Abstract
Adult T-cell leukemia/lymphoma (ATLL) is a generally fatal malignancy. Most ATLL patients fare poorly with conventional chemotherapy; however, antiviral therapy with zidovudine (AZT) and interferon alpha (IFN-α) has produced long-term clinical remissions. We studied primary ATLL tumors and identified molecular features linked to sensitivity and resistance to antiviral therapy. Enhanced expression of the proto-oncogene c-Rel was noted in 9 of 27 tumors. Resistant tumors exhibited c-Rel (6 of 10; 60%) more often than did sensitive variants (1 of 9; 11%). This finding was independent of the disease form. Elevated expression of the putative c-Rel target, interferon regulatory factor-4 (IRF-4), was observed in 10 (91%) of 11 nonresponders and in all tested patients with c-Rel+ tumors and occurred in the absence of the HTLV-1 oncoprotein Tax. In contrast, tumors in complete responders did not express c-Rel or IRF-4. Gene rearrangement studies demonstrated the persistence of circulating T-cell clones in long-term survivors maintained on antiviral therapy. The expression of nuclear c-Rel and IRF-4 occurs in the absence of Tax in primary ATLL and is associated with antiviral resistance. These molecular features may help guide treatment. AZT and IFN-α is a suppressive rather than a curative regimen, and patients in clinical remission should remain on maintenance therapy indefinitely.
Introduction
Adult T-cell leukemia/lymphoma (ATLL) was first described as a distinct clinical entity in 1979, and the association with the human T-cell leukemia virus type 1 (HTLV-1) was reported shortly thereafter.1 The disease may manifest itself in various forms and is generally subclassified into 4 subtypes.2 In the 2 most aggressive variants, lymphoma-type and acute ATLL, patients usually have a very high tumor burden and hypercalcemia. The chronic and smoldering variants of ATLL have a more indolent course, though they often progress to the more malignant forms of the disease.3 Therapy for ATLL, particularly acute and lymphoma types, is disappointing. In a large published series of more than 800 Japanese patients with acute and lymphomatous ATLL who were treated with a variety of chemotherapeutic regimens, the median survival time was 6.2 and 10.2 months, respectively.2 With some of the most intensive chemotherapy regimens, complete response (CR) rates of approximately 35% or more have been reported.4,5 Allogeneic bone marrow transplantation, including reduced-intensity regimens, has been successful in a number of ATLL patients, though severe immunodeficiency resulting from the underlying disease and the preparatory regimens poses a significant problem.6,7 IL-2 receptor–directed therapies (anti-Tac) have proven to be useful in some ATLL patients,8,9 but these are also expensive and unlikely to be feasible in many areas in which HTLV-1 is endemic.
Several phase 2 trials have demonstrated the efficacy of zidovudine (AZT) and interferon alpha (IFN-α) therapy in ATLL.10-13 High response rates were noted in chemotherapy-naive and acute ATLL patients, and some had prolonged periods of remission. The antitumor mechanism of this therapy is unclear but may involve the inhibition of telomerase by AZT.14 IFN-α is known to have antiproliferative properties, and it has been effective in the treatment of some human malignancies, including other non–Hodgkin lymphomas, chronic myelogenous leukemia, Kaposi sarcoma, and melanoma.15,16 However, resistance to this drug has been widely observed, and specific defects in proteins involved in or affecting the IFN signaling pathway have been found in some tumors.17-19
The study of the evolution of ATLL is further complicated by its low incidence (2%-6% lifetime risk) and prolonged latency (more than 30 years) before the development of overt disease in HTLV-1 carriers.20 In addition, the difficulty of establishing representative animal models and primary tumor cell lines has hindered research. In general, published “ATLL” cell lines are either clonal outgrowths that differ from the original tumor or HTLV-1–transformed cells that express the viral oncoprotein Tax.21 Most research on the pathogenesis of HTLV-1–related disease has focused on Tax, a promiscuous transcriptional activator that induces the expression of viral genes (through the viral LTR) and cellular genes through interaction with pleiotropic transcription factors such as NF-κB, CREB, SR-F, and AP-1.22 Primary ATLL and HTLV-1 transformed cell lines share a high constitutive expression of NF-κB and its transactivated genes that exerts a predominant antiapoptotic effect in viral lymphoproliferative disease and other malignancies.23-27 The vital role of NF-κB in ATLL is highlighted by the fact that pharmacologic inhibition of this transcription factor induces apoptosis in primary tumor cells.28-30 One difficulty in the study of the biology of primary ATLL is that Tax expression occurs soon after cells are placed in tissue culture or murine models.23,31 To better understand the mechanisms of malignant growth in ATLL, it is essential to study NF-κB and its activation pathways independently of the effects of Tax in primary unmanipulated tumors.
We studied and characterized the expression of activated NF-κB in a cohort of primary ATLL tumors derived from patients treated with AZT and IFN-α. Here we demonstrate the overexpression of the oncogenic subunit of NF-κB, c-Rel, in a significant percentage of ATLL tumors and its association with interferon regulatory factor-4 (IRF-4) and antiviral therapy resistance. We also demonstrate persistence of T-cell clones in the blood of long-term ATLL survivors who are in clinical remission on maintenance antiviral therapy. Our data indicate that variant expression of NF-κB and IRF-4 may be a predictor of response to antivirals and that the combination of AZT and IFN-α is a suppressive rather than a curative regimen.
Patients, materials, and methods
Patients and tumor samples
We studied a primary ATLL tumor cohort of 38 patients, mostly Caribbean immigrants from Miami, whose disease was diagnosed and treated between 1995 and 2006. Investigators at the Federal University of Bahia in the northeastern Brazilian state of Bahia, a highly endemic region for HTLV-1, also contributed tumor specimens for this study. ATLL was classified into clinical subtypes according to Shimoyama.2 The diagnosis of ATLL was based on serologic evidence of HTLV-1 by ELISA, confirmed by Western blot, and the identification of clonal circulating leukemic cells or tissue lymphocytes of T-cell origin was determined by flow cytometry, immunostains, and gene rearrangement studies. Patients also had one or more of the typical clinical features of the disease, including markedly elevated lactate dehydrogenase (LDH), generalized lymphadenopathy, hepatosplenomegaly, and hypercalcemia. Patient characteristics included in this study are summarized in Table 1.
ATLL patient characteristics, AZT and IFN-α response, c-Rel, and IRF-4 status
| Patient . | Age, y . | ATLL type . | WBC count or solid tissue . | Surface antigen expression . | LDH . | ↑ Ca2+ . | Antiviral response . | c-Rel . | IRF-4 . | Survival, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| ATLL-1 | 48 | Lymph → acute | 13 000 | CD4+CD25+ | > 2N | Yes | CR | − | −WB | 63+ |
| ATLL-2 | 37 | Acute | 37 000 | CD4+CD25+ | > 2N | No | CR | − | −WB | 63+ |
| ATLL-3 | 44 | Unfavorable chronic | 23 000 | CD4+CD25+ | < 2N | No | CR | − | −WB | 16+ |
| ATLL-4 | 46 | Unfavorable chronic | 17 000 | CD4+CD25+CD30+ | Normal | No | NR | + | +WB | 1+ |
| ATLL-5 | 49 | Lymphoma | Bone mass | CD4+CD25+ | Normal | No | NR | + | +WB | 17 |
| ATLL-6 | 36 | Acute | 126 000 | CD4+CD25+ | > 2N | No | NR | + | +WB | 1.4 |
| ATLL-7 | 76 | Acute | 182 000 | CD4+CD25+ | < 2N | Yes | PR | + | +WB | 8 |
| ATLL-8 | 42 | Acute | 49 000 | CD4+CD25+ | > 2N | Yes | PR | − | NA | 2 |
| ATLL-9 | 64 | Acute | 84 000 | CD4+CD25+ | > 2N | Yes | NA | + | NA | 1.6 |
| ATLL-10 | 53 | Acute | 117 000 | CD4+CD25− | > 2N | Yes | NA | − | −IHC LN | 3 |
| ATLL-11 | 50 | Lymph → acute | 53 000 | CD4+CD25+ | > 2N | Yes | NR | − | +IHC skin | 5.5 |
| ATLL-12 | 66 | Acute | 42 000 | CD4+CD25− | > 2N | Yes | NA | − | NA | 4 |
| ATLL-13 | 68 | Acute | 108 000 | CD4+ | > 2N | Yes | NA | + | NA | 0.3 |
| ATLL-14 | 70 | Acute | 144 000 | CD4+CD25+ | > 2N | No | PR | − | −WB | 2.5+ |
| ATLL-15 | 37 | Acute | 70 000 | CD4+CD25+ | > 2N | No | NR | − | −WB | 8 |
| ATLL-16 | 52 | Acute | 127 000 | CD4+CD25+ | > 2N | Yes | NA | − | NA | 2 |
| ATLL-17 | 27 | Acute | 139 000 | CD4+CD25− | > 2N | No | NR | − | −WB | 5 |
| ATLL-18 | 44 | Acute | 63 000 | CD4+ | > 2N | Yes | NA | − | NA | 0.7 |
| ATLL-19 | 74 | Chronic | 21 000 | CD4+CD25+ | NA | No | Untreated | − | NA | 3+ |
| ATLL-20 | 66 | Lymph → acute | 34 000 | CD4+CD25− | > 2N | Yes | NR | + LN | +WB, IHC LN | 3.5 |
| ATLL-21 | 29 | Unfavorable chronic | 61 000 | CD4+CD25+CD30− | < 2N | No | CR | − | −WB | 4+ |
| ATLL-22 | 69 | Acute | 144 000 | CD4+CD25+ | > 2N | Yes | NR | + | +WB | 5 |
| ATLL-23 | 69 | Lymphoma | Colon mass | CD3+CD30+ (IHC) | Normal | No | * | − | +IHC | 6.5 |
| ATLL-24 | 67 | Acute | 81 000 | CD4+CD25− | > 2N | Yes | PR | − | −WB | 4+ |
| ATLL-25 | 52 | Acute | 102 000 | CD4+CD25+ | > 2N | Yes | NR | + | NA | 2.5 |
| ATLL-26 | 50 | Acute | 63 000 | CD4+CD25+ | < 2N | No | NR | − | +WB | 21+ |
| ATLL-27 | 46 | Acute | 89 000 | CD4+CD25+ | > 2N | Yes | CR | − | −WB | 7+ |
| ATLL-28 | 41 | Lymphoma | Bone marrow | CD4+CD25+ | < 2N | Yes | NR | NA | + IHC | 5 |
| ATLL-29 | 57 | Lymphoma | Lymph node | CD3+CD30− (IHC) | < 2N | No | * | NA | + IHC | 21+ |
| ATLL-30 | 50 | Lymphoma | Lymph node | CD4+CD25+ | > 2N | Yes | NR | NA | + IHC | 7+ |
| ATLL-31 | 34 | Lymphoma | Chest mass | CD3+CD30+ (IHC) | > 2N | No | NA | NA | + IHC | 39 |
| ATLL-32 | 43 | Lymphoma | Lymph node | CD4+CD25+ | > 2N | No | * | NA | +IHC | 5 |
| ATLL-33 | 36 | Lymphoma | Lymph node | CD3+ (IHC) | > 2N | No | * | NA | + IHC | 17 |
| ATLL-34 | 45 | Lymphoma | Lymph node | CD4+CD25− | < 2N | No | NR | NA | + IHC | 6.5 |
| ATLL-35 | 34 | Lymphoma | Lymph node | CD4+CD25+CD30− | > 2N | Yes | NA | NA | + IHC | 4+ |
| ATLL-36 | 66 | Lymphoma | Oral mass | CD4+CD25+ | Normal | No | * | NA | + IHC | 30 |
| ATLL-37 | 36 | Lymphoma | Lymph node | CD4+CD25+CD30+ | > 2N | No | NA | NA | + IHC | 10 |
| ATLL-38 | 56 | Lymphoma | Lymph node | CD4+CD25+ | > 2N | No | NA | NA | +IHC | 9 |
| Patient . | Age, y . | ATLL type . | WBC count or solid tissue . | Surface antigen expression . | LDH . | ↑ Ca2+ . | Antiviral response . | c-Rel . | IRF-4 . | Survival, mo . |
|---|---|---|---|---|---|---|---|---|---|---|
| ATLL-1 | 48 | Lymph → acute | 13 000 | CD4+CD25+ | > 2N | Yes | CR | − | −WB | 63+ |
| ATLL-2 | 37 | Acute | 37 000 | CD4+CD25+ | > 2N | No | CR | − | −WB | 63+ |
| ATLL-3 | 44 | Unfavorable chronic | 23 000 | CD4+CD25+ | < 2N | No | CR | − | −WB | 16+ |
| ATLL-4 | 46 | Unfavorable chronic | 17 000 | CD4+CD25+CD30+ | Normal | No | NR | + | +WB | 1+ |
| ATLL-5 | 49 | Lymphoma | Bone mass | CD4+CD25+ | Normal | No | NR | + | +WB | 17 |
| ATLL-6 | 36 | Acute | 126 000 | CD4+CD25+ | > 2N | No | NR | + | +WB | 1.4 |
| ATLL-7 | 76 | Acute | 182 000 | CD4+CD25+ | < 2N | Yes | PR | + | +WB | 8 |
| ATLL-8 | 42 | Acute | 49 000 | CD4+CD25+ | > 2N | Yes | PR | − | NA | 2 |
| ATLL-9 | 64 | Acute | 84 000 | CD4+CD25+ | > 2N | Yes | NA | + | NA | 1.6 |
| ATLL-10 | 53 | Acute | 117 000 | CD4+CD25− | > 2N | Yes | NA | − | −IHC LN | 3 |
| ATLL-11 | 50 | Lymph → acute | 53 000 | CD4+CD25+ | > 2N | Yes | NR | − | +IHC skin | 5.5 |
| ATLL-12 | 66 | Acute | 42 000 | CD4+CD25− | > 2N | Yes | NA | − | NA | 4 |
| ATLL-13 | 68 | Acute | 108 000 | CD4+ | > 2N | Yes | NA | + | NA | 0.3 |
| ATLL-14 | 70 | Acute | 144 000 | CD4+CD25+ | > 2N | No | PR | − | −WB | 2.5+ |
| ATLL-15 | 37 | Acute | 70 000 | CD4+CD25+ | > 2N | No | NR | − | −WB | 8 |
| ATLL-16 | 52 | Acute | 127 000 | CD4+CD25+ | > 2N | Yes | NA | − | NA | 2 |
| ATLL-17 | 27 | Acute | 139 000 | CD4+CD25− | > 2N | No | NR | − | −WB | 5 |
| ATLL-18 | 44 | Acute | 63 000 | CD4+ | > 2N | Yes | NA | − | NA | 0.7 |
| ATLL-19 | 74 | Chronic | 21 000 | CD4+CD25+ | NA | No | Untreated | − | NA | 3+ |
| ATLL-20 | 66 | Lymph → acute | 34 000 | CD4+CD25− | > 2N | Yes | NR | + LN | +WB, IHC LN | 3.5 |
| ATLL-21 | 29 | Unfavorable chronic | 61 000 | CD4+CD25+CD30− | < 2N | No | CR | − | −WB | 4+ |
| ATLL-22 | 69 | Acute | 144 000 | CD4+CD25+ | > 2N | Yes | NR | + | +WB | 5 |
| ATLL-23 | 69 | Lymphoma | Colon mass | CD3+CD30+ (IHC) | Normal | No | * | − | +IHC | 6.5 |
| ATLL-24 | 67 | Acute | 81 000 | CD4+CD25− | > 2N | Yes | PR | − | −WB | 4+ |
| ATLL-25 | 52 | Acute | 102 000 | CD4+CD25+ | > 2N | Yes | NR | + | NA | 2.5 |
| ATLL-26 | 50 | Acute | 63 000 | CD4+CD25+ | < 2N | No | NR | − | +WB | 21+ |
| ATLL-27 | 46 | Acute | 89 000 | CD4+CD25+ | > 2N | Yes | CR | − | −WB | 7+ |
| ATLL-28 | 41 | Lymphoma | Bone marrow | CD4+CD25+ | < 2N | Yes | NR | NA | + IHC | 5 |
| ATLL-29 | 57 | Lymphoma | Lymph node | CD3+CD30− (IHC) | < 2N | No | * | NA | + IHC | 21+ |
| ATLL-30 | 50 | Lymphoma | Lymph node | CD4+CD25+ | > 2N | Yes | NR | NA | + IHC | 7+ |
| ATLL-31 | 34 | Lymphoma | Chest mass | CD3+CD30+ (IHC) | > 2N | No | NA | NA | + IHC | 39 |
| ATLL-32 | 43 | Lymphoma | Lymph node | CD4+CD25+ | > 2N | No | * | NA | +IHC | 5 |
| ATLL-33 | 36 | Lymphoma | Lymph node | CD3+ (IHC) | > 2N | No | * | NA | + IHC | 17 |
| ATLL-34 | 45 | Lymphoma | Lymph node | CD4+CD25− | < 2N | No | NR | NA | + IHC | 6.5 |
| ATLL-35 | 34 | Lymphoma | Lymph node | CD4+CD25+CD30− | > 2N | Yes | NA | NA | + IHC | 4+ |
| ATLL-36 | 66 | Lymphoma | Oral mass | CD4+CD25+ | Normal | No | * | NA | + IHC | 30 |
| ATLL-37 | 36 | Lymphoma | Lymph node | CD4+CD25+CD30+ | > 2N | No | NA | NA | + IHC | 10 |
| ATLL-38 | 56 | Lymphoma | Lymph node | CD4+CD25+ | > 2N | No | NA | NA | +IHC | 9 |
ATLL type classified as acute, lymphoma, chronic, or unfavorable chronic according to Japanese Lymphoma Study Group definitions.2 Lymph → acute indicates previously diagnosed with lymphoma type and progressed to leukemia before AZT and IFN-α as induction therapy; age, patient age at time of diagnosis; WBC, white blood cell count in cells/mm3 immediately before antiviral therapy and blood collection for molecular studies; solid tissue, when specified, biopsy specimens were used for immunophenotypic and molecular studies; surface Ag expression, cell surface antigen (CD3, CD4, CD25, CD30) expression status (indicated by predominantly + or −) by flow cytometry or immunostains (IHC) (these data were available at Jackson Memorial Hospital; if not reported, data were unavailable); lactose dehydrogenase (LDH), > 2N, more than twice the normal value (per Shimoyama2); < 2N, elevated but less than twice the normal value; NA, data not available or not evaluable; ↑Ca2+, hypercalcemia; antiviral response, response to induction with high-dose parenteral AZT and IFN-α; *, patients who received oral AZT and subcutaneous IFN-α as outpatient (not as induction) therapy after previous therapies; c-Rel, nuclear expression by EMSA; +, overexpression; −, comparable to negative controls; IRF-4, protein expression measured by Western blot (WB), immunohistochemistry (IHC), or both; +, expression; −, no expression; NA, data not available; LN, lymph node, and skin biopsy tissues were available for IHC for the specified patients; survival, in months from the time of diagnosis, regardless of therapies received; +, censored survival times.
The study was reviewed and approved by the institutional review boards of the University of Miami and the Federal University of Bahia. Primary ATLL specimens used for molecular studies were obtained from peripheral blood mononuclear cells (PBMCs) from leukemic patients or residual solid tumor tissue from patients with ATLL after informed consent was obtained. Specimens were either processed immediately for protein and nucleic acid extractions or cryopreserved for later use. Also included in this study were cryopreserved tumor samples from deceased patients. Twenty-one samples were derived from acute leukemia-type ATLL, and the other specimens were from chronic (1), unfavorable chronic (3), and lymphoma-type leukemia (13) ATLL (Table 1). In most patients, all tumor characterization studies were performed on the original diagnostic specimens before any treatment. Leukemic ATLL was found in 25 treatment-naive patients. Two patients, ATLL-1 and ATLL-11, initially were given diagnoses of T-cell lymphoma. Both had relapses with leukemia-type ATLL after receiving chemotherapy, at which time their PBMCs were preserved. Conventional chemotherapy failed in 4 patients (ATLL-6, ATLL-12, ATLL-20, ATLL-23), and their original diagnostic specimens were analyzed. ATLL-20 initially had lymphoma and experienced relapse with leukemia, and 5 patients (ATLL-5, ATLL-10, ATLL-32, ATLL-35, ATLL-37) were coinfected with HIV.
Thirty-eight samples were available for our study. Twenty-two patients treated in the same manner with antiviral therapy had evaluable responses. Twenty-seven of 38 samples were evaluated for expression of NF-κB/Rel, and 30 of 38 samples were evaluated for IRF-4 expression. Nineteen samples were examined for both c-Rel and IRF-4.
Antiviral therapy and response criteria
Twenty-eight patients in this study (21 of whom were treatment naive) received high-dose parenteral AZT (1.5 g twice daily) and IFN-α (5-10 million U twice daily) as induction regimen, a standard approach at our institution for ATLL patients with leukemia. Included in this group were 4 patients with lymphoma-type ATLL treated in the same manner. Patients who responded to induction therapy continued to receive outpatient treatment in the form of oral AZT 600 mg twice daily and subcutaneous IFN-α 5 million U once or twice daily. For patients with prolonged clinical response, these drugs were tapered to as low as twice-daily doses of 300 mg AZT oral and 3 million U subcutaneous IFN-α 3 times weekly as maintenance therapy. Twenty-two patients could be evaluated for response to induction antiviral therapy. CR was defined as the disappearance of circulating abnormal lymphocytes, along with the resolution of adenopathy, organomegaly, hypercalcemia, and measurable disease lasting for at least 1 month.10 Partial response was defined as a reduction of white blood cell (WBC) counts or a measurable tumor load by more than 50%, sustained for at least 1 month in the absence of clinical disease progression. Molecular response in leukemic patients was evaluated by flow cytometry and T-cell receptor gene rearrangement studies by multiplex PCR on DNA extracted from PBMCs.
Multiplex polymerase chain reaction
Multiplex polymerase chain reaction (PCR) analysis for T-cell receptor (TCR) gene rearrangement (performed with reagents from Invivoscribe Technologies, San Diego, CA) were carried out as routine diagnostic work-up for lymphoma, as previously described.32,33 Briefly, purified DNA was quantified and amplified using a thermal cycler (ABI Geneamp 9700; Applied Biosystems, Foster City, CA). Detection was performed using an ABI 3100-Avant Genetic Analyzer (Applied Biosystems). Fragment analysis using GeneMapper version 3.7 software was used to detect the fluorescence-labeled amplified products. DNA from normal polyclonal populations gave a unique Gaussian distribution of amplified products in the expected size range for the primers used. Monoclonal populations gave unique, single-sized peaks, with or without a significant polyclonal background.
Antibodies and reagents
The following antibodies were used for electrophoretic mobility shift assay (EMSA) supershift experiments: anti-p50 (H-119), anti-p65 (F-6), anti-c-Rel (N), p52 (C-5), and RelB (C-19) (Santa Cruz Biotechnology, Santa Cruz, CA). For Western blot analysis, the following antibodies were used: anti–IRF-4 (Santa Cruz Biotechnology), mouse monoclonal anti-Tax from hybridoma cell culture (kindly provided by Dr Chou-Zen Giam), anti–β-actin, and anti–α-tubulin (Sigma-Aldrich, St Louis, MO).
Isolation of PBMCs from ATLL patients and cell culture
PBMCs from ATLL patients with leukemia, an HTLV-1–negative patient with peripheral CD4+CD25+ T-cell lymphoma with leukemia phase, and a healthy donor were separated using Lymphoprep solution (MediaTech, Herndon, VA) by centrifugation. On selected patients, isolated PBMCs were placed in culture for 72 hours or less in 10% fetal bovine serum–supplemented RPMI medium (Sigma-Aldrich) in a humidified 5% CO2 incubator at 37°C, and cells were collected at the desired times, centrifuged at 1000g (2000 rpm), washed in sterile 1 × phosphate-binding buffer (PBS), and frozen as pellets for later use.
Electrophoretic mobility shift assays
Nuclear extracts were prepared from fresh or cryopreserved (10% DMSO) PBMCs of ATLL patients with leukemia, total PBMCs, and magnetically isolated CD4+ cells from a healthy donor (CD4+ T Cell Isolation Kit II; Miltenyi Biotec, Bergisch-Gladbach, Germany), leukemic PBMCs from an HTLV-1–negative patient with CD4+CD25+ lymphoma, and fresh lymphoma-type ATLL tumor specimens. Human T-cell lines MT-2 (Tax-expressing, HTLV-1+) and Jurkat (ATCC) were also used as controls. Methods for nuclear protein extraction and EMSA supershift analyses in our laboratory have been described previously.34
Western blot analysis
Whole cell extracts were prepared from MT-2 and Jurkat cells, frozen ATLL solid tumor specimens, and PBMCs from ATLL patients with leukemia and were frozen as pellets or were cryopreserved in 10% DMSO or fresh isolates before and after culture. Protein sample preparation and Western blot analysis were performed as previously described.34 Total protein (25-50 μg) was fractionated on 12% SDS-PAGE and transferred by electroblotting onto nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). Proteins were visualized with the enhanced chemiluminescence (ECL) developing kit (Amersham, Piscataway, NJ).
Immunohistochemistry
Paraffin sections (3-μm–thick) were dewaxed in xylene, rehydrated, and treated with 6% hydrogen peroxide to block endogenous peroxidase. Slides were then pressure cooked for 5 minutes in the presence of target retrieval solution (DAKO, Carpinteria, CA), blocked with avidin/biotin solution, and incubated in rabbit anti–IRF-4 antibody (M7259; DAKO) for 30 minutes in a humidity chamber. Primary antibody was removed from the slides by washing with PBS, and the slides were incubated in biotinylated antirabbit linking solution for 25 minutes, washed with PBS, and incubated in streptavidin peroxidase conjugated for 25 minutes in a humidity chamber. Finally, they were washed with PBS, stained with DAB (DAKO), washed in PBS again, submerged in 1% cupric sulfate, rinsed in H2O, and counterstained with 0.1% Fast Green. Images were visualized with an Olympus BH-2/BHTV microscope (Center Valley, PA). The H&E-stained picture (top panel, Figure 4) was visualized with a 100×/lp25 NA oil-immersion objective lens, and MUM1 (bottom panel, Figure 4) was visualized with a 50×/0.90 NA oil-immersion objective lens. Stains used were hematoxylin-eosin (H&E) and Fast Green. Imaging medium used for immunohistochemical stain was Tissue-TEK Mounting Media #6419 (Sakura Finetek, Torrance, CA). Images were taken with a Nikon Coolpix 5400 (Melville, NY) using Nikon View image acquisition software.
Results
Antiviral therapy response in patients with ATLL and clonal persistence in long-term survivors
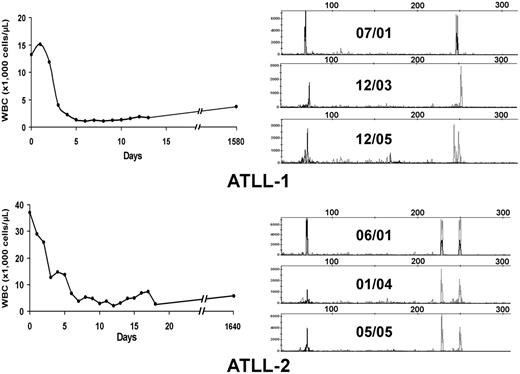
Twenty-eight patients included in this analysis were treated with high-dose parenteral AZT (1.5 g twice daily) and IFN-α (5-10 million U twice daily) as induction therapy (Table 1). Twenty-two patients had evaluable responses. Five (22.7%; 3 acute, 2 unfavorable chronic leukemia) patients achieved CR, and 4 (18.2%) achieved partial response (PR); the overall response rate (CR + PR) was 40.9%. No patients with lymphoma-type ATLL responded to antiviral treatment. All CR patients except ATLL-1 were treatment naive before the beginning of therapy. Three long-term survivors (ATLL-1, ATLL-2, ATLL-3) have remained in clinical remission for 63 months, 63 months, and 16 months, respectively, on maintenance antiviral therapy, and ATLL-7 continues to be in remission after 7 months. Figure 1A–B (left panels) shows the hematologic responses within the first 3 weeks of antiviral therapy in 2 long-term survivors. Despite regression of their measurable disease (including morphologically abnormal peripheral blood lymphocytes and hypercalcemia), multiplex PCR analysis for T-cell receptor (TCR) gene rearrangements performed from PBMCs continued to detect clones for each patient 52 and 48 months, respectively, after therapy initiation (Figure 1A–B, right panels). Persistent clones were also detected in another long-term survivor (data not shown). Southern blot analysis confirmed persistent TCR rearrangement in multiple follow-up studies (data not shown). Complete responders ATLL-3 and ATLL-21 were lost to follow-up but were in clinical remission at the time of their last visit.
Hematologic and molecular response to AZT and IFN-α in ATLL patients. (A, left) WBC counts were plotted over time for 2 ATLL patients with leukemia after induction and during antiviral treatment. (B, right) Analysis of T-cell clones is demonstrated by combined multiplex PCR at the specified dates for these 2 patients using two sets of reaction primers, including those specific to the V1-8, V9, and consensus J1 and J2 regions of βchain TCR genes (peaks to the right) or alternative J1 and J2 regions (peaks to the left).
Hematologic and molecular response to AZT and IFN-α in ATLL patients. (A, left) WBC counts were plotted over time for 2 ATLL patients with leukemia after induction and during antiviral treatment. (B, right) Analysis of T-cell clones is demonstrated by combined multiplex PCR at the specified dates for these 2 patients using two sets of reaction primers, including those specific to the V1-8, V9, and consensus J1 and J2 regions of βchain TCR genes (peaks to the right) or alternative J1 and J2 regions (peaks to the left).
Expression of nuclear NF-κB/Rel in primary ATLL
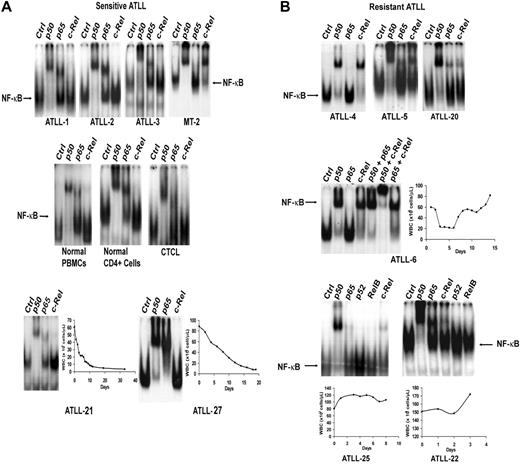
NF-κB exerts a predominant antiapoptotic effect and is associated with resistance to chemotherapy agents in different tumor types.35 To investigate the role of this transcription factor in AZT and IFN-α–sensitive versus resistant forms of ATLL, we used EMSA to analyze the composition of activated NF-κB complexes in primary ATLLs. Nuclear overexpression of activated c-Rel was detected in 9 (33%) of 27 tumor samples analyzed (Table 1). Tumors from the 5 patients who achieved CR (ATLL-1, ATLL-2, ATLL-3, ATLL-21, ATLL-27) did not express significant c-Rel on EMSA (Figure 2A) and contained mainly p50/p50 and p50/p65 heterodimers. In contrast, 6 of 7 c-Rel+ tumors (ATLL-4, ATLL-5, ATLL-6, ATLL-20, ATLL-22, ATLL-25) were from patients in whom induction therapy with AZT and IFN-α failed (Figure 2B). These tumors expressed c-Rel/p50 heterodimers at various levels. Nuclear extracts from ATLL-4 and ATLL-6 contained p50/c-Rel heterodimers exclusively, whereas p50/p50 constituted most of the NF-κB complex in others (Figure 2B). Tumor specimens from patients ATLL-20 and ATLL-5 also contained c-Rel (data not shown). c-Rel expression was insignificant in healthy or CTCL controls. Supershift bands on c-Rel lanes were faint but showed undiminished and unshifted baseline NF-κB complexes (indicated by arrows) compared with control lanes (Figure 2A). NF-κB subunits p52 and RelB were not detected in any of the samples analyzed.
Analysis of expression of NF-κB/Rel subunits in primary ATLL cells by EMSA. Nuclear extracts from leukemic cells of (A) antiviral-sensitive (ATLL-1, ATLL-2, ATLL-3, ATLL-21, ATLL-27) and (B) antiviral-resistant (ATLL-4, ATLL-5, ATLL-6, ATLL-20, ATLL-22, ATLL-25) tumors were incubated with the indicated antibodies specific for the different NF-κB subunits and 32P-labeled oligonucleotide containing a consensus NF-κB–binding sequence and were separated by gel electrophoresis. PBMCs from a healthy subject, isolated CD4+ cells from normal PBMCs, a leukemia patient with CD4+CD25+ mycosis fungoides/CTCL (HTLV-1 negative) (WBCs, 70 × 109/L), and MT-2 cells (c-Rel+, HTLV-1+ line) were used as controls. The NF-κB complex was further characterized by supershifting with several antibody combinations in one patient (ATLL-6). WBC counts over time during induction AZT and IFN-α therapy curves are demonstrated in the selected leukemia patients.
Analysis of expression of NF-κB/Rel subunits in primary ATLL cells by EMSA. Nuclear extracts from leukemic cells of (A) antiviral-sensitive (ATLL-1, ATLL-2, ATLL-3, ATLL-21, ATLL-27) and (B) antiviral-resistant (ATLL-4, ATLL-5, ATLL-6, ATLL-20, ATLL-22, ATLL-25) tumors were incubated with the indicated antibodies specific for the different NF-κB subunits and 32P-labeled oligonucleotide containing a consensus NF-κB–binding sequence and were separated by gel electrophoresis. PBMCs from a healthy subject, isolated CD4+ cells from normal PBMCs, a leukemia patient with CD4+CD25+ mycosis fungoides/CTCL (HTLV-1 negative) (WBCs, 70 × 109/L), and MT-2 cells (c-Rel+, HTLV-1+ line) were used as controls. The NF-κB complex was further characterized by supershifting with several antibody combinations in one patient (ATLL-6). WBC counts over time during induction AZT and IFN-α therapy curves are demonstrated in the selected leukemia patients.
c-Rel expression did not correlate with tumor burden, ATLL subtype, or T-cell phenotype because 1 chronic and 2 lymphoma ATLL specimens expressed c-Rel (Table 1). In the 19 patients for whom we measured c-Rel expression, the response rate for c-Rel− patients was 66.7% (8 of 12) compared with 14.3% (1 of 7) for c-Rel+ patients, and the difference was marginally significant (P = .057; 2-sided Fisher exact test) (Table 2, left panel). Six of 7 c-Rel+ patients did not respond to AZT and IFN-α. ATLL-7 was a c-Rel+ patient who achieved PR according to our criteria but who never achieved a WBC count below 18 × 109/L. All 5 patients who achieved CR were in the c-Rel− group. CR rates for c-Rel− and c-Rel+ groups were 41.7% and 0%, respectively (P = .106; 2-sided Fisher exact test).
Comparison of response to AZT and IFN-α by c-Rel and by IRF-4 expression
| Response . | c-Rel− (%) . | c-Rel+ (%) . | P . | IRF-4− (%) . | IRF4+ (%) . | P . |
|---|---|---|---|---|---|---|
| CR | 5 (41.7) | 0 (0.0) | .106 | 5 (55.6) | 0 (0.0) | .008 |
| PR | 3 (0.25) | 1 (14.3) | — | 2 (22.2) | 1 (9.1) | — |
| CR + PR | 8 (66.7) | 1 (14.3) | .057 | 7 (77.8) | 1 (9.1) | .005 |
| NR | 4 (0.33) | 6 (85.7) | — | 2 (22.2) | 10 (90.9) | — |
| Total | 12 (100) | 7 (100) | — | 9 (100) | 11 (100) | — |
| Response . | c-Rel− (%) . | c-Rel+ (%) . | P . | IRF-4− (%) . | IRF4+ (%) . | P . |
|---|---|---|---|---|---|---|
| CR | 5 (41.7) | 0 (0.0) | .106 | 5 (55.6) | 0 (0.0) | .008 |
| PR | 3 (0.25) | 1 (14.3) | — | 2 (22.2) | 1 (9.1) | — |
| CR + PR | 8 (66.7) | 1 (14.3) | .057 | 7 (77.8) | 1 (9.1) | .005 |
| NR | 4 (0.33) | 6 (85.7) | — | 2 (22.2) | 10 (90.9) | — |
| Total | 12 (100) | 7 (100) | — | 9 (100) | 11 (100) | — |
P values were determined by 2-sided Fisher exact test. Whole numbers = number of patients.
CR indicates complete response; PR, partial response; NR, no response; —, not determined.
IRF-4 expression correlates with c-Rel and antiviral therapy resistance in ATLL
c-Rel has been associated with the expression of IRF-4, which has been implicated in interferon resistance.36 IRF-4 has been shown to play an important role in T-cell function, has oncogenic activity in vitro, and is expressed in patients with aggressive lymphomas, including ATLL, of poor prognosis.37-45 IRF-4 is also thought to function as a repressor of IFN-regulated genes, and IRF-4–deficient c-Rel−/− cells are markedly sensitive to the antiproliferative effects of IFN.36,46,47 IRF-4 is readily detected in HTLV-1–infected cell lines and primary ATLL cells that express the viral oncoprotein Tax.47-49 However, uncultured primary ATLLs do not express Tax.23 Because of the observed c-Rel expression in several antiviral (IFN-α)–insensitive tumors, we hypothesized that IRF-4 might also play a role in the resistance of primary ATLL to IFN.
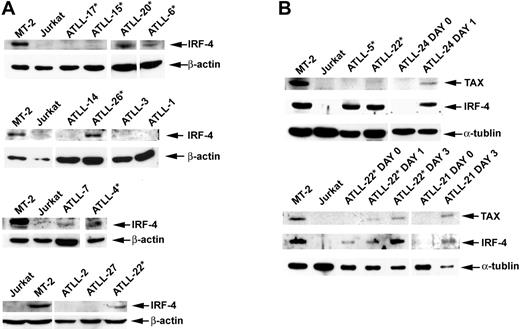
We examined IRF-4 protein expression in tumors for which sample material was available by Western blot analysis or immunohistochemistry (IHC). Data on IRF-4 expression were available for 20 of the 22 patients assessed for response to AZT and IFN-α therapy. Response (CR + PR) rates were 77.8% in the IRF-4− group and only 9.1% in the IRF-4+ group (Table 2, right panel). This difference was statistically significant (P = .005; 2-sided Fisher exact test). No significant IRF-4 protein was detected in 7 (87.5%) of 8 patients whose tumors were sensitive to antiviral therapy (5 CR, 2 PR). CR rates for IRF-4− and IRF-4+ patients were 55.6% and 0%, respectively, and the difference was statistically significant (P = .008; 2-sided Fisher exact test). Induction therapy with AZT and IFN-α failed in 10 (90.9%) of 11 patients with IRF-4+ tumors (4 lymphomas, 5 acute, and 1 unfavorable chronic leukemia) (Tables 1, 2). Six (60%) of 10 IRF-4+ nonresponders (3 lymphomas, 2 acute, 1 unfavorable chronic) were treatment naive before they received AZT and IFN-α induction therapy. Patient ATLL-7 (acute type) had an IRF-4+ tumor and achieved PR, but leukemia persisted (minimum WBC count, 18 × 109/L) despite antiviral treatment. High IRF-4 protein expression correlated with high mRNA expression, as determined by quantitative RT-PCR assays (data not shown). IRF-4 protein (tested by IHC) was expressed in 100% of patients with lymphoma-type ATLL, including the 4 patients in whom parenteral antiviral therapy failed (ATLL-5, ATLL-28, ATLL-30, ATLL-34). In all specimens, IRF-4 was expressed homogenously in tumor cells only. The disease of 5 additional patients with lymphoma (ATLL-23, ATLL-29, ATLL-32, ATLL-33, ATLL-36) who had IRF-4+ tumors also progressed with oral AZT and subcutaneous IFN-α therapy after previous therapies had failed (Table 1, asterisks). IHC results were confirmed by Western blot in patients ATLL-5 and ATLL-20 (Figure 3).
IRF-4 and Tax protein expression in primary ATLL tumors before and after cell culture. (A) Western blot analysis demonstrating Tax and IRF-4 protein status in primary unmanipulated and cultured ATLL tumors. (B) In selected patients, IRF-4 and Tax protein expression was measured ex vivo before cell culture (day 0) and on in vitro culture for the indicated time periods (day 1, 24 hours; day 2, 48 hours; day 3, 72 hours). Whole cell lysates from MT-2 and Jurkat lines were used as positive and negative controls, respectively. *Tumors from patients in whom AZT and IFN-α therapy failed.
IRF-4 and Tax protein expression in primary ATLL tumors before and after cell culture. (A) Western blot analysis demonstrating Tax and IRF-4 protein status in primary unmanipulated and cultured ATLL tumors. (B) In selected patients, IRF-4 and Tax protein expression was measured ex vivo before cell culture (day 0) and on in vitro culture for the indicated time periods (day 1, 24 hours; day 2, 48 hours; day 3, 72 hours). Whole cell lysates from MT-2 and Jurkat lines were used as positive and negative controls, respectively. *Tumors from patients in whom AZT and IFN-α therapy failed.
IRF-4 was detected by Western blot in all 6 c-Rel+ tumors tested (Figure 3A–B), including a lymph node specimen (ATLL-20) analyzed by IHC (Figure 4). In all but one patient (ATLL-7), tumors were resistant to AZT and IFN-α (Table 1). IRF-4 was also detected in 3 c-Rel− tumors from patients in whom antiviral therapy failed (ATLL-11, ATLL-23, ATLL-26). In contrast, no significant IRF-4 protein was detected in 9 (69%) of 13 c-Rel− tumors, including 7 from patients whose tumors were antiviral sensitive and all 5 complete responders (ATLL-1, ATLL-2, ATLL-3, ATLL-14, ATLL-21, ATLL-24, ATLL-27). In summary, almost all antiviral therapy–resistant tumors, most of which were c-Rel+, had detectable IRF-4 protein levels, whereas the antiviral-sensitive tumors (including all complete responders) were c-Rel− and lacked IRF-4 protein (Table 1). The association between IRF-4 and c-Rel expression was statistically significant (P = .03). Kaplan-Meier survival plots indicated significance and a tendency toward better survival in ATLL patients lacking c-Rel and IRF-4 in their tumors, respectively (P = .021 and P = .061, respectively; log rank test) (Figure 5).
Expression of nuclear IRF-4/MUM-1 in ATLL lymphoma specimen. (top) Hematoxylin and eosin–stained paraffin-embedded section of tumor-infiltrated lymph node from patient ATLL-20. (bottom) IRF-4/MUM-1 antibody (Dako) staining by immunohistochemistry of mirror section.
Expression of nuclear IRF-4/MUM-1 in ATLL lymphoma specimen. (top) Hematoxylin and eosin–stained paraffin-embedded section of tumor-infiltrated lymph node from patient ATLL-20. (bottom) IRF-4/MUM-1 antibody (Dako) staining by immunohistochemistry of mirror section.
Survival by c-Rel and IRF-4 status among patients evaluated for response. P values, determined by 2-sided log rank test (tick marks), indicate censored survival times.
Survival by c-Rel and IRF-4 status among patients evaluated for response. P values, determined by 2-sided log rank test (tick marks), indicate censored survival times.
We then analyzed whether IRF-4 was associated with Tax expression in ATLL. IRF-4 is highly expressed in Tax-positive HTLV-1–infected cells, though no significant Tax expression occurs in primary unmanipulated ATLL tumors.23,47-50 In fact, low anti-Tax antibody titers have been associated with the development of ATLL in HTLV-1 carriers.51 ATLL cells do, however, begin to express Tax spontaneously after introduction into tissue culture.23,28 We observed high IRF-4 protein levels at baseline in MT-2– and non-Tax–expressing tumors (ATLL-5, ATLL-22) (Figure 3B). However, Tax protein expression in ATLL cells (ATLL-21, ATLL-22, ATLL-24) occurred only after in vitro culture, which coincided with the expression of IRF-4. In summary, IRF-4 was expressed in the absence of Tax in primary unmanipulated antiviral-resistant ATLL cells, but it was coinduced with Tax only after culture in antiviral-sensitive tumors.
Discussion
ATLL generally remains an incurable illness that recurs even after treatment with intensive chemotherapy regimens. Several studies have shown that IFN-α–based regimens, in combination with either AZT or arsenic trioxide, may benefit a subset of patients with ATLL.10-13,52 The molecular mechanisms that predict response to antiviral-based therapy and the mechanisms of action of these drugs in ATLL are likely complex and involve a variety of factors. Mutations in tumor suppressors (p15, p16, p53) and overexpression of prosurvival molecules, including NF-κB, survivin, and IRF-4, have been found in ATLL tumors of poor prognosis.23,24,45,53-55 Alterations of death receptor signaling (FAS, ligand, and TRAIL receptors, c-FLIP) have also been observed in ATLL cells,55-57 and IFN signaling defects have been identified in several malignancies, including T-cell lymphomas.17-19 In this study, we found that lack of response to antiviral therapy correlated with the nuclear expression of the NF-κB subunit c-Rel, particularly with its putative target, IRF-4. Resistance to AZT and IFN-α correlated independently with the expression of these 2 oncogenic proteins, but coexpression in the resistant tumors was common. Conversely, lack of IRF-4 and c-Rel was associated with response to antiviral therapy, particularly complete response and long-term survival. IRF-4 alone may be a more useful predictor of antiviral therapy response because it was overexpressed in resistant tumors lacking c-Rel. IRF-4 immunohistochemistry is also widely available in many centers.
NF-κB complexes containing c-Rel (p50/c-Rel and c-Rel/c-Rel) have been demonstrated predominantly in hematopoietic lineages.58 c-Rel is the cellular homologue of the avian oncogene v-Rel from the REV-T retrovirus, which induces aggressive B- and T-cell lymphomas59 and is the only known subunit of NF-κB/Rel capable of transforming cells in vitro and of generating fatal tumors in vivo in its wild-type form.60 c-Rel is amplified in a growing number of human Hodgkin and non-Hodgkin lymphomas.61-63 Of relevance to the pathogenesis of ATLL is that c-Rel is critical in the regulation of IL-2 expression.64,65 In 2 studies from Japan, HTLV-1–transformed cells expressed p50/c-Rel, whereas a small number of primary ATLL samples expressed mainly p50/p50 and p50/p65 heterodimers.23,24 Recently, the expression of p50 and c-Rel, rather than of p65, has been observed in ATLL-like tumors developed in a tax transgenic murine model and ATLL-derived xenographs in NOD-SCID mice.66,67 In our study, c-Rel was associated with antiviral therapy resistance and appeared to be independent of ATLL subtype, as observed in 1 patient with chronic-type disease and in 2 patients with lymphoma that expressed c-Rel/p50 heterodimers. Combination AZT and IFN-α was ineffective in most patients with evaluable c-Rel+ tumors. We noted a markedly disparate response to IFN-based therapy in several ATLL patients with clinically similar forms of the disease. For instance, female patient ATLL-27 (acute-type, c-Rel−) had sustained leukemia regression after induction with AZT and IFN-α, but 2 other women with c-Rel+ tumors and comparable tumor burdens (ATLL-6, ATLL-22) had progressive disease while receiving antiviral therapy. These data suggest that NF-κB subunit analysis might be one factor predictive of response to antiviral therapy in ATLL.
We also investigated the expression of IRF-4 in primary ATLL tumors. IRF-4 has transforming and oncogenic potential in vitro39 and is overexpressed in non-Hodgkin lymphomas of poor prognosis, including aggressive-type ATLL.40-45 IRF-4 is a member of the interferon regulatory factor family of transcription factors whose expression is restricted to the lymphoid and myeloid compartments.68,69 In normal lymphocytes, IRF-4 is important in gene expression, proliferation, and differentiation.37,70 In mature human CD4+ T cells, IRF-4 is essential for cytokine production and cell survival.37 IRF-4 is also thought to function as a repressor of toll-like receptor (TLR)–dependent inflammatory cytokines and IFN-regulated genes, and IRF-4–deficient c-Rel−/− cells demonstrate unusual sensitivity to the antiproliferative effects of IFN.46,47,71 IRF-4 is constitutively upregulated in HTLV-1–infected cell lines and primary ATLL cells that express the oncoprotein Tax.47-49 However, uncultured primary ATLL does not express Tax.23 We detected IRF-4 protein in most antiviral therapy–resistant ATLL tumors tested and in one c-Rel+ partial responder, but no IRF-4 protein was detected in the tumors of the other patients responsive to AZT and IFN-α, including all 5 complete responders. Furthermore, IRF-4 overexpression was observed in virtually all lymphoma-type ATLL tumors, which are known to be generally resistant to antiviral therapy.3 Most lymphomas tested could not be analyzed for c-Rel because of the lack of nuclear protein available for EMSA. Our early experience with antiviral therapy in lymphoma patients (and in others) suggested this treatment was ineffective in this ATLL subtype, so we preferentially treat with chemotherapy. IRF-4 expression in c-Rel− tumors supports the activation of its promoter through a different mechanism or by other NF-κB subunits (ie, p50/p65), as has been demonstrated by other investigators.49 Alternatively, IRF-4 in these tumors may be activated by other cellular pathways and by mechanisms yet to be described. Ideally, we would like to perform confirmatory experiments with siRNA-mediated knockdown of c-Rel (or IRF-4). These types of studies have proven to be difficult because of the rapid induction of Tax (and Tax-driven IRF-4) in primary cells placed in culture.
IRF-4 expression in primary antiviral-resistant ATLL was independent of the HTLV-1 oncoprotein Tax, which was not detected in primary unmanipulated tumors that exhibited IRF-4. Our findings do confirm that primary ATLL cells can coexpress Tax and IRF-4—but only after they are placed in culture—and support the role of Tax in the induction of IRF-4.47,48
It is likely that NF-κB and its target genes can be upregulated in multiple ways in ATLL in the absence of Tax expression. Among the NF-κB (c-Rel) target genes recently identified that may be significant in the pathogenesis of ATLL are IL-2 and IL-15, which are cytokine ligands for CD25, a receptor expressed ubiquitously in ATLL, and the antiapoptotic protein of the bcl family.9,65,72 Gene expression analysis may be useful for analyzing targets of c-Rel, Tax, or IRF-4 in cultured ATLL or established cell lines; however, primary tumors differ substantially from these lines, as we noted in Affymetrix (Santa Clara, CA) microarray experiments (data not shown).
Antiviral therapy proved to be effective in a subset of our leukemic patients, some of whom have been maintained in clinical remission for years. In contrast, essentially all patients with lymphoma-type ATLL have fared poorly. Similar experiences have been reported by other investigators.3 We found that the peripheral blood of long-term ATLL survivors in stable remission continued to have low levels of detectable T-cell clones. Other investigators have demonstrated that persistence of tumor clones in ATLL patients invariably correlated with disease relapse.73 These data indicate that AZT and IFN-α is a suppressive rather than a curative regimen; patients in clinical remission should remain on this therapy long term. Recently, a number of ATLL patients have been reported to be in continuous remission after allogeneic stem cell transplantation.6,7 Our studies and those of other investigators demonstrate that specific molecular signatures are associated with sensitivity or resistance to antiviral therapy that may useful in deciding on initial therapy for ATLL patients. In the presence of poor prognostic molecular features, early allogeneic stem cell transplantation should probably be considered when feasible for ATLL patients and perhaps for those who have responded to antiviral therapy and have persistent molecular evidence of the disease and a suitable stem cell donor. In the future, larger prospective studies that use these molecular markers may be necessary to better characterize their role as predictors for treatment outcome.
Authorship
Contribution: J.C.R. performed most of the work, analyzed the data, and coauthored the paper. P.R. performed clonality assays. L.R. provided resources and reagents. I.R. performed statistical analysis. C.B. contributed specimens. C.P. contributed and processed specimens. G.B. performed immunohistochemical analysis. L.T. performed experiments. V.A. assisted in NF-κB experiments. E.H. contributed reagents. I.L. assisted in gene expression and data analysis. W.J.H. designed the research, analyzed the data, and coauthored the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William J. Harrington Jr, University of Miami Miller School of Medicine, Sylvester Comprehensive Cancer Center, Rm 3400 (D8-4), 1475 NW 12th Ave, Miami, FL 33136; e-mail: wharring@med.miami.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Supported by National Institutes of Health grants UO1-CA-070058 (AIDS Malignancy Consortium), CA-082274, and CA-10521.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal