21), +13 |
| Patient 28 | 65 | M | AML, M5 | Refractory relapse 1 | 46XY |
| Patient 29 | 67 | M | MDS/AML | Relapse 1 | 45XY, t(1;9), −7, 20q− |
| 10 mg/m2 × 4/6 wk (DL 5) | |||||
| Patient 30 | 55 | M | MDS/AML | Refractory relapse 1 | 47XY, inv(1), t(10:15), 13q−, +21 |
| Patient 31 | 73 | M | AML, M6 | Primary refractory | 42-51XY, 7q−, der(11), −12, −17, −18, add(19), t(12;20), +3-5mar |
| Patient 32 | 57 | M | AML, M4 | Primary refractory | 45XY, add(3), add(4), −5, 7q−, add(10), −12, add(13), del(16), −17, +2mar |
| Patient 33 | 70 | M | MDS/AML | Primary refractory | 46XY |
| Patient 34 | 72 | M | MDS/AML | Primary refractory | 48XY, +13, +21 |
| Patient 35 | 55 | M | AML, M4 | Refractory relapse 1 | 46XY |
| Patient 36 | 67 | M | AML, M5 | Relapse 1 | 48XY, +5, +7 |
| Patient 37 | 60 | M | AML, M7 | Primary refractory | 46XY, −1, add(1), del(2), add(3), add(5), −6, del(6), −7, del(8), del(10), del(12), add(15), add(16), add(17), del(18), +1-3mar |
| Patient 38 | 72 | M | MDS/AML | Primary refractory | 47XY, dup(4), dup(7), +8 |
| Patient 39 | 52 | F | MDS/AML | Primary refractory | 47XY, +8 |
AML indicates acute myeloid leukemia; CML-BC, chronic myeloid leukemia-blast crisis; AUL, acute undifferentiated leukemia; 2°, secondary; MDS/AML, AML arising from myelodysplasia.
Toxicity
The total number of patients enrolled and cycles administered at each dose level are depicted in Table 2. The summary of all drug-related toxicities is provided in Table 3. Initially, MS-275 was administered weekly for 2 weeks followed by 2 weeks of rest (28-day cycle). Dose escalation progressed from DL 1 to DL 3 without the achievement of MTD. Only one patient treated at DL 2 did not complete the first cycle because of rapidly progressive disease. As depicted in Table 2, one DLT grade 3 fatigue was recorded at DL 1. This patient had underlying disease-related fatigue, and the contribution of MS-275 could not be clearly assessed.
Dose levels
| Dose level/schedule . | No. of patients (no. who completed first cycle) . | Total no. of cycles . | Median no. of cycles (range) . | DLTs . |
|---|---|---|---|---|
| DL 1: 4 mg/m2 × 2/4 wk | 5 (5) | 7 | 2 (1-4)* | 1† |
| DL 2: 6 mg/m2 × 2/4 wk | 5 (4) | 9* | 1 | 0 |
| DL 3: 8 mg/m2 × 2/4 wk | 3 (3) | 3 | 1 | 0 |
| DL 4: 8 mg/m2 × 4/6 wk | 15 (9) | 27 | 1 (1-5) | 0 |
| DL 5: 10 mg/m2 × 4/6 wk | 10 (5) | 12 | 1 (1-2) | 5‡ |
| Dose level/schedule . | No. of patients (no. who completed first cycle) . | Total no. of cycles . | Median no. of cycles (range) . | DLTs . |
|---|---|---|---|---|
| DL 1: 4 mg/m2 × 2/4 wk | 5 (5) | 7 | 2 (1-4)* | 1† |
| DL 2: 6 mg/m2 × 2/4 wk | 5 (4) | 9* | 1 | 0 |
| DL 3: 8 mg/m2 × 2/4 wk | 3 (3) | 3 | 1 | 0 |
| DL 4: 8 mg/m2 × 4/6 wk | 15 (9) | 27 | 1 (1-5) | 0 |
| DL 5: 10 mg/m2 × 4/6 wk | 10 (5) | 12 | 1 (1-2) | 5‡ |
Two patients enrolled at level 1 each received 2 subsequent cycles at level 2.
Fatigue.
Infections (3), neurologic toxicity (1), and abnormal laboratory parameters (1).
Toxicity summary for all treatment cycles (possibly, probably, definitely related to MS-275)
| Adverse events . | All grades . | Grade 3-4, no. (5, no.) . | |
|---|---|---|---|
| No. of cycles . | % . | ||
| General | |||
| Dehydration, depression, fever, dizziness, insomnia | 5 | 9 | 0 |
| Fatigue | 12 | 21 | 1 |
| Gastrointestinal | |||
| Anorexia | 5 | 9 | 0 |
| Nausea | 8 | 14 | 0 |
| Vomiting | 6 | 10 | 0 |
| Abdominal pain/gas | 1 | 2 | 0 |
| Infections | |||
| Neutropenic bacteremia, sepsis, pneumonia, other | 9 | 16 | 8 (2) |
| Laboratory | |||
| Hypoalbuminemia | 31 | 53 | 0 |
| Hypocalcemia | 32 | 55 | 0 |
| Hyponatremia, hyperkalemia | 2 | 3 | 0 |
| LDH | 1 | 2 | 1 |
| Bilirubin | 1 | 2 | 0 |
| Glucose | 1 | 2 | 1 |
| Triglycerides | 1 | 2 | 1 |
| Hematologic | |||
| Cytopenias | 9 | 16 | 6 |
| Neurologic | |||
| Unsteady gait, lower extremities weakness | 3 | 5 | 1 |
| Somnolence/lethargy | 1 | 2 | 1 |
| Adverse events . | All grades . | Grade 3-4, no. (5, no.) . | |
|---|---|---|---|
| No. of cycles . | % . | ||
| General | |||
| Dehydration, depression, fever, dizziness, insomnia | 5 | 9 | 0 |
| Fatigue | 12 | 21 | 1 |
| Gastrointestinal | |||
| Anorexia | 5 | 9 | 0 |
| Nausea | 8 | 14 | 0 |
| Vomiting | 6 | 10 | 0 |
| Abdominal pain/gas | 1 | 2 | 0 |
| Infections | |||
| Neutropenic bacteremia, sepsis, pneumonia, other | 9 | 16 | 8 (2) |
| Laboratory | |||
| Hypoalbuminemia | 31 | 53 | 0 |
| Hypocalcemia | 32 | 55 | 0 |
| Hyponatremia, hyperkalemia | 2 | 3 | 0 |
| LDH | 1 | 2 | 1 |
| Bilirubin | 1 | 2 | 0 |
| Glucose | 1 | 2 | 1 |
| Triglycerides | 1 | 2 | 1 |
| Hematologic | |||
| Cytopenias | 9 | 16 | 6 |
| Neurologic | |||
| Unsteady gait, lower extremities weakness | 3 | 5 | 1 |
| Somnolence/lethargy | 1 | 2 | 1 |
In the absence of DLTs at DL 2 and 3, the schedule of drug administration was changed to weekly for 4 weeks followed by 2 weeks of rest (42-day cycle). Initially, 8 patients were treated at DL 4; 2 patients did not complete the first cycle because of death (one died on day 2 from PD/leukostasis, one died on day 13 from progressive fungal pneumonia). An additional 2 patients died while on study: one on day 36 of cycle 1 from sepsis, and another with sudden death by day 20 of cycle 2 thought to be related to his underlying heart disease (history of mitral valve prolapse, aortic stenosis, atrial fibrillation, hypertension) in the setting of diarrhea and dehydration related to his chronic Clostridium difficile colitis and pancreatic insufficiency as a consequence of previous Whipple surgery for duodenal carcinoma. Because none of the above-mentioned events were considered MS-275 related, dose escalation progressed to DL 5. Five patients (50%) at DL 5 were unable to complete the first cycle of treatment because of PD (1 patient), progressive fungal pneumonia and early death on days 5 and 8 (2 patients), or overwhelming Staphylococcus aureus sepsis and ultimate death on days 16 and 36 (2 patients). An additional patient died on day 35 from PD; however, this patient developed grade 4 neurologic toxicity manifesting as a somnolence, weakness, and unsteady gait, and grade 3 laboratory abnormalities such as elevated LDH, hypertriglyceridemia, and hyperglycemia, possibly related to MS-275 and as such were considered DLTs. As shown in Table 4, infections, such as bacteremia, sepsis, pneumonia, were encountered at all dose levels and were primarily related to the underlying disease, as almost all patients were neutropenic for a prolonged period of time, underwent extensive treatments prior to MS-275, and had central venous catheters or previous pneumonias. However, only at DL 5, we observed 2 overwhelming S aureus bacteremias and, in an additional patient with bone marrow CR, grade 3 pneumonia and bacteremia in the absence of neutropenia. These episodes, although possibly explicable by underlying disease, must be also considered possibly related to MS-275. As such, we terminated further dose escalation and expanded the DL 4 cohort for an additional 7 patients, 4 patients did not complete the full cycle because of PD. Two patients completed 5 cycles and 1 patient completed 2 cycles of treatment before their disease progressed with no evidence of DLT. Therefore, the MTD of MS-275 was 8 mg/m2 administered weekly × 4 every 6 weeks. Because of frequent progressive disease in this patient population, only 8 (21%) patients received 2 or more cycles of treatment. Therefore, chronic toxicities associated with repetitive cycles and tolerability of the drug administered at MTD in subsequent cycles are not well defined by this study population.
Infectious episodes
| Dose level . | Bacteremia, no.* . | Sepsis, no. . | Pneumonia, no.† . |
|---|---|---|---|
| 1 | 2 | 1 | 0 |
| 2 | 2 | 0 | 1 |
| 3 | 2 | 0 | 0 |
| 4 | 3 | 1 | 5 |
| 5 | 2 | 2 | 3 |
| Dose level . | Bacteremia, no.* . | Sepsis, no. . | Pneumonia, no.† . |
|---|---|---|---|
| 1 | 2 | 1 | 0 |
| 2 | 2 | 0 | 1 |
| 3 | 2 | 0 | 0 |
| 4 | 3 | 1 | 5 |
| 5 | 2 | 2 | 3 |
In most instances bacteremia was line related and caused by Staphylococcus epidermidis. Two patients developed Staphylococcus aureus bacteremia at DL 5. There was no evidence of mucositis.
Most frequently it was reactivation of known fungal pneumonia.
As depicted in Table 3, frequent grade 1 and 2 toxicities were anorexia, nausea, and vomiting occurring in almost one third of patients independent of dose level. Fatigue occurred in 21% of treatment cycles and more frequently at DL 4. Laboratory abnormalities such as hypoalbuminemia and accompanying hypocalcemia were recorded in greater than 50% of cycles each. However, most of the patients were hypoalbuminemic at the start of the treatment, and the decrease in albumin was brief, with no clinical consequences, and independent of dose. Three patients who developed grade 3 hypoalbuminemia had grade 2 hypoalbuminemia at the start of the treatment.
Clinical outcome
No patients achieved a CR or PR by standard criteria. We have summarized the biologic effects of MS-275 in Table 5. Among the patients who experienced improvements in ANC, the time to response and duration of response varied. Although some patients experienced improvement in ANC already in the first week of the treatment, some patients required 4 or more weeks to achieve response. Duration of ANC response varied from 1 to 10 weeks, and, occasionally, patients with temporary improvement would experience recurrent increase in ANC in subsequent cycles.
Evidence of MS-275 biologic effect
| Patient no. . | Dose level . | Type of response . | No. of cycles . |
|---|---|---|---|
| 2 | 4 mg/m2 × 2/4 wk | Bone marrow PR (>50% → 12% blasts, cycle 1) | 4 |
| Differentiation in myeloid lineage (ANC 504/μL → ANCmax 1410/μL, cycle 1) | |||
| 3 | 4 mg/m2 × 2/4 wk | Stable disease (bone marrow >20% → 11% blasts, cycle 1) | 2 |
| 5 | 4 mg/m2 × 2/4 wk (cycle 1); | Stable disease | 3 |
| 6 mg/m2 × 2/4 wk (cycle 2 and 3) | |||
| 11 | 8 mg/m2 × 2/4 wk | Decreased transfusion requirements | 1 |
| 14 | 8 mg/m2 × 4/6 wk | Resolution of bone pain | 1 |
| 15 | 8 mg/m2 × 4/6 wk | Bone marrow PR (35% → 14% blasts, cycle 1) | 1.75 |
| Differentiation in myeloid lineage (ANC 288/μL → ANCmax 2052/μL, cycle 1; ANCmax 5546/μL, cycle 2) | |||
| 18 | 8 mg/m2 × 4/6 wk | Differentiation in myeloid lineage (ANC 124/μL → ANCmax 680/μL, cycle 1) | 1.25 |
| 20 | 8 mg/m2 × 4/6 wk | Decreased transfusion requirements | 1 |
| 24 | 8 mg/m2 × 4/6 wk | Bone marrow PR (30% → 20% blasts, cycle 1) | 4.75 |
| Differentiation in myeloid lineage (ANC 520/μL → ANCmax 3637/μL, cycle 1; ANCmax 4456/μL, cycle 3) | |||
| 27 | 8 mg/m2 × 4/6 wk | Stable disease (peripheral blood 21% → 0% blasts, cycles 1 to 3; Bone marrow 68% → 34% blasts, cycle 1, 67% blast, cycle 4, 87% blasts, cycle 5) | 5 |
| 33 | 10 mg/m2 × 4/6 wk | Resolution of extramedullary chloroma | 2 |
| 34 | 10 mg/m2 × 4/6 wk | Bone marrow CR (54% → 3% blasts, cycle 1) | 2 |
| Differentiation in myeloid lineage (ANC 103/μL → ANCmax 854/μL, cycle 1; ANCmax 2929/μL, cycle 2) |
| Patient no. . | Dose level . | Type of response . | No. of cycles . |
|---|---|---|---|
| 2 | 4 mg/m2 × 2/4 wk | Bone marrow PR (>50% → 12% blasts, cycle 1) | 4 |
| Differentiation in myeloid lineage (ANC 504/μL → ANCmax 1410/μL, cycle 1) | |||
| 3 | 4 mg/m2 × 2/4 wk | Stable disease (bone marrow >20% → 11% blasts, cycle 1) | 2 |
| 5 | 4 mg/m2 × 2/4 wk (cycle 1); | Stable disease | 3 |
| 6 mg/m2 × 2/4 wk (cycle 2 and 3) | |||
| 11 | 8 mg/m2 × 2/4 wk | Decreased transfusion requirements | 1 |
| 14 | 8 mg/m2 × 4/6 wk | Resolution of bone pain | 1 |
| 15 | 8 mg/m2 × 4/6 wk | Bone marrow PR (35% → 14% blasts, cycle 1) | 1.75 |
| Differentiation in myeloid lineage (ANC 288/μL → ANCmax 2052/μL, cycle 1; ANCmax 5546/μL, cycle 2) | |||
| 18 | 8 mg/m2 × 4/6 wk | Differentiation in myeloid lineage (ANC 124/μL → ANCmax 680/μL, cycle 1) | 1.25 |
| 20 | 8 mg/m2 × 4/6 wk | Decreased transfusion requirements | 1 |
| 24 | 8 mg/m2 × 4/6 wk | Bone marrow PR (30% → 20% blasts, cycle 1) | 4.75 |
| Differentiation in myeloid lineage (ANC 520/μL → ANCmax 3637/μL, cycle 1; ANCmax 4456/μL, cycle 3) | |||
| 27 | 8 mg/m2 × 4/6 wk | Stable disease (peripheral blood 21% → 0% blasts, cycles 1 to 3; Bone marrow 68% → 34% blasts, cycle 1, 67% blast, cycle 4, 87% blasts, cycle 5) | 5 |
| 33 | 10 mg/m2 × 4/6 wk | Resolution of extramedullary chloroma | 2 |
| 34 | 10 mg/m2 × 4/6 wk | Bone marrow CR (54% → 3% blasts, cycle 1) | 2 |
| Differentiation in myeloid lineage (ANC 103/μL → ANCmax 854/μL, cycle 1; ANCmax 2929/μL, cycle 2) |
The median baseline peripheral white blood cell (WBC) count was 3.3 × 109/L (range, 0.4-41.8 × 109/L), with 33 (84.6%) of 39 and 10 (25.6%) of 39 patients having a baseline peripheral WBC count greater than 1 × 109/L and greater than 10 × 109/L, respectively. Fifteen (38.5%) patients had a greater than 50% reduction in WBC counts occurring at a median 6 days (range, 3-28 days) and 10 of 15 patients having a greater than 50% WBC counts reduction by day 7.
Increase in protein acetylation
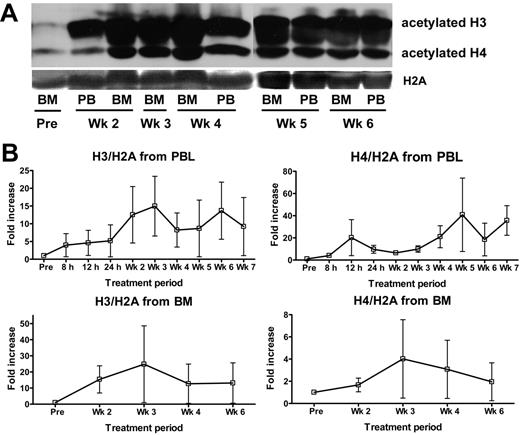
To examine whether MS-275 inhibited HDAC activity in leukemia cells, we performed a comprehensive analysis of the histone/protein acetylation status of PBMCs and BMMCs before and following MS-275 treatment. As indicated in Figure 1, increase in histone H3 and H4 acetylation was detected by Western blotting in PBMCs and BMMCs collected from 8 patients treated at DL 4 and 1 patient at DL 5. Consistent patterns of induction of histone acetylation were observed. First, an increase in histone H3 and H4 acetylation was evident at 8 to 12 hours following MS-275 treatment in all 4 patients who had PB specimens collected at these early time points. Second, almost all patients had increase in histone acetylation in either PBMCs or BMMCs documented by week 2 to 3 (day 8 and 15) of treatment. Third, in most instances, increase in acetylation was seen for both histones H3 and H4, although there was substantial intrapatient and interpatient variability. Fourth, histone acetylation increased with time and persisted for at least 2 to 3 weeks following the last MS-275 administration. Similar increase in protein acetylation was also observed by flow cytometry in the majority of BM specimens obtained from patients treated at DL 4 or 5 (Figure 2A-E). Of 4 specimens analyzed by both techniques, results were consistent for 3 of them. Five patients who achieved clinical response and had MCs examined for protein/histone acetylation status had increase in acetylation. However, increase in acetylation was observed also in patients without response, and the pattern of acetylation (time, intensity) appeared similar for responders and nonresponders.
In vivo effects of MS-275 on histone acetylation as determined by Western blotting. (A) Serial analysis of histone acetylation in BMMCs and PBMCs of patient 26 treated at DL 4 as determined by Western blotting using antibodies against acetyl-histone H3 and acetyl-histone H4. Histone H2A served as a control protein. (B) Serial PBMCs and BMMCs from 9 patients treated at DL 4 (n = 8) and 5 (n = 1) were analyzed by Western blotting for histone acetylation as described above. Intensity index was calculated as a ratio of intensity of acetylated histone H3 or H4 band to band intensity of nonacetylated H2A as determined by Western blot analysis using antibodies against acetyl-histone H3, acetyl-histone H4, and histone H2A and expressed as fold increase ± SEM over the baseline value.
In vivo effects of MS-275 on histone acetylation as determined by Western blotting. (A) Serial analysis of histone acetylation in BMMCs and PBMCs of patient 26 treated at DL 4 as determined by Western blotting using antibodies against acetyl-histone H3 and acetyl-histone H4. Histone H2A served as a control protein. (B) Serial PBMCs and BMMCs from 9 patients treated at DL 4 (n = 8) and 5 (n = 1) were analyzed by Western blotting for histone acetylation as described above. Intensity index was calculated as a ratio of intensity of acetylated histone H3 or H4 band to band intensity of nonacetylated H2A as determined by Western blot analysis using antibodies against acetyl-histone H3, acetyl-histone H4, and histone H2A and expressed as fold increase ± SEM over the baseline value.
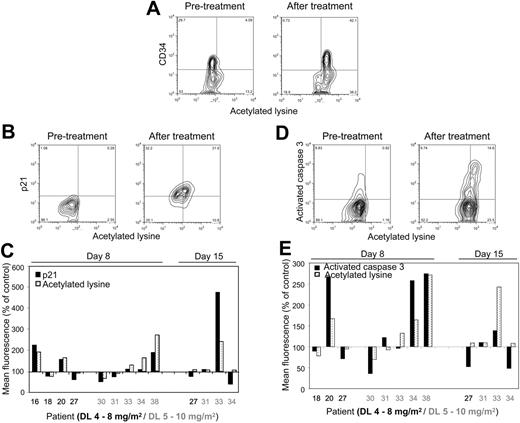
MS-275–induced increase in protein acetylation correlates with increase in p21 expression and caspase-3 activation in BMMCs as determined by flow cytometry. (A) Protein hyperacetylation versus CD34 expression before and after MS-275 (day 8, patient 38, DL 5). Note that in this patient all of the CD34+ cells became hyperacetylated but not all of the CD34− cells. (B) Protein hyperacetylation versus p21 expression in BMMCs before and after MS-275 (day 15, patient 33, DL 5). (C) Comparison in individual patients of the effect of MS-275 on protein hyperacetylation and p21 expression in BMMCs. (D) Protein hyperacetylation versus caspase-3–activated form expression in BMMCs before and after MS-275 (day 8, patient 20, DL 4). (E) Comparison in individual patients of the effect of MS-275 on protein hyperacetylation and caspase-3 activation in BMMCs. Correlations were performed in at least 7000 individual cells by multiparameter flow cytometry using a FACSCalibur flow cytometer (Becton Dickinson).
MS-275–induced increase in protein acetylation correlates with increase in p21 expression and caspase-3 activation in BMMCs as determined by flow cytometry. (A) Protein hyperacetylation versus CD34 expression before and after MS-275 (day 8, patient 38, DL 5). Note that in this patient all of the CD34+ cells became hyperacetylated but not all of the CD34− cells. (B) Protein hyperacetylation versus p21 expression in BMMCs before and after MS-275 (day 15, patient 33, DL 5). (C) Comparison in individual patients of the effect of MS-275 on protein hyperacetylation and p21 expression in BMMCs. (D) Protein hyperacetylation versus caspase-3–activated form expression in BMMCs before and after MS-275 (day 8, patient 20, DL 4). (E) Comparison in individual patients of the effect of MS-275 on protein hyperacetylation and caspase-3 activation in BMMCs. Correlations were performed in at least 7000 individual cells by multiparameter flow cytometry using a FACSCalibur flow cytometer (Becton Dickinson).
Furthermore, flow cytometric analysis allowed us to confirm that increase in acetylation indeed occurred in CD34+ cells in 4 patients' specimens examined (Figure 2A), and that in most instances it correlates with increase in p21 expression (85% of specimens; Figure 2B-C) and caspase-3 activation (67% of specimens; Figure 2D-E). These findings are in agreement with observed in vitro effects of MS-275 on leukemia cell lines and primary patient samples.19,20,24
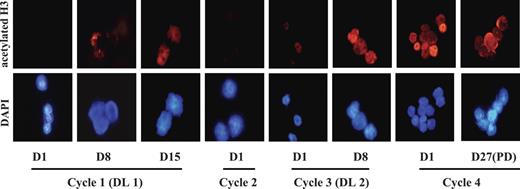
As the Western blotting and flow cytometric assays were developed and validated later on in the study, we initially analyzed samples for histone H3 acetylation status by immunocytochemistry. As indicated in Figure 3, increase in histone H3 acetylation was already detected at the lowest dose level of MS-275. Additional specimens from a single patient treated at DL 2 were analyzed by flow cytometry which detected the increase in protein acetylation following MS-275 administration (data not shown). Similar increase in histone H3 acetylation in PBMCs at all dose levels was reported in MS-275 solid-tumor study with no clear demonstration of dose-effect.27
MS-275–induced histone H3 hyperacetylation in BMMCs. Changes in histone acetylation were monitored sequentially in BMMCs by staining with polyclonal antiacetylated histone H3 antibody (top) and counterstaining with 4,6-diamidoino-2-phenylindole (DAPI; bottom). The images were collected with a Zeiss Axioskop fluorescence microscope (Carl Zeiss, Oberkochen, Germany) equipped with a Hamamatsu C4742-95 camera (Hamamatsu Photonics, Hamamatsu City, Japan) and a Zeiss 63×/1.25 NA oil-immersion objective lens, and were processed with Openlab Software version 4.0.4 (Improvision, Lexington, MA). Note increase in histone H3 acetylation in patient 2 treated at DL 1 for first 2 cycles and at DL 2 for last 2 cycles. Increase in histone H3 acetylation was observed at day 8 and persisted through subsequent cycles.
MS-275–induced histone H3 hyperacetylation in BMMCs. Changes in histone acetylation were monitored sequentially in BMMCs by staining with polyclonal antiacetylated histone H3 antibody (top) and counterstaining with 4,6-diamidoino-2-phenylindole (DAPI; bottom). The images were collected with a Zeiss Axioskop fluorescence microscope (Carl Zeiss, Oberkochen, Germany) equipped with a Hamamatsu C4742-95 camera (Hamamatsu Photonics, Hamamatsu City, Japan) and a Zeiss 63×/1.25 NA oil-immersion objective lens, and were processed with Openlab Software version 4.0.4 (Improvision, Lexington, MA). Note increase in histone H3 acetylation in patient 2 treated at DL 1 for first 2 cycles and at DL 2 for last 2 cycles. Increase in histone H3 acetylation was observed at day 8 and persisted through subsequent cycles.
Pharmacokinetics
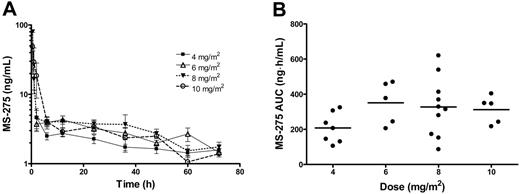
Complete pharmacokinetic data were available from 35 patients. As shown in Figure 4A, the plasma concentration versus time profiles of MS-275 were very similar at each dose level and consistent with those observed previously with the drug administered at similar dose levels.27 For 8 patients (2 at 6 mg/m2, 5 at 8 mg/m2, and 1 at 10 mg/m2), the percentage of the AUC extrapolated beyond the last sampling time point with measurable concentrations was greater than 50%. For these individuals, only Cmax and Tmax were considered. A summary of the pharmacokinetic parameters of MS-275 obtained as a function of DL is provided in Table 6.
Pharmacokinetic profile of MS-275. Data were obtained from 35 patients with leukemia treated with MS-275 at dose levels ranging from 4 to 10 mg/m2. (A) Concentration-time profiles of MS-275 administered orally by dose group. Data from patients treated at the same dose levels were grouped and are presented as mean values (symbol) ± SE (error bar). The legend insert shows the different symbols/lines used for each of the test dose levels. (B) Effect of drug dose on the area under the plasma concentration versus time curve of MS-275 (AUC). Each symbol represents data from an individual patient, and horizontal lines represent the median value in each dose group.
Pharmacokinetic profile of MS-275. Data were obtained from 35 patients with leukemia treated with MS-275 at dose levels ranging from 4 to 10 mg/m2. (A) Concentration-time profiles of MS-275 administered orally by dose group. Data from patients treated at the same dose levels were grouped and are presented as mean values (symbol) ± SE (error bar). The legend insert shows the different symbols/lines used for each of the test dose levels. (B) Effect of drug dose on the area under the plasma concentration versus time curve of MS-275 (AUC). Each symbol represents data from an individual patient, and horizontal lines represent the median value in each dose group.
Summary of pharmacokinetic parameters for MS-275
| Dose, mg/m2 . | No. of patients . | Cmax, ng/mL (range) . | AUC, ng h/mL (range) . | CL/F, L/h (range) . | T1/2, h (range) . | Tmax, h (range) . |
|---|---|---|---|---|---|---|
| 4 | 7 | 4.74 ± 3.41 (2.20-10.7) | 208 ± 86.1 (106-324) | 40.9 ± 17.2 (24.7-75.8) | 36.6 ± 18.6 (16.2-64.2) | 12 (2-24) |
| 6 | 7 | 4.85 ± 1.76 (2.10-7.60) | 351 ± 121* (206-470) | 34.4 ± 11.5* (25.5-48.7) | 43.8 ± 16.4* (28.3-69.3) | 12 (2-36) |
| 8 | 15 | 53.1 ± 92.4 (2.71-302) | 328 ± 168† (86.0-620) | 66.7 ± 54.5† (25.8-209) | 33.4 ± 12.7† (16.7-53.3) | 1 (0.5-24) |
| 10 | 6 | 53.2 ± 58.0 (4.55-164) | 312 ± 78.8‡ (217-404) | 68.2 ± 25.4‡ (48.7-103) | 30.4 ± 13.9‡ (14.6-49.5) | 1 (0.5-2) |
| All doses | 35 | NA | NA | 54.0 ± 37.2§ (24.7-209) | 35.6 ± 15.0§ (14.6-69.3) | 2 (0.5-36) |
| Dose, mg/m2 . | No. of patients . | Cmax, ng/mL (range) . | AUC, ng h/mL (range) . | CL/F, L/h (range) . | T1/2, h (range) . | Tmax, h (range) . |
|---|---|---|---|---|---|---|
| 4 | 7 | 4.74 ± 3.41 (2.20-10.7) | 208 ± 86.1 (106-324) | 40.9 ± 17.2 (24.7-75.8) | 36.6 ± 18.6 (16.2-64.2) | 12 (2-24) |
| 6 | 7 | 4.85 ± 1.76 (2.10-7.60) | 351 ± 121* (206-470) | 34.4 ± 11.5* (25.5-48.7) | 43.8 ± 16.4* (28.3-69.3) | 12 (2-36) |
| 8 | 15 | 53.1 ± 92.4 (2.71-302) | 328 ± 168† (86.0-620) | 66.7 ± 54.5† (25.8-209) | 33.4 ± 12.7† (16.7-53.3) | 1 (0.5-24) |
| 10 | 6 | 53.2 ± 58.0 (4.55-164) | 312 ± 78.8‡ (217-404) | 68.2 ± 25.4‡ (48.7-103) | 30.4 ± 13.9‡ (14.6-49.5) | 1 (0.5-2) |
| All doses | 35 | NA | NA | 54.0 ± 37.2§ (24.7-209) | 35.6 ± 15.0§ (14.6-69.3) | 2 (0.5-36) |
Data are shown as mean ± SD, with observed range in parentheses, except for Tmax (median with range in parentheses).
Cmax indicates peak plasma concentration; AUC, area under the plasma concentration versus time curve extrapolated to infinity; CL/F, apparent oral clearance; T1/2, half-life of the terminal phase; Tmax, time to Cmax; and NA, not applicable.
n = 5.
n = 10.
n = 5.
n = 27.
Typical for oral anticancer drugs, the interindividual pharmacokinetic variability was substantial at all dose levels, with a CV for the CL/F as high as 69.8%, suggesting varied systemic exposure to MS-275 during the treatment. The Tmax was highly variable, with values ranging between 30 minutes and 36 hours, median value approaching 2 hours. Because the terminal half-life was relatively consistent in all patients, exhibiting an overall mean value of 35.6 ± 15.0 hours (CV = 42.2%), this suggests that interindividual differences in the rate of gastrointestinal absorption is the main contributor to variability in the observed concentration-time profiles. The same variability in gastrointestinal absorption of MS-275 was observed previously.27 For 9 patients whose Tmax of MS-275 was 30 minutes, the extent of drug uptake could be possibly underestimated in the absence of earlier sampling time points.
The AUC reached a plateau with increasing doses of MS-275 (Table 6; Figure 4B). Furthermore, the median CL/F of MS-275 was dependent on drug dose (P = .045), with values obtained at 10 mg/m2 being statistically significantly higher than those observed at the 2 lowest dose levels. However, because of the relatively small number of patients studied at each dose level in combination with the extensive interindividual pharmacokinetic variability, it is most likely that this dose dependence is a spurious finding.
Discussion
This phase 1 study represents the first clinical trial of HDI MS-275 in patients with advanced acute leukemias, mainly AML. In this cohort of patients, we found that MS-275 can be safely given on a weekly schedule for 2 or 4 weeks in the dose range explored. The most frequently encountered toxicities were fatigue and gastrointestinal symptoms such as anorexia, nausea, vomiting, occurring at all dose levels, with fatigue being more frequently documented at DL 4 and higher. These toxicities appear to be a hallmark of all HDIs developed so far37-45 and were noted as well in a phase 1 study of MS-275 in patients with solid tumors, in which they represented DLTs at DL 12 mg/m2.27 In contrast, however, in our study we did not consider these effects to be dose limiting, in part because many of our patients had baseline fatigue and nausea which correlated clinically with disease progression prior to MS-275 administration. Furthermore, because of advanced leukemia in our patient population and frequent early removal from the study because of disease progression, our study provides only limited information on the tolerability of MS-275 with chronic administration.
The MTD defined in our study was 8 mg/m2 × 4 weeks with a 2-week washout period. However, DLTs may be viewed as slightly unusual for this patient population. As already presented in “Results,” infections, such as bacteremias/sepsis (line related or not) or reactivation/progression of pneumonia, which are inherent to this patient population, were encountered at all dose levels. Nonetheless, at least 2 infectious episodes at DL 5 occurred in patients without neutropenia or in the setting of early neutrophil recovery. One potential mechanism underlying these events could be MS-275–induced changes in phagocytic respiratory burst function of myeloid cells46 because none of the patients on the study had any evidence of mucositis to account for infectious events. Lymphopenia and herpes virus reactivation were reported in the MS-275 solid-tumor study; however, those patients did not experience any unusual or increased frequency of such infections at the highest dose level.27 We can only speculate that the profound and long-standing myelosuppression present in our patient population in combination with potential MS-275–associated phagocytic dysfunction or unexplained immunosupression may be responsible for the reported events. However, it is also conceivable that these infections may have had nothing to do with the drug itself and that MTD was wrongly selected. Furthermore, pharmacokinetic parameters in our study suggested similar Cmax and AUC at DLs 4 and 5, arguing against dose-related toxicity. In regard to neurotoxicity, this DLT occurred in a patient with progressive disease and other comorbidities. An additional episode of lower extremity weakness was likely related to CNS leukemia. Thus, similar to the solid-tumor study, the incidence of neurotoxicity was low, but further observation is warranted.
Overall, peak plasma concentrations achieved at MTD in our study and in the solid-tumor study are similar and above levels required to achieve in vitro or in vivo growth inhibition of various human tumors in different models.19,20 Although none of the patients achieved responses by classical criteria, this is not surprising because more than 80% of patients on this study had refractory disease. Although assessment of clinical response was not the primary objective of this study, we did observe clear evidence of biologic effect on hematologic parameters or at least stable disease in 12 patients with documented transient at least 50% reduction in peripheral WBC count in 40% of patients. Different classes of HDAC inhibitors have been recently tested in clinical trials in patients with acute myeloid leukemia.31,45,47-50 Early results from these single-agent studies suggest similar efficacy. Only SAHA so far has been reported to induce CR or CRp in approximately 10% of patients with AML,47 whereas depsipeptide appears to induce responses preferentially in patients with core binding factor leukemias, known to recruit HDACs.50 In our study, we did not observe increased efficacy of MS-275 in 5 patients with t(15;17) or inv(16)/del 16q. All 5 patients had advanced leukemia and complex karyotype, and, as such, it is not surprising that MS-275 could not overcome inherent resistance of their leukemia cells.
Histone H3, H4, and overall protein acetylation status in PBMCs and BMMCs were measured as a surrogate of the ability of MS-275 to induce HDAC inhibition. Although immunocytochemistry and Western blotting allowed us to examine specifically acetylation of histones H3 and H4, known targets of MS-275, by flow cytometry, we were able to examine and quantify the overall increase in protein acetylation. So far, more than 40 different nuclear and cytoplasmic proteins have been identified as substrates of acetylation, many of which are important in cancer biology.14 As such, use of multiparameter flow cytometry allowed us to detect most cellular acetylated proteins and to attempt to relate any MS-275–induced changes with specific parameters such as induction of p21, caspase-3 activation, and CD34 expression, making it a very attractive method for use in future studies of HDIs alone or in combination with other therapeutics.34 Consistent with phase 1 solid-tumor study,27 our data suggest that HDAC inhibition was achieved at the lowest dose level tested but, because of substantial intrapatient and interpatient variability, we could not identify a specific pattern or magnitude of histone or protein acetylation that related to clinical response. Although we did not specifically select for leukemia cells (except for 4 specimens examined by flow cytometry for CD34 expression and protein acetylation), most of our patients had advanced leukemia. Thus, it is likely that at least the BMMCs were leukemic blasts, and, as such, that histone/protein hyperacetylation occurred in the malignant cohort. Our data also suggest that treatment with MS-275 leads to increase in p21 expression which correlates with an increase in protein acetylation and caspase-3 activity. Although the question of whether protein hyperacetylation and induction of apoptosis will be biologically relevant end points for prediction of response to MS-275 or to other HDIs remains unanswered by our data, achievement of this end point is encouraging and might be a basis for combining MS-275 with other modulators of cell signaling.
There was substantial interindividual variability in the pharmacokinetic behavior of MS-275, which is not unexpected for oral anticancer drugs and has been reported in a previous study of MS-275 in patients with solid tumors.51 In the setting of relatively stable T1/2 and highly variable T max (30 minutes to 36 hours), the individual difference in the rate of GI absorption remains the most likely explanation for observed variability. It is also conceivable that intercurrent illnesses and medications such as antibiotics administered to this patient population may in part affect the rate of GI absorption. Our study also confirms that the mean T1/2 of 36 hours in humans is substantially longer than that observed in laboratory animals. Interestingly, the AUC in our study did not increase proportionally with the dose of MS-275 because the median apparent CL/F of MS-275 appeared to be dose dependent and increased with higher doses of MS-275. This finding could be spurious because of the relatively small number of patients studied and extensive interindividual pharmacokinetic variability. This possibility is supported by previously obtained pharmacokinetic data from a study in which MS-275 was escalated from 2 to 12 mg/m2 and where no clear dose dependence of pharmacokinetic parameters was observed.27
In conclusion, our data demonstrate that MS-275 has cellular and molecular effects in AML, as evidenced by increased histone/protein acetylation, p21 expression, and caspase-3 activation in vivo, consistent with its already observed effects in vitro.19-25 Although the MTD of MS-275 given weekly × 4 was determined to be 8 mg/m2, the observation of biologic and pharmacodynamic effects at all dose levels coupled with substantial pharmacokinetic variability suggests that even lower doses may be effective and may be more readily tolerated. This is especially relevant for future combination studies with a different class of epigenetic modulators such as demethylating agents, which have a similar toxicity profile and which require chronic administration.15,52 Ease of administration, and recent laboratory studies suggesting synergism between HDIs and other classical chemotherapeutics or biologic agents15,52-68 makes MS-275 an excellent candidate for further development in hematologic malignancies, preferably in combinations and in patients with less-advanced disease or MDS, where the effects of prolonged drug administration can be better assessed.
Authorship
Contribution: I.G. and J.E.K. designed and performed research, analyzed data, and wrote the paper. A.J., J.B.T., A.S., W.D.F., S.R., E.J.C., M.-J.L., and S.D.G. performed laboratory research and analyzed laboratory correlates. M.L.T. and S.D.G. performed clinical research; J.G. coordinated the study. E.A.S. and J.Z. participated in the design of the study, E.A.S. critically reviewed the data, and J.Z. helped with the study conduct. All authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ivana Gojo, University of Maryland Marlene and Stewart Greenebaum Cancer Center, 22 S Greene St, Baltimore, MD 21201; e-mail: igojo@umm.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by cooperative agreements from the National Cancer Institute (U01 CA69854) (I.G. and J.E.K.) and (U01 CA70095) (J.E.K.) and by research funding from ScheringAG (J.B.T.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal