Abstract
Several groups, including our own, have independently demonstrated that effector memory T cells from non–alloantigen-primed donors do not cause graft-versus-host disease (GVHD). In the current study, we further investigated whether this approach could be extended to all memory T cells, and we studied the underlying mechanisms. Neither total memory T cells nor purified central memory T cells were able to induce GVHD. Memory T cells were at least 3-log less potent than bulk T cells in mediating GVHD. As expected, memory T cells failed to elicit cytotoxicity and proliferated poorly against alloantigens in standard 5-day mixed-lymphocyte cultures. However, the proliferative responses of memory T cells were more comparable with those of bulk and naive T cells when the culture time was shortened. Moreover, the frequencies of IL-2–secreting cells measured by 42-hour enzyme-linked immunosorbent spot (ELISPOT) assay were similar among naive, memory, and bulk T cells. These data indicated that memory T cells are able to respond to alloantigens initially but fail to develop to full potential. The abortive immune response, which was mediated by non–alloantigen-specific memory T cells in response to alloantigens, may explain why memory T cells from unprimed and non–alloantigen-primed donors could not induce GVHD.
Introduction
Allogeneic stem cell transplantation offers the hope of cure for a wide variety of otherwise lethal malignant and nonmalignant diseases.1 However, this procedure is associated with substantial risks, including graft-versus-host disease (GVHD), infection, and disease relapse.1 These complications are intricately related to the function of mature T cells contained in the graft.1,2 T cells mediate GVHD,3 a life-threatening adverse effect that can occur after allogeneic stem cell transplantation.1 Depletion of T cells from the graft is effective in preventing GVHD but is associated with increased risk for graft failure, leukemia relapse, and opportunistic infection.2 Thus, the major challenge in allogeneic stem cell transplantation is how to transfer the T cell–mediated beneficial effects without causing GVHD.
Because the antigen specificity of each T lymphocyte is determined before its contact with antigen by random gene rearrangements in the thymus,4 mature T cells can be divided into naive and memory subsets based on whether they have encountered their corresponding antigens.5 Naive and memory T cells can be distinguished by cell surface markers.5-7 We hypothesized that, if a donor has never encountered the host alloantigens, all host alloantigen–specific T cells are exclusively contained in the naive T-cell compartment and that memory T cells from these animals would not cause GVHD. Based on this hypothesis, we have demonstrated that CD62L− T cells (effector memory T cells [TEM]) from unprimed or non–host alloantigen–primed donors do not induce GVHD.8 Similar results have been reported by several other groups using different animal systems.9-12 Based on the expression of homing molecules, such as CD62L, memory T cells can be further divided into central memory (TCM; all CD62L+ memory T cells) and effector memory (TEM; all CD62L− T cells) subsets.13,14 We asked whether the findings with TEM could be extended to all memory T cells. If our hypothesis is correct, all memory T cells, including TCM and TEM, should be unable to induce GVHD. To answer this question directly, we tested the ability of all memory T cells to induce GVHD. Moreover, we explored the mechanisms by which memory T cells failed to cause GVHD.
Material and methods
Mice
BALB/c (H2d), C57BL/6 (H2b), and C3H/HeJ (H2k) were purchased from The Jackson Laboratories (Bar Harbor, ME). C57BL/Ka, CD45.1, Thy1.1 mice (gifts from Dr Jos Domen, Duke University, Durham, NC), which are congeneic to C57BL/6, were bred at Duke University. Female mice were primarily used in this study. Most of the unprimed donor mice were between 10 and 30 weeks of age. Some of the repeated experiments were performed using male or older donor mice (up to 20 months of age) or C57BL/Ka, CD45.1, Thy1.1 mice. No differences were observed when male, older, or C57BL/Ka, CD45.1, Thy1.1 donor mice were used (data not shown). Recipient mice were 7 to 12 weeks old when they underwent transplantation. The mice were housed in a specific pathogen-free facility throughout the study.
T-cell depletion from bone marrow
T cells were depleted from bone marrow using anti–Thy1.2 antibody and complement according to a published protocol from our laboratory.15
T-cell purification
Splenic T cells were negatively selected by using T-cell enrichment columns (R&D Systems, Minneapolis, MN) followed by a negative selection procedure using anti–major histocompatibility complex (MHC) class II (Ia), CD11b, and DX5 microbeads (Miltenyi Biotec, Auburn, CA). The purity of T cells was always greater than 95%.
Sorting of memory T cells
Purified T cells were first stained with biotinylated anti–CD4 (RM4-5) and anti–CD8 (53-6.7) Cy-Chrome conjugated anti–CD44 (IM7), phycoerythrin (PE)–conjugated anti–CD45RB (16A), and fluorescein isothiocyanate (FITC)–conjugated anti–CD62L (MEL-14) antibodies followed by PE-Texas Red–conjugated streptavidin (Caltag, South San Francisco, CA). Except where indicated, antibodies were purchased from BD PharMingen (San Jose, CA). Stained cells were sorted into naive (CD45RB+CD62L+CD44−/low) and memory T-cell fractions (all other T cells except those with naive phenotype, including all the T cells with the following phenotypes: CD45RB+CD62L+CD44high, CD45RB−CD62L+, and CD62L−) using FACS Vantage SE equipped with FACSDiva software (Becton Dickinson, San Jose, CA).5,6 In some experiments, memory T cells (total) were sorted into TCM (all CD62L+ memory T cells, including T cells with the phenotypes CD45RB+CD62L+CD44high and CD45RB−CD62L+) and TEM (all CD62L− T cells) through 4-way sorting.13,14,16 To sort CD25− memory T cells, allophycocyanin (APC)–conjugated anti–CD25 (PC61) and PE-Cy5.5–conjugated anti–CD44 (IM7; eBioscience, San Diego, CA) antibodies were used. To sort CD4+ memory T cells, APC-conjugated anti–CD4 (RM4-5; Caltag) antibody was used. The sorting strategy is shown in Figures 1A and 7A, and the post-sort analyses and the phenotypes of different T-cell subsets are shown in Figures 1B and 7B. Most of the unsorted control bulk T cells used in the study were not stained with antibodies. However, we tested the antibodies and found that they do not affect the ability of bulk T cells to induce GVHD or to proliferate against alloantigens (data not shown). Naive T cells, which were stained with antibodies and sorted together with memory T-cell subsets, were always included as a control. Responder cell doses were always adjusted based on T-cell content.
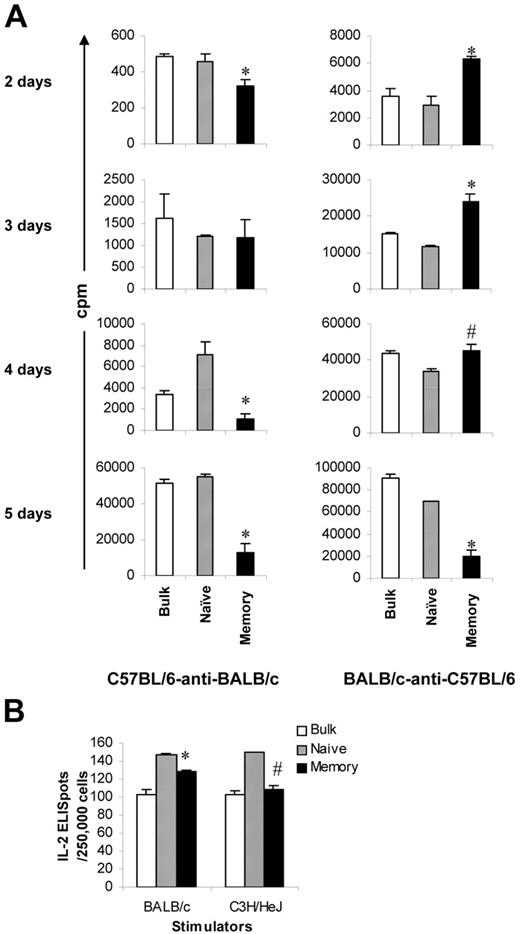
Proliferation and cytotoxicity. Sorted T-cell subsets were cultured with irradiated allogeneic spleen cells for 5 days before they were tested for proliferation and cytotoxicity. Results represent mean ± SD of triplicate wells. (A) Sorting strategy. Enriched T cells were stained with anti-CD4, anti-CD8, anti-CD44, anti-CD45RB, and anti-CD62L antibodies. We first gated on CD4/CD8+ T cells. CD45RB+CD62L+ T cells (region A) were further separated into 2 populations based on the expression of CD44 (A1, CD45RB+CD62L+CD44−/low; A2, CD45RB+CD62L+CD44high). Cells were sorted into naive (A1) and memory (all other T cells except naive [B+C+A2]) phenotype T cells. In some experiments (Figure 5), memory (total) T cells were further separated into TEM (region B) and TCM (C+A2) based on the expression of CD62L. Shown in the third column are cells from region A in the second column. Numbers represent percentages of parent gates. Donor mice for this sort were 4 to 6 months old. (B) Phenotypes of sorted T-cell subsets. Shown in the second column are cells from region A in the first column. Data presented in the last 2 columns were not from post-sort analyses. Logarithmic displays were used in all histograms or dot plots except those in the last column in which “logicle” displays (biexponential displays) were used. These data represent the results of at least 4 similar experiments. (C) Mixed lymphocyte reaction. Proliferation was determined by 3H-thymidine incorporation. The responder alone was always lower than 200 cpm. Data represent the results of 3 to 4 similar experiments. (D) Cytotoxic T-lymphocyte assay. Cytotoxicity was measured by 4-hour 51Cr release assay. *P < .01, memory versus other groups; #P < .01, naive versus bulk.
Proliferation and cytotoxicity. Sorted T-cell subsets were cultured with irradiated allogeneic spleen cells for 5 days before they were tested for proliferation and cytotoxicity. Results represent mean ± SD of triplicate wells. (A) Sorting strategy. Enriched T cells were stained with anti-CD4, anti-CD8, anti-CD44, anti-CD45RB, and anti-CD62L antibodies. We first gated on CD4/CD8+ T cells. CD45RB+CD62L+ T cells (region A) were further separated into 2 populations based on the expression of CD44 (A1, CD45RB+CD62L+CD44−/low; A2, CD45RB+CD62L+CD44high). Cells were sorted into naive (A1) and memory (all other T cells except naive [B+C+A2]) phenotype T cells. In some experiments (Figure 5), memory (total) T cells were further separated into TEM (region B) and TCM (C+A2) based on the expression of CD62L. Shown in the third column are cells from region A in the second column. Numbers represent percentages of parent gates. Donor mice for this sort were 4 to 6 months old. (B) Phenotypes of sorted T-cell subsets. Shown in the second column are cells from region A in the first column. Data presented in the last 2 columns were not from post-sort analyses. Logarithmic displays were used in all histograms or dot plots except those in the last column in which “logicle” displays (biexponential displays) were used. These data represent the results of at least 4 similar experiments. (C) Mixed lymphocyte reaction. Proliferation was determined by 3H-thymidine incorporation. The responder alone was always lower than 200 cpm. Data represent the results of 3 to 4 similar experiments. (D) Cytotoxic T-lymphocyte assay. Cytotoxicity was measured by 4-hour 51Cr release assay. *P < .01, memory versus other groups; #P < .01, naive versus bulk.
Proliferation assay
Proliferation assay has been described previously.8 Graded numbers of purified T cells (0.0156-2.5 × 105) were plated in 96-well, flat-bottom culture plates with 5 × 105 irradiated (20 Gy) allogeneic spleen cells or keyhole limpet hemocyanin (KLH; 100 μg/mL, Pierce, Rockford, IL) plus 5 × 105 irradiated (20 Gy) syngeneic spleen cells in a final volume of 200 μL. After incubation at 37°C and 5% CO2 for a certain period of time (96 hours, except where indicated), cultures were pulsed with 3H-thymidine (1 μCi [0.037 MBq]/well). After another 16 hours of incubation, the cultures were harvested and counted in a MicroBeta Trilux liquid scintillation counter (EG&G Wallac, Turku, Finland). Triplicate cultures were set up for every cell population tested.
Cytotoxic T-cell assay
Cytotoxic T-cell assay has been described previously.17 Graded numbers of responder cells were plated with 5 × 105 irradiated (20 Gy) allogeneic spleen cells per well in 96-well, round-bottom plates. Plates were cultured at 37°C in a 5% CO2 incubator for 112 hours. The cells were then tested in situ for lysis of 51Cr-labeled 2-day concanavalin A (Con A) blast cells. After 4 hours of culture, supernatant was removed and counted in a MicroBeta Trilux liquid scintillation counter (EG&G Wallac) after it was mixed with SuperMix scintillator (EG&G Wallac). In each experiment, cultures were tested in parallel for lysis of irrelevant targets to ensure the specificity of the killing. Triplicate cultures were set up for every cell population tested. Spontaneous release was obtained by incubating target cells with stimulator cells only. Maximal release was obtained after treatment with 1% Igepal CA-630 (Sigma, St Louis, MO). The percentage of specific release was calculated as follows: ([Experimental release − spontaneous release]/[Maximal release − spontaneous release]) × 100 = % specific release.
Enzyme-linked immunosorbent spot assay
Enzyme-linked immunosorbent spot (ELISPOT) assay was performed using ELISPOT kits (R&D Systems) according to the manufacturer's protocol. Graded numbers of C57BL/6 responder cells were incubated with 5 × 105/well irradiated (20 Gy) BALB/c spleen cells at 37°C and 5% CO2 for 42 hours in the microplates. Wells were washed 3 times before the detection anti–cytokine antibody was added. Microplates were then incubated at 4°C overnight. At the end of incubation, the plates were washed 3 times again, then 100μL streptavidin-AP was added to each well. After 2 hours of incubation at room temperature, the microplates were washed 3 times, and the substrate solution (BCIP/NBT chromogen) was added. Plates were further incubated in the dark at room temperature for 1 hour. The chromogen solution was then discarded from the microplates, and the microplates were rinsed with distilled water. After the microplates were dry, the ELISPOTS, which represented the number of cytokine-secreting cells, were scored using a KS ELISPOT reader (Carl Zeiss Vision, Hallbergmoos, Germany). Triplicate cultures were set up for every cell population tested.
GVHD model
Lethally irradiated (BALB/c, 8.5 Gy; C3H/HeJ, 9.5 Gy; C57BL/6, 10.5 Gy) mice were injected intravenously through the tail vein with 1 × 107 T-cell–depleted bone marrow (TCD BM) cells and purified T cells (1 × 106 were used for most of the experiments, except where indicated). Recipients were weighed once every 7 days. Survival and clinical evidence of GVHD, such as skin changes (hair loss and erythema), diarrhea, and hunched posture, were monitored daily. Moribund mice were humanely killed according to an animal protocol approved by the Duke University Institutional Animal Care and Use Committee. Except where indicated, the experiments were performed in the C57BL/6→BALB/c system.
Histopathological analysis of GVHD
Biopsy samples were taken from skin, tongue, lung, intestine, liver, and spleen and were stored in buffered formalin. Specimens were then embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin-eosin. Slides were examined under a light microscope (Zeiss Standard 25; Carl Zeiss, Thornwood, NY) by B.J.C. in a blinded manner and further verified independently by N.T.L. (blinded to experimental groups). To detect subclinical acute and chronic GVHD, we scored histological GVHD using a semiquantitative scoring system adapted from systems previously published by other groups,18-21 with additional measurements of histological changes of chronic GVHD. Slides were scored on the basis of inflammation, tissue damage, and fibrosis. The scoring system for each category measured the extent and the severity of the histological changes: 0, normal; 0.5, focal and rare; 1, focal and mild; 2, diffuse and mild; 3, diffuse and moderate; 4, diffuse and severe. Scores were subsequently added to provide a total score for each specimen. Minimum score was 0, and maximum score was 12 for each target organ. A score greater than 0.5 in any category or a total score more than 1.5 was considered positive for GVHD. Specific changes examined included inflammatory infiltrates, apoptosis of keratinocytes, separation of dermo-epidermal junction, and formation of cleft, follicular dropout, and fibrosis in the skin; inflammation, apoptosis of bile duct epithelial cells, apoptosis of hepatocytes, cholestasis, fibrosis, and parenchyma in the liver; and lamina propria inflammatory cell infiltrate, crypt regeneration, crypt epithelial cell apoptosis, crypt loss, mucosal ulceration, and fibrosis in the intestine.18,19,22 Only skin, liver, and intestine slides were scored. Slides from other tissues (tongue, lung, and spleen) were examined but not scored because the criteria for GVHD in these tissues have not yet been established. The histologic pictures were taken under an Olympus Vanox-S light microscope (Olympus, Center Valley, PA) using an Olympus DP11C camera. We used a 40×/0.7 NA objective lens. The total magnification of the picture was × 100 (objective, ×40; photolens, × 2.5). The original, unmodified pictures were used in this paper.
Immunization
Each donor mouse was injected intraperitoneally with 50μg KLH emulsified in complete Freund adjuvant (Pierce). Donor animals were used more than 4 weeks after immunization.
Coculture assay
The assay was performed as previously described.23 Briefly, T cells with putative regulatory activity were added to the mixed lymphocyte cultures. Cocultured cells were added at different ratios to the responder cells. Bulk T cells and irradiated (20 Gy) responder spleen cells were included as controls. Experiments were performed in 96-well, flat-bottom culture plates with a total volume of 200 μL/well. Proliferation was measured as described after 112-hour culture.
Statistical analysis
Data are represented as mean ± SD or as mean + SD. Comparison between groups was performed by analysis of variance (ANOVA) because all the experiments involved more than 2 groups in this study. When a difference was detected by ANOVA among the groups, the Fisher protected least significant difference test was used to determine the difference between 2 groups. Survival data were analyzed by log rank test. All statistical analyses were performed using StatView software (SAS Institution, Cary, NC). P values below .05 were considered significant.
Results
Memory T cells from unprimed mice respond poorly to alloantigens in 5-day mixed-lymphocyte cultures
Others and we have demonstrated that TEM cannot cause GVHD.8-12 In this study, we further explored whether these observations could be extended to all memory T cells. To test this, we sorted T cells into naive and memory (defined as all T cells except naive T cells, including TEM and TCM) populations (Figure 1A-B) and first evaluated their ability to respond to alloantigens using optimal 5-day proliferation and CTL assays. As demonstrated in 2 different systems (Figure 1C), memory T cells proliferated poorly against alloantigens compared with naive and bulk T-cell controls. Similar results were observed in 2 other animal systems (C57BL/6-anti-C3H/HeJ, BALB/c-anti-C3H/HeJ; data not shown). Consistent with the results in the proliferation assays, memory T cells were unable to elicit cytotoxicity in response to alloantigens, whereas the cytotoxicity elicited by naive and bulk T cells was readily detectable (Figure 1D). It is important to point out that, unlike FVB/N mice, as previously reported,24 young (8-12 weeks old) and old (9-10 months old) C57BL/6 mice responded similarly against BALB/c and C3H/HeJ antigens in proliferation assays (data not shown).
Memory T cells from unprimed donors were unable to induce GVHD
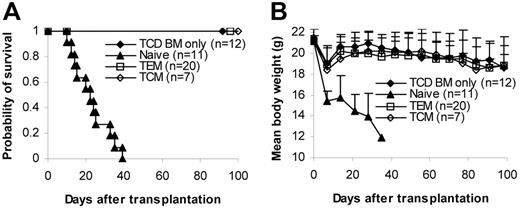
We then tested whether memory T cells could induce GVHD in several different systems (C57BL/6→BALB/c, C57BL/6→C3H/HeJ, BALB/c→C57BL/6). T cells (1 × 106/mouse) were transplanted into lethally irradiated recipients together with 1 × 107 T-cell–depleted bone marrow (TCD BM) cells. GVHD did not develop in any of the recipients of memory T cells. In contrast, lethal GVHD developed in almost all recipients of naive and bulk T cells. As a result, almost all memory T-cell recipients survived more than 100 days after transplantation, whereas all naive and almost all bulk T-cell recipients died of GVHD within 80 days of transplantation (Figure 2A). Body weights in some of the memory T-cell recipients were lower (though not statistically significant) than in the recipients of T-cell–depleted bone marrow, but body weights on day 98 were not different from those on day 0 (Figure 2A). Moreover, no histological evidence was found in recipients of memory T cells (Figure 2B; Table 1)These data demonstrate that memory T cells from unprimed mice are unable to induce GVHD in major histocompatibility complex (MHC)–mismatched recipients.
Memory T cells do not cause GVHD. Sorted T-cell subsets were transplanted into lethally irradiated recipients along with T-cell–depleted BM cells. Mice were monitored for the development of GVHD. (A) Survival and weight curves. Body weight data are presented as mean + SD. For survival, P < .05 (memory versus bulk or naive). For body weight, P was not significant (memory versus TCD BM only in all time points after day 7). No signs of GVHD were observed in the single memory T-cell recipient (C57BL/6→C3H/HeJ) that died during observation. Data were pooled from several independent similar experiments (3 for C57BL/6→BALB/c, 1 for BALB/c→C57BL/6, 2 for C57BL/6→C3H/HeJ). (B) Histological analysis (hematoxylin and eosin stain, × 100). Biopsy specimens were obtained from BALB/c recipients of C57BL/6 T cells on day 32 for bulk and naive T-cell groups and day 120 for TCD BM only and memory T-cell groups. (C) Dose-dependent T-cell response in GVHD. Graded numbers of purified C57BL/6 T cells were transplanted into lethally irradiated BALB/c recipients along with T-cell–depleted BM. Mice were monitored for the development of GVHD. For survival, P < .05 (1 × 106 versus other groups). For body weight, P < .05 (TCD BM only versus 1 × 104, 1 × 105, and 1 × 106 at all time points later than day 14 except 1 × 104 on day 70). *P < .05 (1 × 103 versus TCD BM only on days 77, 84, and 98). TCD BM indicates T-cell–depleted BM.
Memory T cells do not cause GVHD. Sorted T-cell subsets were transplanted into lethally irradiated recipients along with T-cell–depleted BM cells. Mice were monitored for the development of GVHD. (A) Survival and weight curves. Body weight data are presented as mean + SD. For survival, P < .05 (memory versus bulk or naive). For body weight, P was not significant (memory versus TCD BM only in all time points after day 7). No signs of GVHD were observed in the single memory T-cell recipient (C57BL/6→C3H/HeJ) that died during observation. Data were pooled from several independent similar experiments (3 for C57BL/6→BALB/c, 1 for BALB/c→C57BL/6, 2 for C57BL/6→C3H/HeJ). (B) Histological analysis (hematoxylin and eosin stain, × 100). Biopsy specimens were obtained from BALB/c recipients of C57BL/6 T cells on day 32 for bulk and naive T-cell groups and day 120 for TCD BM only and memory T-cell groups. (C) Dose-dependent T-cell response in GVHD. Graded numbers of purified C57BL/6 T cells were transplanted into lethally irradiated BALB/c recipients along with T-cell–depleted BM. Mice were monitored for the development of GVHD. For survival, P < .05 (1 × 106 versus other groups). For body weight, P < .05 (TCD BM only versus 1 × 104, 1 × 105, and 1 × 106 at all time points later than day 14 except 1 × 104 on day 70). *P < .05 (1 × 103 versus TCD BM only on days 77, 84, and 98). TCD BM indicates T-cell–depleted BM.
Recipients of memory T cells do not have histological GVHD
| Group . | GVHD scores . | ||
|---|---|---|---|
| Skin . | Liver . | Colon . | |
| TCD BM only, n = 4 | |||
| Mouse 1 | 0.5 | 0.5 | 0 |
| Mouse 2 | 0 | 0.5 | 0 |
| Mouse 3 | 0.5 | 0.5 | 0 |
| Mouse 4 | 0.5 | 0 | 0 |
| Bulk, n = 3 | |||
| Mouse 1 | 5 | 9 | 5 |
| Mouse 2 | 0.5 | 6.5 | 7 |
| Mouse 3 | 0 | 2.5 | 7 |
| Naive, n = 3 | |||
| Mouse 1 | 3.5 | 6.5 | 7 |
| Mouse 2 | 0 | 8 | 7 |
| Mouse 3 | 2 | 1.5 | 8 |
| Memory, n = 4 | |||
| Mouse 1 | 0 | 0.5 | 0 |
| Mouse 2 | 0 | 0.5 | 0 |
| Mouse 3 | 0.5 | 0.5 | 0 |
| Mouse 4 | 0.5 | 0 | 0 |
| Group . | GVHD scores . | ||
|---|---|---|---|
| Skin . | Liver . | Colon . | |
| TCD BM only, n = 4 | |||
| Mouse 1 | 0.5 | 0.5 | 0 |
| Mouse 2 | 0 | 0.5 | 0 |
| Mouse 3 | 0.5 | 0.5 | 0 |
| Mouse 4 | 0.5 | 0 | 0 |
| Bulk, n = 3 | |||
| Mouse 1 | 5 | 9 | 5 |
| Mouse 2 | 0.5 | 6.5 | 7 |
| Mouse 3 | 0 | 2.5 | 7 |
| Naive, n = 3 | |||
| Mouse 1 | 3.5 | 6.5 | 7 |
| Mouse 2 | 0 | 8 | 7 |
| Mouse 3 | 2 | 1.5 | 8 |
| Memory, n = 4 | |||
| Mouse 1 | 0 | 0.5 | 0 |
| Mouse 2 | 0 | 0.5 | 0 |
| Mouse 3 | 0.5 | 0.5 | 0 |
| Mouse 4 | 0.5 | 0 | 0 |
Tissues were harvested from representative mice shown in Figure 2A (C57BL/6→BALB/c). Similar results were observed in 2 other systems (BALB/c→C57BL/6 and C57BL/6→C3H/HeJ). Tissues from recipients of bulk and naive T cells were harvested between days 23 and 36. Tissues from TCD BM only and memory T-cell recipients were harvested after recipients were electively killed on day 120. Shown are individual scores of maximum scores of 12 for each target organ.
TCD BM indicates T-cell-depleted BM.
To quantify the degree of GVHD reduction after depletion of naive T cells, we titrated the cell dose down in the control bulk T-cell group but did not increase the memory T-cell dose because it was technically difficult to sort greater numbers of cells than 1 × 106 cells/mouse for transplantation. As demonstrated in Figure 2C, purified bulk T cells induced GVHD in a dose-dependent manner. As expected, lethal GVHD developed in all recipients of 1 × 106 T cells, and they died within 1 month of transplantation. GVHD developed in 4 of 5 mice in the 1 × 105 T cell group, and 1 died on day 98. GVHD developed in 3 of 5 of the recipients of 1 × 104 T cells, and 2 of them died within 200 days of transplantation. GVHD developed in 2 of 5 recipients of 1 × 103 T cells, and 1 of them died on day 162. Clinical data were further confirmed by histological examination (data not shown). These data suggest that memory T cells are at least 3-log less potent at inducing GVHD than bulk T cells because GVHD did not develop in any of the memory T-cell recipients receiving 1 × 106 T cells (Figure 2A-B; Table 1).
Memory T cells from KLH-primed donors are unable to induce GVHD
To be certain that the true functional memory T cells are unable to induce GVHD, we repeated the experiments using T cells from KLH-primed donors. Although memory T cells from KLH-primed mice proliferated vigorously against KLH (Figure 3A), proliferation against alloantigens remained low (Figure 3B). In contrast, naive T cells responded vigorously to alloantigens (Figure 3B) but not to KLH (Figure 3A). These data demonstrate that the memory phenotype T cells used in the experiments were true functional memory T cells. We next tested their ability to induce GVHD in vivo. Similar to those from unprimed donors (Figure 2A-B), memory T cells from KLH-primed donors were unable to induce GVHD (Figure 3C-D). In contrast, naive and bulk T cells from KLH-primed donors induced lethal GVHD (Figure 3C-D). Histological analyses were consistent with the clinical observations (data not shown), indicating that functional memory T cells from non–alloantigen-primed animals were unable to induce GVHD.
Memory T cells from KLH-primed donors do not cause GVHD. Donor C57BL/6 mice were first immunized with KLH antigens. More than 4 weeks later, T cells were obtained from these mice and were sorted into different T-cell subsets. Responses against KLH antigens and alloantigens were evaluated by in vitro proliferation assays and in vivo GVHD assay (C57BL/6→BALB/c). Proliferation was detected by 3H-thymidine incorporation after 5 days in culture. Proliferation assays have been repeated twice with similar results. GVHD data were from a single experiment. (A) Proliferation against KLH antigens. Results represent mean ± SD of triplicate wells. (B) Proliferation against alloantigens. Results represent mean ± SD of triplicate wells. (C) Survival in GVHD assay. P < .05 (memory versus bulk or naive). (D) Weight curve in GVHD assay. Data are represented as mean + SD. P < .05 (memory versus other groups after day 21). TCD BM indicates T-cell–depleted BM. KLH indicates keyhole limpet hemocyanin. *P < .05, memory versus other groups; #P < .05, naive versus bulk.
Memory T cells from KLH-primed donors do not cause GVHD. Donor C57BL/6 mice were first immunized with KLH antigens. More than 4 weeks later, T cells were obtained from these mice and were sorted into different T-cell subsets. Responses against KLH antigens and alloantigens were evaluated by in vitro proliferation assays and in vivo GVHD assay (C57BL/6→BALB/c). Proliferation was detected by 3H-thymidine incorporation after 5 days in culture. Proliferation assays have been repeated twice with similar results. GVHD data were from a single experiment. (A) Proliferation against KLH antigens. Results represent mean ± SD of triplicate wells. (B) Proliferation against alloantigens. Results represent mean ± SD of triplicate wells. (C) Survival in GVHD assay. P < .05 (memory versus bulk or naive). (D) Weight curve in GVHD assay. Data are represented as mean + SD. P < .05 (memory versus other groups after day 21). TCD BM indicates T-cell–depleted BM. KLH indicates keyhole limpet hemocyanin. *P < .05, memory versus other groups; #P < .05, naive versus bulk.
Early alloresponses mediated by memory T cells from unprimed mice
Our next question was why memory T cells lack the ability to induce GVHD. Although proliferative responses in the memory T-cell group were very low (Figure 1C), we observed some counts in the standard optimal 5-day MLR assays (eg, 11 364 cpm for 2.5 × 105/well in the C57BL/6–anti-BALB/c model). Moreover, microscopy revealed that memory T cells did expand initially on challenge with alloantigens. However, when observed on day 5 after the initiation of culture, the colonies were smaller and most of the cells appeared to be dying. These observations prompted us to study the kinetics of the proliferative response mediated by memory T cells. We performed MLR assays again and harvested the cells at various time points after the initiation of culture. In C57BL/6–anti-BALB/c mice, the proliferative responses mediated by memory T cells were close to those mediated by naive and bulk T cells when cultured for 2 and 3 days (Figure 4A). In BALB/c-anti–C57BL/6 mice, the proliferative responses mediated by memory T cells were even higher than those mediated by naive T cells when cultured for 2, 3, and 4 days (Figure 4A). Similar to the data presented in Figure 1C, the proliferative responses mediated by memory T cells were significantly lower than those mediated by naive and bulk T cells when measured after 5 days of culture (Figure 4A), the peak proliferation time for bulk T cells (data not shown). Because memory T cells have similar ability to proliferate in the first several days, we considered whether memory T cells were able to secrete IL-2 in response to alloantigens because IL-2 production is an important event in T-cell activation and expansion.25 As illustrated in Figure 4B, the frequencies of alloantigen-specific IL-2 production cells in memory T cells were similar to those in naive T cells (128 vs 147 for BALB/c stimulators, 109 vs 150 for C3H/HeJ stimulators) and were not different from those in bulk T cells. Taken together, these data strongly suggest that memory T cells are able to respond initially but fail to reach their full potential on challenge with alloantigens.
Early alloresponses mediated by memory T cells from unprimed mice. Sorted T-cell subsets were cultured with irradiated allogeneic stimulators. All experiments were repeated at least twice and yielded similar results. Results represent mean + SD of triplicate wells. (A) Kinetics of proliferative response. Cells were harvested at different times after the initiation of culture. Proliferation was determined by 3H-thymidine incorporation. Proliferation rates in the syngeneic controls were less than 10%, 5%, and 1% of the experimental groups for 2-, 3-, and 4-day cultures, respectively. (B) IL-2 production. Frequencies of IL-2–producing cells were determined by ELISPOT assay. IL-2 indicates interleukin-2; ELISpot, enzyme-linked immunosorbent spot assay. *P < .05, memory versus other groups; #P < .05, memory versus naive.
Early alloresponses mediated by memory T cells from unprimed mice. Sorted T-cell subsets were cultured with irradiated allogeneic stimulators. All experiments were repeated at least twice and yielded similar results. Results represent mean + SD of triplicate wells. (A) Kinetics of proliferative response. Cells were harvested at different times after the initiation of culture. Proliferation was determined by 3H-thymidine incorporation. Proliferation rates in the syngeneic controls were less than 10%, 5%, and 1% of the experimental groups for 2-, 3-, and 4-day cultures, respectively. (B) IL-2 production. Frequencies of IL-2–producing cells were determined by ELISPOT assay. IL-2 indicates interleukin-2; ELISpot, enzyme-linked immunosorbent spot assay. *P < .05, memory versus other groups; #P < .05, memory versus naive.
Purified central memory T cells are unable to induce GVHD
The inability of TEM to induce GVHD was thought to result from their inability to home to secondary lymphoid organs, such as lymph nodes and Peyer patches.24,26 Moreover, it was demonstrated that CD8+ TEM had a decreased ability to mediate protective immunity against viruses compared with CD8+ TCM.14 Thus, it was of interest to test whether TCM cells, which have the capacity to home to secondary lymphoid organs26 and are more efficient in eliciting protective immunity,14 could induce GVHD. Data presented in Figure 2A-B demonstrate that 1 × 106 total memory T cells, which contain approximately 20% (2 × 105) TCM and 80% (8 × 105) TEM (Figure 1A-B), do not cause GVHD. These data strongly suggest that TCM cells also lack the ability to induce GVHD because as few as 1 × 103 bulk T cells are able to induce GVHD in this model (Figure 2C). To determine the role of TCM in GVHD more definitively, we isolated TCM cells by cell sorting (Figure 1A-B) and tested their ability to induce GVHD in vivo. C57BL/6 splenic TCM cells (1 × 106/mouse) were transplanted into lethally irradiated BALB/c recipients along with T-cell–depleted bone marrow. Similar to T-cell–depleted bone marrow alone and TEM recipients, TCM recipients did not develop GVHD, and all the mice in this group survived more than 100 days after transplantation (Figure 5A-B). In contrast, all naive T-cell recipients developed lethal GVHD and died within 40 days of transplantation (Figure 5A-B). These clinical observations were further confirmed by histological analyses (data not shown). Our data definitively demonstrated that TCM cells are unable to induce GVHD, as has been found with TEM cells8 .
Purified central memory T cells do not cause GVHD. Sorted T-cell subsets were transplanted into lethally irradiated recipients, along with T-cell–depleted BM cells. Mice were monitored for the development of GVHD. Data were pooled from 3 independent experiments. (A) Survival. P < .05, TCM or TEM versus naive. (B) Weight curve. P not significant, TCD BM only versus TEM or TCM.
Purified central memory T cells do not cause GVHD. Sorted T-cell subsets were transplanted into lethally irradiated recipients, along with T-cell–depleted BM cells. Mice were monitored for the development of GVHD. Data were pooled from 3 independent experiments. (A) Survival. P < .05, TCM or TEM versus naive. (B) Weight curve. P not significant, TCD BM only versus TEM or TCM.
CD4+ memory T cells are unable to induce GVHD
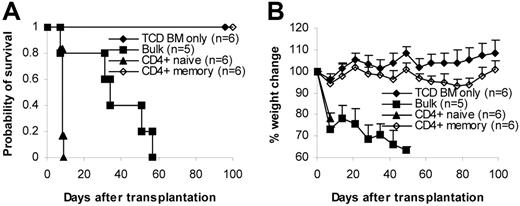
Although proliferation mediated by memory T cells was low, it was detectable in 5-day culture (Figure 1C). However, CTL activity was completely undetectable (Figure 1D). These observations might suggest that the lack of capability of memory T cells to induce GVHD was the result of defective CTLs. To exclude this possibility, we tested the effect of purified CD4+ memory T cells on GVHD. CD8+ CTL cells were not involved. We isolated CD4+ memory T cells by cell sorting and tested them in the C57BL/6→BALB/c model. Sorted CD4+ memory T cells (1 × 106/mouse) were transplanted into lethally irradiated recipients together with T-cell–depleted bone marrow. As demonstrated in Figure 6, lethal GVHD developed in all recipients of CD4+ naive and bulk T cells, and they died shortly after transplantation (naive, less than 10 days; bulk, less than 60 days). In contrast, lethal GVHD did not develop in any of the recipients of memory T cells; all survived more than 100 days after transplantation. Body weights in CD4+ recipients of memory T cells were significantly lower than in recipients of TCD BM only, suggesting that the former might have mild GVHD. However, these mice did not have any other signs of GVHD. Moreover, the body weights of CD4+ memory T cell recipients were the same as baseline (Figure 6B, day 0). The difference in the body weights was in fact a result of increased body weights in the TCD BM group (Figure 6B). In addition, we performed detailed histological analyses on these mice and did not find any histological evidence of GVHD (Table 2), indicating that CD4+ memory T cells alone were unable to induce GVHD and suggesting that the lack of ability of memory T cells to induce GVHD was not a result of defective CTLs.
CD4+ memory T cells do not cause GVHD. Sorted CD4+ T-cell subsets were transplanted into lethally irradiated recipients along with T-cell–depleted BM cells. Mice were monitored for the development of GVHD. (A) Survival. P < .05, CD4+ memory versus bulk or CD4+ naive; P < .05, bulk versus CD4+ naive. (B) Weight curve. P < .05, CD4+ memory versus TCD BM only after day 42.
CD4+ memory T cells do not cause GVHD. Sorted CD4+ T-cell subsets were transplanted into lethally irradiated recipients along with T-cell–depleted BM cells. Mice were monitored for the development of GVHD. (A) Survival. P < .05, CD4+ memory versus bulk or CD4+ naive; P < .05, bulk versus CD4+ naive. (B) Weight curve. P < .05, CD4+ memory versus TCD BM only after day 42.
Recipients of CD4+ memory T cells do not have histological GVHD
| Group . | GVHD scores . | ||
|---|---|---|---|
| Skin . | Liver . | Colon . | |
| TCD BM only, n = 5 | |||
| Mouse 1 | 0 | 0 | 0 |
| Mouse 2 | 0 | 0.5 | 0 |
| Mouse 3 | 0 | 0.5 | 0 |
| Mouse 4 | 0.5 | 0 | 0 |
| Mouse 5 | 0 | 0 | 0 |
| CD4+ naive, n = 5 | |||
| Mouse 1 | 0.5 | 8 | 7 |
| Mouse 2 | 0.5 | 8 | 8 |
| Mouse 3 | 1 | 6 | ND |
| Mouse 4 | 1 | 6 | ND |
| Mouse 5 | 0 | 6 | 8 |
| CD4+ memory, n = 5 | |||
| Mouse 1 | 0.5 | 0 | 0 |
| Mouse 2 | 0 | 0.5 | 0 |
| Mouse 3 | 0 | 0 | 0 |
| Mouse 4 | 0.5 | 0 | 0.5 |
| Mouse 5 | ND | 0 | 0 |
| Group . | GVHD scores . | ||
|---|---|---|---|
| Skin . | Liver . | Colon . | |
| TCD BM only, n = 5 | |||
| Mouse 1 | 0 | 0 | 0 |
| Mouse 2 | 0 | 0.5 | 0 |
| Mouse 3 | 0 | 0.5 | 0 |
| Mouse 4 | 0.5 | 0 | 0 |
| Mouse 5 | 0 | 0 | 0 |
| CD4+ naive, n = 5 | |||
| Mouse 1 | 0.5 | 8 | 7 |
| Mouse 2 | 0.5 | 8 | 8 |
| Mouse 3 | 1 | 6 | ND |
| Mouse 4 | 1 | 6 | ND |
| Mouse 5 | 0 | 6 | 8 |
| CD4+ memory, n = 5 | |||
| Mouse 1 | 0.5 | 0 | 0 |
| Mouse 2 | 0 | 0.5 | 0 |
| Mouse 3 | 0 | 0 | 0 |
| Mouse 4 | 0.5 | 0 | 0.5 |
| Mouse 5 | ND | 0 | 0 |
Tissues were harvested from representative mice shown in Figure 6. Tissues from recipients of CD4+ naive T cells were harvested between day 8 and day 9. Tissues from recipients of TCD BM only and memory T cells were harvested after recipients were electively killed on day 182. Shown are individual scores of maximum scores of 12 for each target organ.
TCD BM indicates T-cell-depleted BM; ND, not determined.
Regulatory T cells are not responsible for the low alloresponse mediated by memory T cells
Memory T cells from KLH-primed animals were able to proliferate against KLH (Figure 3A), making it unlikely that regulatory T cells, especially CD62L-expressing CD4+CD25+ cells,27,28 were responsible for the low alloresponse mediated by the memory T-cell population (Figures 1,Figure 2,Figure 3,Figure 4,Figure 5–6). To exclude this possibility, we performed several experiments. First, we examined whether CD4+CD25+ cells were enriched in memory T-cell subsets. As illustrated in Figure 1A-B, the percentages of CD4+CD25+ cells among total T cells (5% vs 5%-10%) and among CD4+ T cells (6% vs 6%-18%; data not shown) were only slightly increased in all memory T-cell subsets. Second, we depleted CD25+ cells from the memory T-cell fraction (Figure 7A-B) and tested their ability to respond to alloantigens through in vitro proliferation and in vivo GVHD assays. As demonstrated in Figure 7C, CD25− TEM and TCM responded poorly to alloantigens. CD25− TCM cells were defective in causing GVHD (Figure 7D), as were TCM cells (Figure 5). We did not study CD25− TEM (CD62L−) in the in vivo assays because it is known that CD62L− regulatory T cells have limited ability to inhibit GVHD27,28 and that CD4+CD25+ regulatory T cells do not account for the inability of CD4+ TEM cells to induce GVHD.10 Data from these experiments indicated that CD4+CD25+ regulatory T cells were not responsible for the memory T-cell–mediated low alloresponse. Finally, we performed a coculture experiment to exclude the involvement not only of CD4+CD25+ but also of other putative subsets of regulatory T cells. We added sorted memory T cells from C57BL/6 mice to a mixed lymphocyte culture consisting of 1.25 × 105 C57BL/6 T cells and 5 × 105 irradiated BALB/c spleen cell stimulators. As demonstrated in Figure 7E, memory T cells could not inhibit the alloresponses, indicating that regulatory T cell do not play a major role in low alloresponse mediated by memory T cells.
Regulatory T cells are not responsible for low alloresponse mediated by memory phenotype T cells. (A) Sorting strategy for CD25− memory T cells. We first gated on CD25− T cells. The rest of the sorting strategies for naive and memory phenotype T cells are the same as those described in Figure 1A. Shown in the third column are cells from region A in the second column. Numbers represent percentages of parent gates. (B) Phenotypes of sorted T-cell subsets. Shown in the third column are cells from region A in the second column. (C) Mixed lymphocyte reaction (C57BL/6–anti-BALB/c). Proliferation was determined by 3H-thymidine incorporation. Results represent mean ± SD of triplicate wells. Data are representative of 2 experiments with similar results. *P < .01, memory versus other groups; #P < .01, naive versus bulk. (D) In vivo GVHD (C57BL/6→BALB/c) assay. Sorted T-cell subsets were transplanted into lethally irradiated recipients along with T-cell–depleted BM cells. Mice were monitored for the development of GVHD. Data were pooled from 2 independent experiments. For survival, P < .05 (TCM versus naive). For body weight, P was not significant for TCD BM only versus TCM. (E) Coculture assay. T cells with putative regulatory activity from C57BL/6 mice were added to the 5-day mixed lymphocyte cultures (C57BL/6–anti-BALB/c). Cocultured cells were added to the responder cells at 2 different ratios. Proliferation was determined by 3H-thymidine incorporation. Results represent mean ± SD of triplicate wells. Data are representative of 3 experiments with similar results. *P < .05, bulk versus medium.
Regulatory T cells are not responsible for low alloresponse mediated by memory phenotype T cells. (A) Sorting strategy for CD25− memory T cells. We first gated on CD25− T cells. The rest of the sorting strategies for naive and memory phenotype T cells are the same as those described in Figure 1A. Shown in the third column are cells from region A in the second column. Numbers represent percentages of parent gates. (B) Phenotypes of sorted T-cell subsets. Shown in the third column are cells from region A in the second column. (C) Mixed lymphocyte reaction (C57BL/6–anti-BALB/c). Proliferation was determined by 3H-thymidine incorporation. Results represent mean ± SD of triplicate wells. Data are representative of 2 experiments with similar results. *P < .01, memory versus other groups; #P < .01, naive versus bulk. (D) In vivo GVHD (C57BL/6→BALB/c) assay. Sorted T-cell subsets were transplanted into lethally irradiated recipients along with T-cell–depleted BM cells. Mice were monitored for the development of GVHD. Data were pooled from 2 independent experiments. For survival, P < .05 (TCM versus naive). For body weight, P was not significant for TCD BM only versus TCM. (E) Coculture assay. T cells with putative regulatory activity from C57BL/6 mice were added to the 5-day mixed lymphocyte cultures (C57BL/6–anti-BALB/c). Cocultured cells were added to the responder cells at 2 different ratios. Proliferation was determined by 3H-thymidine incorporation. Results represent mean ± SD of triplicate wells. Data are representative of 3 experiments with similar results. *P < .05, bulk versus medium.
Discussion
Several groups, including our own, have independently demonstrated that TEM cells are unable to induce GVHD in different animal models.8-12 The first goal of the current study was to determine whether the observations on TEM could be extended to all memory T cells. Our in vivo data clearly demonstrated that the GVHD-inducing potential was exclusively contained in naive phenotype T cells and that memory phenotype T cells did not cause GVHD in unprimed (Figure 2A-B; Table 1) and non–alloantigen-primed mice (Figure 3). Moreover, sorted TCM (Figure 5) and CD4+ memory T (Figure 6; Table 2) cells were unable to induce GVHD. Because it is technically difficult to sort more cells than 1 × 106 cells/mouse for transplantation, we titrated the cell dose down in the control bulk T-cell group rather than increase the cell dose of memory T cells to quantify the degree of GVHD reduction. Titration data (Figure 2C) demonstrated that the ability of memory T cells to induce GVHD is at least 3-log decreased because as few as 1 × 103 bulk T cells were able to induce GVHD whereas as many as 1 × 106 memory T cells were unable to cause GVHD (Figure 2A-B; Table 1). Such dramatic decrease in the ability to induce GVHD may allow the transfer of higher number of T cells than is currently possible, leading to stronger immune effects. Thus, this strategy may have a positive impact on the safety and broader application of allogeneic stem cell transplantation.
Another major goal of the current study was to understand why memory T cells are unable to induce GVHD. Our working hypothesis was that memory T cells from unprimed and non–alloantigen-primed animals do not contain alloantigen-specific T cells and, therefore, are unable to induce GVHD. However, even if our hypothesis is correct, the question remains: why is it that non–alloantigen-specific memory T cells, which likely contain T cells cross-reactive with MHC antigens,29 do not induce GVHD in MHC-mismatched recipients? Low responses mediated by memory T cells in 5-day MLR and CTL assays (Figure 1) seem to corroborate our hypothesis. However, when cultured for shorter periods of time, the responses mediated by memory T cells in response to alloantigens were not different from those mediated by naive and bulk T cells (Figure 4). These data indicated that memory T cells are able to respond to alloantigens initially but are unable to maintain the proliferative response. These data further suggest that unprimed memory T cells do contain “alloreactive” T cells defined by in vitro proliferation. However, these cells do not appear to be alloantigen specific because the responses mediated by these cells are different from those mediated by true alloantigen-specific memory T cells30 (Figure 4). Even though we do not fully understand the mechanism(s), we speculate that these are non–allospecific memory T cells cross-reacting with alloantigens at a level that does not lead to full activation. If they are reflecting non–allospecific memory T cells cross-reacting with alloantigens, these observations may explain why the proliferative alloresponses mediated by unprimed memory T cells are so different between different models24,31 (Figure 4A) because different cross-reactive T cells are likely involved in different systems. Thus, the inability of memory T cells from unprimed donors to maintain the responses on challenge with alloantigens (Figure 4) may explain why they are unable to induce GVHD.
It was proposed that the lack of capability of TEM cells to induce GVHD is the result of their inability to home to lymph nodes and Peyer patches.24 Our data using purified TCM cells (Figure 5), which are able to home to lymph nodes and Peyer patches,26 suggest that other mechanisms are involved. In fact, defects in homing cannot explain why the in vitro responses were dramatically decreased (Figures 1C-D, 7C). It was noted that TCM cells contain fewer CD4+ T cells than other T-cell subsets (Figure 1B), which may contribute to the lack of GVHD in TCM recipients (Figure 5) because CD8+ T cells have less ability to induce GVHD than do CD4+ T cells in this model.32,33 However, the decreased percentage of CD4+ T cells cannot completely explain why TCM recipients are free of GVHD (Figure 5) because TCM recipients do contain sufficient numbers of CD4+ T cells, which have been demonstrated to be able to induce lethal GVHD in this model33 (Figure 2C). CTLs play a critical role in tissue damage in GVHD.34 Decreased CTL activity in memory T cells (Figure 1D) may result in reduced GVHD. However, our data, presented in Figure 6 and Table 2, strongly suggest that defective CTLs did not play a pivotal role because purified CD4+ memory T cells, which do not contain CD8+ CTLs, were also unable to induce GVHD. Lack of GVHD could be attributed to increased regulatory T-cell activity in the graft.35 However, memory T cells depleted of CD4+CD25+ regulatory T cells remained low responsive against alloantigens (Figure 7C-D). Moreover, the coculture assay failed to detect any regulatory activity in memory T cells (Figure 7E). These data indicate that regulatory T cells are not responsible for the inability of memory phenotype T cells to induce GVHD.
In conclusion, we have demonstrated that all memory T cells, including TEM and TCM, are unable to induce GVHD and that GVHD-inducing T cells exclusively express the naive phenotype in unprimed donors. The lack of capability of memory T cells from unprimed donors to induce GVHD may be a result of “abortive” alloresponses mediated by non–alloantigen-specific memory T cells. Regulatory T cells do not play a major role in this process. This novel approach may allow the transfer of recall T-cell immunity without causing GVHD. The better understanding of memory T-cell responses in GVHD will facilitate the clinical translation of this approach to improve the safety, broaden the scope, and enhance the effectiveness of allogeneic stem cell transplantation.
Authorship
Contribution: B.J.C. designed the research, performed the research, analyzed the data, and wrote the paper. D.D. performed the research. X.C. designed the research, performed the research, and analyzed the data. N.T.L. performed the research and analyzed the data. J.S. designed the research, performed the research, and analyzed the data. J.W.F. designed the research, performed the research, and analyzed the data. N.J.C. provided advice on experimental design and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Benny J. Chen, Division of Cellular Therapy/BMT, Department of Medicine, Duke University Medical Center, Box 3289, 247A Carl Bldg, Durham, NC 27710; e-mail: chen0032@mc.duke.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health grants P01-HL67314 and P01-CA47741.
We thank Patti McDermott and Danielle King from the Duke Human Vaccine Institute Flow Cytometry Core Facility (supported by National Institute of Health grant AI-051445) for cell sorting and Dr Gwynn Long for critical reading of the manuscript.

![Figure 1. Proliferation and cytotoxicity. Sorted T-cell subsets were cultured with irradiated allogeneic spleen cells for 5 days before they were tested for proliferation and cytotoxicity. Results represent mean ± SD of triplicate wells. (A) Sorting strategy. Enriched T cells were stained with anti-CD4, anti-CD8, anti-CD44, anti-CD45RB, and anti-CD62L antibodies. We first gated on CD4/CD8+ T cells. CD45RB+CD62L+ T cells (region A) were further separated into 2 populations based on the expression of CD44 (A1, CD45RB+CD62L+CD44−/low; A2, CD45RB+CD62L+CD44high). Cells were sorted into naive (A1) and memory (all other T cells except naive [B+C+A2]) phenotype T cells. In some experiments (Figure 5), memory (total) T cells were further separated into TEM (region B) and TCM (C+A2) based on the expression of CD62L. Shown in the third column are cells from region A in the second column. Numbers represent percentages of parent gates. Donor mice for this sort were 4 to 6 months old. (B) Phenotypes of sorted T-cell subsets. Shown in the second column are cells from region A in the first column. Data presented in the last 2 columns were not from post-sort analyses. Logarithmic displays were used in all histograms or dot plots except those in the last column in which “logicle” displays (biexponential displays) were used. These data represent the results of at least 4 similar experiments. (C) Mixed lymphocyte reaction. Proliferation was determined by 3H-thymidine incorporation. The responder alone was always lower than 200 cpm. Data represent the results of 3 to 4 similar experiments. (D) Cytotoxic T-lymphocyte assay. Cytotoxicity was measured by 4-hour 51Cr release assay. *P < .01, memory versus other groups; #P < .01, naive versus bulk.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/7/10.1182_blood-2006-04-016410/4/m_zh80070710490001.jpeg?Expires=1767736542&Signature=u7ENYL7QR-hf68xxPZ2gb5RZL4~fXW5JPz2rt7npdtmYdgoDqAUtzlQD9mfSkBF~pFQqJUOK9PgfUE613XsefcQigif7m-lE76yOcnmz2stFt-vz1fqLsChLzuKro7EPkGtZ9z19c6ezRXVQQKfoCCvScUYKOQXiZ8wtB~Ausipfr6FvUqnv0NMMaDQpAg01atEZI9m65AzO6Oh7KE5PBqihNIJ~XuXAIK0oUu~hz~2GkYopu3nX7mQlTZIXAg1PvIjxxHjhj5Sg2R4Oxp-l0F9N-wc890WtFHS-0C6n3dOShgEb9iidkfNiYSFbn8XgrH1PMfJwoVB7kWdAamR3rQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal