Abstract
Bone homeostasis is regulated by a delicate balance between osteoblastic bone formation and osteoclastic bone resorption. Osteoclastogenesis is controlled by the ratio of receptor activator of NF-κB ligand (RANKL) relative to its decoy receptor, osteoprotegerin (OPG). The source of OPG has historically been attributed to osteoblasts (OBs). While activated lymphocytes play established roles in pathological bone destruction, no role for lymphocytes in basal bone homeostasis in vivo has been described. Using immunomagnetic isolation of bone marrow (BM) B cells and B-cell precursor populations and quantitation of their OPG production by enzyme-linked immunosorbent assay (ELISA) and real-time reverse transcriptase–polymerase chain reaction (RT-PCR), cells of the B lineage were found to be responsible for 64% of total BM OPG production, with 45% derived from mature B cells. Consistently B-cell knockout (KO) mice were found to be osteoporotic and deficient in BM OPG, phenomena rescued by B-cell reconstitution. Furthermore, T cells, through CD40 ligand (CD40L) to CD40 costimulation, promote OPG production by B cells in vivo. Consequently, T-cell–deficient nude mice, CD40 KO mice, and CD40L KO mice display osteoporosis and diminished BM OPG production. Our data suggest that lymphocytes are essential stabilizers of basal bone turnover and critical regulators of peak bone mass in vivo.
Introduction
Bone homeostasis is maintained by a delicate balance between bone-forming cells, the osteoblasts (OBs), and bone-resorbing cells, the osteoclasts (OCs). Physiological OC renewal is driven by the exposure of receptor activator of NFκB (RANK)–bearing OC precursors to the key osteoclastogenic cytokine RANK ligand (RANKL). OC formation is negatively regulated by osteoprotegerin (OPG), a soluble decoy receptor of RANKL and a potent physiological inhibitor of osteoclastogenesis.1,2 The physiological importance of OPG is attested to by the fact that overexpression of OPG in mice results in severe osteopetrosis3 while OPG-null mice are osteoporotic.4,5 Interestingly, OPG is not required for embryonic bone formation, because OPG knockout (KO) mice are born with normal bone mass. However, OPG is essential for the maintenance of postnatal bone mass, and decreased bone mineral density (BMD) becomes evident radiographically at 1 month of age in OPG KO mice, increasing progressively with age. Loss of a single OPG allele in the heterozygous mouse results in a slower loss of BMD, achieving statistical significance over a period of several months.4 Although many cell types are capable of producing OPG, the OB and its precursor, the bone marrow (BM) stromal cell, are generally considered the key source in the BM.6–10
Activated T cells may undermine bone homeostasis and stimulate bone destruction under pathological conditions such as estrogen deficiency11–13 and in inflammatory conditions14,15 as they become a significant source of RANKL14 and TNFα.12
Interestingly, T cells are also capable of mediating antiosteoclastogenic signals, as depletion of CD4+ and CD8+ T lymphocytes in mice in vivo enhances vitamin D3–stimulated OC formation in vitro by a mechanism involving decreased OPG production.16 The source of OPG and the mechanism of its regulation are not fully elucidated. In addition, CD8+ T cells have been reported to be antiosteoclastogenic.17,18 Interestingly, we have reported that although BMD is normal or elevated in very young C57BL/6 T-cell–deficient nude mice,12 BMD decreases as the mice age.19 This suggests that T cells may play a protective role in postembryonic basal bone modeling; however, the mechanisms of OC repression by T cells in vivo remain to be elucidated.
Like T cells, the role of B cells in bone turnover is poorly understood. B cells and B-cell–derived plasma cells in multiple myeloma have been reported to have the potential to support osteoclastogenesis, possibly via direct expression of RANKL18,20,21 or as an indirect consequence of IL-7 secretion,22,23 a potent stimulator of bone resorption in vivo.19,24
B lymphopoiesis is stimulated during estrogen deficiency25 while estrogen treatment down-regulates B lymphopoiesis but up-regulates immunoglobulin production.26 B-lineage cells have consequently been suggested to play a role in ovariectomy-induced bone loss.24 B-cell precursors are capable of differentiating into OCs in vitro,19,27 suggesting that estrogen deficiency may expand the reservoir of OC precursors.
In contrast, Pax5 KO mice, which have a developmental arrest in the B-cell lineage at the pro-B-cell stage, are severely osteopenic, although osteopenia has been attributed directly to Pax5 deficiency rather than being a consequence of B-cell depletion.28
We previously reported that peripheral blood B cells inhibit OC formation in a human in vitro model of osteoclastogenesis, in part through secretion of TGFβ,29 a cytokine that induces apoptosis of OCs29–31 and that is reported to stimulate OPG production.32 Depletion of B cells in vivo also aggravates bone loss in an animal model of periodontitis, suggesting that B cells may act to limit bone resorption under certain pathological conditions.33
Furthermore, B-cell to T-cell crosstalk may regulate B-cell production of bone-active cytokines, because B cells suppress osteoclastogenesis when activated by Th1 cytokines while promoting osteoclastogenesis when stimulated with Th2 cytokines.34 In vitro ligation of the costimulatory molecule CD40 on human tonsil-derived B cells with an activating antibody is reported to stimulate B-cell OPG production.35 Physiologically CD40 interacts with its cognate ligand, CD40 ligand (CD40L), a molecule expressed on activated T cells during antigen presentation by antigen-presenting cells (APCs) such as B cells, macrophages, and dendritic cells36 and acts in priming of naive CD8+ cells.37 Whether T-cell to B-cell crosstalk contributes to regulate basal bone turnover in vivo is presently unknown.
In this study we have investigated the involvement of both T cells and B cells in the process of basal bone turnover. We provide novel data showing that in addition to the well-documented roles of lymphocytes in bone destruction under pathological conditions, both T and B cells cooperate to play a critical role in limiting basal bone resorption in vivo. This protective effect is centered on a mechanism involving the production of OPG by B-lineage cells, and augmented by T cells, via CD40/CD40L costimulation.
Materials and methods
All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University. All reagents were purchased from the Sigma Chemical (St Louis, MO) unless otherwise indicated.
Mice
B-cell–deficient mice (B6.129S2-Igh-6tm1Cgn/J), nude mice (B6.Cg-Foxn1nu/J), CD40 KO mice (B6.129P2-Tnfrsf5tm1Kik/J), and CD40L KO mice (B6.129S2-Tnfsf5tm1Imx/J) were purchased from Jackson Laboratories (Bar Harbor, ME) and housed under sterile conditions. All stains were on the C57BL/6 background, and age- and genetically matched C57BL/6 wild-type (WT) mice were used as controls.
Bone mineral density
In vivo BMD measurements of total body, lumbar spine, and femurs (left and right femurs were averaged for each mouse) were made by dual-energy x-ray absorptiometry (DXA) using a PIXImus2 bone densitometer (GE Medical Systems, Piscataway, NJ) as previously described.11
Microcomputed tomography
Microcomputed tomography (μCT) was performed using a μCT 40 scanner (Scanco Medical, Bassersdorf, Switzerland) as previously described.38 Bones were scanned at a resolution of 10 μm. Fifty slices were taken covering a total length of 500 μm. Cortical bone was measured at the mid diathesis by taking 80 slices at a resolution of 12 μm. For each group, 1 high-resolution (6 μm) 3D reconstruction was generated for visual representation.
Biochemical indices of bone resorption
C-terminal telopeptide of collagen (CTx) was measured in mouse serum, following an overnight fast, by rodent-specific enzyme-linked immunosorbent assays (ELISAs), RatLaps (Nordic Bioscience Diagnostics, Herlev, Denmark).
Cell purification and RANKL and OPG ELISAs
BM was extracted from long bones by insertion into a 0.5 mL centrifuge tube with a hole pierced into the bottom with an 18 gauge needle and the tube inserted into a 1.5 mL centrifuge tube and spun at 13 500g for 60 seconds. Despite their strong adherence to bone surfaces, this procedure recovers 95% of OBs and stromal cells, as verified by quantitative bone histomorphometry. Recovered BM was gently resuspended in 15 vol red-cell lysis buffer (1 mM EDTA; 100 mM KHCO3, 1.7 M NH4Cl [pH 7.3]) for 10 minutes at room temperature followed by 2 washes with PBS.
B-lineage cells were systematically purified from BM at different stages of differentiation based on the expression of specific makers. IgD+ mature B cells were isolated using biotinylated IgD antibody and streptavidin-coupled immunomagnetic beads (Miltenyi Biotec, Auburn, CA). IgM+ (immature) B cells were isolated using IgM-conjugated immunomagnetic beads following IgD depletion. The IgM antibody used is nonactivating. B-cell precursor cells (pre-proB, proB, large preB, and small preB) were purified using B220-coated immunomagnetic beads following IgM depletion. Plasma cells were isolated by CD138-conjugated immunomagnetic beads following B220 depletion using a CD138+ Plasma Cell Isolation Kit (Miltenyi Biotec).
Immunomagnetic bead isolation led to populations that were 94% pure, while depletion was 96% efficient as assessed by flow cytometry.
Conditioned media were generated by plating 1 × 107 purified B-lineage cells into 48-well plates in 1 mL per well of RPMI 1640 supplemented with 10% FBS and antibiotics. After 48 hours media were collected for analysis of OPG or RANKL by mouse-specific ELISAs (R&D Systems, Minneapolis, MN).
B-cell adoptive transfer
B cells were immunomagnetically purified from the spleens of WT mice, as described above, and adoptively transferred into 4-week-old μMT/μMT B-cell KO mice at a concentration of 2 × 106 cells per mouse in 100 μL sterile phosphate-buffered saline (PBS). Mice were killed 8 weeks later and whole bone marrow flushed as described above, red cells lysed, and total RNA extracted in Trizol Reagent (Sigma Chemical) for reverse transcriptase–polymerase chain reaction (RT-PCR) analysis as described under “Cell purification and RANKL and OPG ELISAs.” BM reconstitution of B cells was verified at killing by flow cytometry using CD19 antibody conjugated to phycoerythrin (PE).
Real-time RT-PCR
Total RNA was extracted from purified B cells or whole nucleated BM, flushed from long bones as described above, and dissolved in Trizol Reagent (Sigma Chemical). Real-time RT-PCR was performed on an ABI Prism 7300 instrument (Applied Biosystems, Foster City, CA) as described38 using commercial (Applied Biosystems) master mix and primer sets and probes for murine OPG (Mm 00435452), RANKL (Mm 00441908), and β-actin (Mm 00607939). Changes in relative gene expression between KO versus WT were calculated using the 2−ΔΔCT method with normalization to β-actin.39
Statistical analysis
Statistical significance was determined using GraphPad InStat version 3 for Windows XP (GraphPad Software, San Diego, CA). Simple comparisons were made using unpaired 2-tailed Student t test. Multiple comparisons were performed by 1-way analysis of variance (ANOVA) with Tukey-Kramer posttest. P ≤ .05 was considered statistically significant. All data are presented as mean ± SD. All in vitro and ex vivo data are representative of at least 2 independent experiments.
Results
B-cell KO mice are osteopenic, a consequence of elevated bone resorption
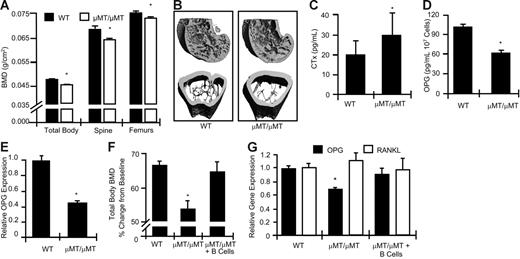
The role of B cells in vivo in the regulation of basal bone turnover is unknown. In C57BL/6 mice peak bone density occurs at around 16 weeks of age,40 a time point at which skeletal modeling is complete and transitioning to remodeling. We thus quantitated BMD at 16 weeks of age in WT and genetically matched μMT/μMT mice, a strain of B-cell KO mouse that produces no mature B cells.41 Quantitation of BMD by DXA, a technique that provides an integrated measurement of both cortical and trabecular bone, revealed (Figure 1A) that μMT/μMT mice have significantly decreased BMD relative to WT mice at the total body, spine, and femurs.
B-cell KO mice display decreased baseline BMD and increase bone resorption, concurrent with decreased BM OPG concentrations. (A) BMD was determined by DXA in μMT/μMT B-cell KO mice (□) and age-matched WT controls (▪) at the total body, spine, and femurs. (B) Longitudinal (top) and cross-sectional (bottom) μCT reconstructions of distal femurs from WT and μMT/μMT B-cell KO mice. (C) Elevated biochemical indices of bone resorption (CTx) were observed in B-cell KO mice. (D) Decreased OPG production was detected in μMT/μMT B-cell KO BM compared with equivalent amounts of WT BM. Mean ± SD of 8 mice per group; *P < .05. (E) Real-time RT-PCR quantitation of OPG mRNA expression in 3 independent WT and B-cell KO mice. *P < .01. (F) B-cell reconstitution of μMT/μMT B-cell KO rescues BMD (*P < .01 versus WT) and (G) OPG production (*P < .05 versus WT). Mice were reconstituted at 4 weeks of age and BMD and RT-PCR studies preformed at 12 weeks of age.
B-cell KO mice display decreased baseline BMD and increase bone resorption, concurrent with decreased BM OPG concentrations. (A) BMD was determined by DXA in μMT/μMT B-cell KO mice (□) and age-matched WT controls (▪) at the total body, spine, and femurs. (B) Longitudinal (top) and cross-sectional (bottom) μCT reconstructions of distal femurs from WT and μMT/μMT B-cell KO mice. (C) Elevated biochemical indices of bone resorption (CTx) were observed in B-cell KO mice. (D) Decreased OPG production was detected in μMT/μMT B-cell KO BM compared with equivalent amounts of WT BM. Mean ± SD of 8 mice per group; *P < .05. (E) Real-time RT-PCR quantitation of OPG mRNA expression in 3 independent WT and B-cell KO mice. *P < .01. (F) B-cell reconstitution of μMT/μMT B-cell KO rescues BMD (*P < .01 versus WT) and (G) OPG production (*P < .05 versus WT). Mice were reconstituted at 4 weeks of age and BMD and RT-PCR studies preformed at 12 weeks of age.
We further evaluated the femoral trabecular and cortical bone compartments of WT and μMT/μMT mice by μCT. Longitudinal (Figure 1B, top images) and cross-sectional (Figure 1B, bottom images) high-resolution (6 μm) reconstructions from WT and B-cell KO femurs show severely reduced trabecular bone structure and moderate thinning of the cortices in μMT/μMT B-cell KO mice relative to WT controls. Morphological indices of femoral trabecular and cortical bone structure were quantitated by μCT (Table 1). The data demonstrate a significant decrease in trabecular bone volume per tissue volume (BV/TV). This decrease resulted from reduced BV, because TV was unchanged. Decreased BV in B-cell KO mice resulted from a significant decrease in trabecular thickness (Tb. Th.), trabecular number (Tb. N.), and trabecular connection density (Conn. D.). A corresponding increase in trabecular spacing (Tb. Sp.) is evident. Bone architecture denoted by the structure model index (SMI) was not significantly altered. Cortical thickness (Co. Th.) and cortical volume (Co. Vol.) were also significantly decreased.
Morphological analysis by μCT of femurs from μMT/μMT B-cell KO mice and WT controls
| Morphological parameters . | WT mice . | B-cell KO mice . | % change . | P . |
|---|---|---|---|---|
| TV, mm3 | 1.28 ± 0.06 | 1.29 ± 0.07 | 0.8 | ≤ .685 |
| BV, mm3 | 0.30 ± 0.03 | 0.25 ± 0.28 | −16.7 | ≤ .003* |
| BV/TV, % | 23.96 ± 2.76 | 19.87 ± 2.51 | −17.1 | ≤ .008* |
| Tb. Th., μm | 60.49 ± 2.78 | 56.21 ± 5.09 | −7.1 | ≤ .011* |
| Tb. Sp., μm | 222.23 ± 7.99 | 244.45 ± 13.66 | 10.0 | ≤ .001* |
| Tb. N./mm | 4.54 ± 0.16 | 4.17 ± 0.19 | −8.1 | ≤ .001* |
| SMI | 0.69 ± 0.24 | 0.79 ± 0.21 | 14.5 | ≤ .402 |
| Conn. D./mm3 | 133.48 ± 15.85 | 102.62 ± 18.46 | −23.1 | ≤ .003* |
| Co. Th., μm | 91.1 ± 3.1 | 82.9 ± 8.4 | −9.0 | ≤ .002* |
| Co. Vol., mm3 | 0.47 ± 0.03 | 0.42 ± 0.04 | −10.6 | ≤ .010* |
| Morphological parameters . | WT mice . | B-cell KO mice . | % change . | P . |
|---|---|---|---|---|
| TV, mm3 | 1.28 ± 0.06 | 1.29 ± 0.07 | 0.8 | ≤ .685 |
| BV, mm3 | 0.30 ± 0.03 | 0.25 ± 0.28 | −16.7 | ≤ .003* |
| BV/TV, % | 23.96 ± 2.76 | 19.87 ± 2.51 | −17.1 | ≤ .008* |
| Tb. Th., μm | 60.49 ± 2.78 | 56.21 ± 5.09 | −7.1 | ≤ .011* |
| Tb. Sp., μm | 222.23 ± 7.99 | 244.45 ± 13.66 | 10.0 | ≤ .001* |
| Tb. N./mm | 4.54 ± 0.16 | 4.17 ± 0.19 | −8.1 | ≤ .001* |
| SMI | 0.69 ± 0.24 | 0.79 ± 0.21 | 14.5 | ≤ .402 |
| Conn. D./mm3 | 133.48 ± 15.85 | 102.62 ± 18.46 | −23.1 | ≤ .003* |
| Co. Th., μm | 91.1 ± 3.1 | 82.9 ± 8.4 | −9.0 | ≤ .002* |
| Co. Vol., mm3 | 0.47 ± 0.03 | 0.42 ± 0.04 | −10.6 | ≤ .010* |
Trabecular indices, including TV, BV, BV/TV, Tb. Th., Tb. Sp., Tb. N., SMI, and Conn. D., and the cortical indices Co. Th. and Co. Vol. were computed from μCT scans. The data are presented as the mean ± SD of 8 mice per group. Percentage change between WT and KO mice is indicated as “% change.” Asterisks indicate significantly different results (P ≤ .05).
Quantitation of serum CTx, a specific and sensitive marker of in vivo bone resorption, revealed a significantly elevated rate of bone resorption in B-cell KO mice (Figure 1C).
B cells have been reported to have the ability to make OPG in vitro,35 and a deficit in B-cell–derived OPG could thus account for the elevated rate of bone resorption. We consequently compared BM OPG protein and mRNA levels in WT and B-cell KO mice. Whole BM was extracted from femurs and tibias by centrifugation. Following red-cell lysis, mRNA was isolated from nucleated cells for real-time RT-PCR quantitation, or cells were cultured ex vivo for 48 hours and OPG protein production was quantitated in the conditioned medium by ELISA. Our data revealed a significant deficit (about 40%) in OPG protein concentrations in B-cell KO BM (Figure 1D). We similarly identified a significant reduction (about 55%) in OPG mRNA transcripts in whole BM using real-time RT-PCR (Figure 1E). The data suggest that B cells directly regulate BMD and bone mass in vivo by either secreting OPG or by indirectly regulating its production.
Finally, to demonstrate that B-cell deficiency is the specific defect leading to reduced OPG levels in B-cell KO mice rather than the consequence of an unrelated defect resulting from genetic manipulation, we performed a rescue experiment in which μMT/μMT mice of 4 week of age were reconstituted with B cells by adoptive transfer. BMD was examined 8 weeks after B-cell reconstitution in WT controls, μMT/μMT B-cell–null mice, and B-cell–reconstituted μMT/μMT mice. The data show (Figure 1F) that at 3 months of age μMT/μMT mice exhibit a significant decrease in total body BMD relative to WT mice. By contrast, B-cell reconstitution completely rescued BMD.
Whole BM was extracted from all mice and total mRNA isolated, and real-time RT-PCR was performed for OPG and RANKL. The data show (Figure 1G) that μMT/μMT mice have a significant deficit in OPG; however, B-cell reconstitution completely normalized OPG production. Consistent with our previous studies we observed a significant reduction in OPG levels in B-cell KO mice compared with WT mice. OPG levels were completely normalized by adoptive transfer of B cells back into B-cell KO mice. By contrast, total BM RANKL mRNA was not significantly affected by B-cell depletion, suggesting that B cells are not a significant source of RANKL under basal conditions.
B cells are a major source of OPG in the bone microenvironment
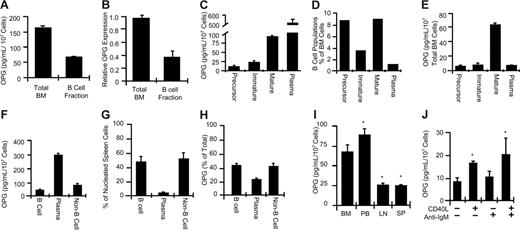
To quantitate the contribution of B cells to BM OPG production under basal conditions, we collected nucleated BM cells from C57BL/6 mice or the B-cell fraction contained within an equivalent amount of BM using an excess of anti-IgM–coated immunomagnetic beads, which specifically isolate the 2 most mature B-cell lineage populations in the BM (immature B cells and mature B cells), without affecting the metabolism thereof. B-cell–replete total nucleated BM and purified B cells isolated from an equivalent portion thereof were used to isolate mRNA for real-time RT-PCR quantitation. Aliquots of total nucleated BM and purified B cells were further cultured ex vivo for 48 hours, and OPG protein secretion was quantitated in the conditioned medium by ELISA. The data show (Figure 2A) that the IgM+ B-cell fraction of whole BM accounts for about 40% of the total OPG produced in the BM under basal conditions. No RANKL secretion by purified B cells was detected (data not shown). Using real-time RT-PCR (Figure 2B) we verified that about 40% of OPG mRNA in BM is B-cell derived. Our data demonstrate that B cells account for a significant proportion of OPG in the BM.
B-lineage cells are a major source of OPG production in the BM and spleen. (A) OPG secretion by total BM white cells or the B-cell fraction contained therein was quantitated by ELISA. Mean ± SD of triplicates. Data are representative of more than 6 independent experiments. (B) Relative OPG transcription was quantitated by RT-PCR in total BM white cells or B-cell fraction contained therein. Data are presented as mean ± SD of 3 independent mice per group. (C) OPG production by equivalent numbers (1 × 107 cells) of purified BM B-cell precursors, immature B cells, mature B cells, and plasma cells. (D) B-lineage cell composition as a percentage of total nucleated BM cells. (E) Actual concentration of OPG secreted by specific B-lineage cells per 107 total nucleated BM cells. The total OPG production was 134 ± 26.1 pg/mL/107 total white BM cells. (F) OPG production by equivalent numbers of mature spleen B cells was quantitated relative to plasma cells and non–B-lineage cells (non-B cell). (G) Spleen B-cell composition as a percentage of total nucleated spleen cells. (H) Percentage spleen OPG production for each cell population in the spleen. Mean ± SD of 2 independent experiments measured in triplicate. *P < .05 relative to unstimulated. (I) Comparison of OPG production by equivalent numbers of mature B cells derived from BM, peripheral blood (PB), lymph nodes (LN), or spleen (SP). BM from 5 independent mice was measured in triplicate. *P < .001 relative to BM (ANOVA). (J) OPG production by mature splenic B cells stimulated in vitro with CD40L (500 ng/mL) and/or anti-IgM activating antibody (5 ng/mL).
B-lineage cells are a major source of OPG production in the BM and spleen. (A) OPG secretion by total BM white cells or the B-cell fraction contained therein was quantitated by ELISA. Mean ± SD of triplicates. Data are representative of more than 6 independent experiments. (B) Relative OPG transcription was quantitated by RT-PCR in total BM white cells or B-cell fraction contained therein. Data are presented as mean ± SD of 3 independent mice per group. (C) OPG production by equivalent numbers (1 × 107 cells) of purified BM B-cell precursors, immature B cells, mature B cells, and plasma cells. (D) B-lineage cell composition as a percentage of total nucleated BM cells. (E) Actual concentration of OPG secreted by specific B-lineage cells per 107 total nucleated BM cells. The total OPG production was 134 ± 26.1 pg/mL/107 total white BM cells. (F) OPG production by equivalent numbers of mature spleen B cells was quantitated relative to plasma cells and non–B-lineage cells (non-B cell). (G) Spleen B-cell composition as a percentage of total nucleated spleen cells. (H) Percentage spleen OPG production for each cell population in the spleen. Mean ± SD of 2 independent experiments measured in triplicate. *P < .05 relative to unstimulated. (I) Comparison of OPG production by equivalent numbers of mature B cells derived from BM, peripheral blood (PB), lymph nodes (LN), or spleen (SP). BM from 5 independent mice was measured in triplicate. *P < .001 relative to BM (ANOVA). (J) OPG production by mature splenic B cells stimulated in vitro with CD40L (500 ng/mL) and/or anti-IgM activating antibody (5 ng/mL).
Among B-lineage cells, plasma cells produce the highest concentrations per cell of OPG, but mature B cells are the major source of B-lineage OPG in the BM
B cells at all stages of development and maturation are found in BM. To access OPG production by the different B-cell populations, we immunomagnetically isolated combined pre-proB, proB, large preB, and small preB B-cell precursors (B220+ IgM− IgD−), immature B cells (B220+ IgM+ IgD−), mature B cells (B220+ IgM+ IgD+), and plasma cells (B220− CD138+). OPG production was quantitated by ELISA in conditioned medium from 1 × 107 purified cells from each population. Our data show (Figure 2C) that plasma cells produced the highest concentrations of OPG of all B-lineage cells (about 500 pg/mL/107 cells), followed by mature B cells (about 80 pg/mL/107 cells). B-cell precursors and immature B cells secreted only low concentrations of OPG (about 10 and 25 pg/mL/107 cells, respectively).
When the percentage of each B-lineage population present in BM was quantitated, we found that early B-cell precursors and mature B cells each represented about 9% of total nucleated BM cells (Figure 1D). Immature B cells constituted about 3.5% while plasma cells represented only about 1.4% of BM white cells. B-lineage cells together accounted for about 23% of the total BM.
When OPG production per 107 cells by each specific B-lineage population was normalized to the proportion of each cell type found in the BM, the data revealed (Figure 2E) that of the 134 ± 26.1 pg/mL/107 cells of OPG produced by total whole BM, 85 pg/mL/107 cells was derived from the B-cell lineage (64%).
Splenic B cells secrete less OPG than BM or peripheral blood B cells, but OPG production can be up-regulated by CD40L stimulation
Because about 50% of the spleen is composed of B cells,42 we further quantitated splenic B-cell OPG production and the contribution of mature B cells and plasma cells to total splenic OPG levels. Spleens from C57BL/6 mice were disaggregated and nucleated cells recovered following red-cell lysis. OPG production was quantitated from an equal quantity (1 × 107) of mature B cells (B220+ CD138+), plasma cells (B220− CD138+), and residual non–B-cell splenocytes (B220− CD138−) purified by immunomagnetic isolation from total nucleated spleen cells. The data revealed (Figure 2F) a 7-fold higher production of OPG by plasma cells relative to mature B cells. When the total number of recovered B cells and plasma cells was quantitated following immunomagnetic isolation, mature B cells were found to constitute 48% of the total recovered nucleated cells in mouse spleen while plasma cells accounted for only 3% (Figure 2G). These numbers are in good agreement with flow cytometric studies of human43 and mouse42 spleen composition. When OPG production by each cell population was normalized to the percentage of each cell type found in the spleen, the data showed (Figure 2H) that the mature B-cell fraction contributed 41% of total splenic OPG. Plasma cells, despite their small number, accounted for an additional 20%.
We further quantitated OPG production by mature B cells isolated from BM, peripheral blood, lymph nodes, and spleen. BM- and peripheral blood–derived B cells produced almost 3 times as much OPG as equivalent numbers of B cells derived from lymph nodes and spleens (Figure 2I).
Because antibody-induced activation of the CD40 costimulatory molecule on human tonsil B cells is reported to stimulate OPG production,35 we next investigated whether murine splenic B cells could be induced to produce higher concentrations of OPG by ligation of CD40 by its physiological ligand, CD40L. Recombinant CD40L was added to purified IgM+ splenic B cells, alone or in combination with B-cell antigen receptor (BCR) cross-linking IgM antibody, a well-established mechanism for activating B cells.44 CD40L induced a 2-fold stimulation of OPG production by splenic B cells (Figure 2J). IgM antibody had no significant stimulatory effect on OPG secretion, either alone or in combination with CD40L. BM B cells were not further up-regulated by CD40L (data not shown), suggesting that B cells in the BM are already maximally stimulated, consistent with Figure 2I.
T-cell–deficient nude mice have decreased basal mass and increased bone resorption
Our data in Figure 2I demonstrate that B-cell activation by CD40L through the costimulatory molecule CD40 up-regulates murine B-cell OPG production. Because the physiological ligand of CD40 in vivo is T-cell–expressed CD40L, these data suggested that T cells may collaborate with B cells to maintain physiological bone homeostasis by promoting B-cell OPG production in vivo. In fact, we have previously reported that T-cell–deficient nude mice have increased BMD 4 weeks after birth12 but rapidly lose BMD relative to WT mice as they age.19 We thus performed a detailed prospective analysis of BMD in nude mice and genetically matched C57BL/6 WT controls between 12 and 16 weeks of age. At this age nude mice are fertile and have normal sex hormones and parathyroid hormone (PTH) levels (data not shown). Our data show a significant decrease in BMD (Figure 3A) in T-cell–deficient nude mice relative to WT mice at 12 weeks of age with a percentage change (Δ) of −11.7% at the total body, −15.4% at the spine, and −12.9% at the femurs. While BMD in WT mice continued to accrue between 12 and 16 weeks, nude mice failed to gain additional BMD over this same time period, leading to a further widening of the difference in BMD between the groups. By 16 weeks of age the percentage change in BMD between WT and nude mice had fallen to −19.3% at the total body, −22.4% at the spine, and −16.2% at the femurs.
T-cell–deficient nude mice display decreased baseline BMD and increased bone resorption, concurrent with decreased total BM OPG, and B-cell–specific OPG production. (A) BMD was determined in WT mice at the total body, spine, and femurs, 12 weeks of age (black bars) and 16 weeks of age (white bars), and in nude mice at 12 weeks of age (vertical lines) and 16 weeks (horizontal lines) of age. Data are presented as mean ± SD of 29 nude mice and 18 WT mice. *P ≤ .001 relative to age-matched WT. Percentage changes (Δ) between nude and WT mice at 12 and 16 weeks are indicated above the appropriate bars. (B) μCT reconstructions showing reduced trabecular and cortical structure of representative tibias in nude mice relative to WT. (C) Increased biochemical indices of bone resorption (CTx) were observed in the serum of nude mice compared with WT. *P < .05; n = 9 mice per group. (D) Decreased OPG production by BM derived from nude mice compared with WT (ELISA). (E) Decreased OPG production by purified IgM+ B cells from nude mice relative to WT, quantitated in 48-hour–conditioned medium by ELISA; n = 6 mice per group. *P ≤ .01. (F) Real-time RT-PCR quantitation of OPG and RANKL mRNA expression in WT and nude mice. Mean ± SD of 3 independent WT and nude mice. *P < .01.
T-cell–deficient nude mice display decreased baseline BMD and increased bone resorption, concurrent with decreased total BM OPG, and B-cell–specific OPG production. (A) BMD was determined in WT mice at the total body, spine, and femurs, 12 weeks of age (black bars) and 16 weeks of age (white bars), and in nude mice at 12 weeks of age (vertical lines) and 16 weeks (horizontal lines) of age. Data are presented as mean ± SD of 29 nude mice and 18 WT mice. *P ≤ .001 relative to age-matched WT. Percentage changes (Δ) between nude and WT mice at 12 and 16 weeks are indicated above the appropriate bars. (B) μCT reconstructions showing reduced trabecular and cortical structure of representative tibias in nude mice relative to WT. (C) Increased biochemical indices of bone resorption (CTx) were observed in the serum of nude mice compared with WT. *P < .05; n = 9 mice per group. (D) Decreased OPG production by BM derived from nude mice compared with WT (ELISA). (E) Decreased OPG production by purified IgM+ B cells from nude mice relative to WT, quantitated in 48-hour–conditioned medium by ELISA; n = 6 mice per group. *P ≤ .01. (F) Real-time RT-PCR quantitation of OPG and RANKL mRNA expression in WT and nude mice. Mean ± SD of 3 independent WT and nude mice. *P < .01.
We further analyzed the trabecular and cortical bone compartments of WT and nude mice at 16 weeks of age by high-resolution (6 μm) μCT of the tibias. Representative cross-sectional images show reduced trabecular and cortical structure in nude mice (Figure 3B).
Morphological measurements (Table 2) were computed from μCT reconstructions of WT and nude mouse tibias. The data show a significant decrease in BV, BV/TV, and Tb. Th. Although Conn. D. was reduced by 12% in nude mice, this index did not reach statistical significance (P = .113). Tb. N. and Tb. Sp. likewise were not significantly changed, possibly a result of the dissolution of plates into rods as evidenced by the elevated SMI. However, Co. Th. and Co. Vol. were dramatically reduced, accounting for the large decreases in BMD observed by DXA.
Morphological analysis by μCT of tibias from nude mice and WT controls
| Morphological parameters . | WT mice . | Nude mice . | % change . | P . |
|---|---|---|---|---|
| TV, mm3 | 1.27 ± 0.15 | 1.28 ± 0.08 | 0.8 | ≤ .870 |
| BV, mm3 | 0.20 ± 0.04 | 0.17 ± 0.03 | −15.0 | ≤ .046* |
| BV/TV, % | 15.8 ± 1.18 | 13.1 ± 1.88 | −17.1 | ≤ .004* |
| Tb. Th., μm | 46.0 ± 2.11 | 41.1 ± 1.95 | −10.7 | ≤ .001* |
| Tb. Sp., μm | 192.1 ± 19.5 | 193.8 ± 11.5 | 0.9 | ≤ .835 |
| Tb. N./mm | 5.33 ± 0.46 | 5.24 ± 0.27 | −1.7 | ≤ .641 |
| SMI | 2.08 ± 0.13 | 2.3 ± 0.10 | 10.6 | ≤ .002* |
| Conn. D./mm3 | 242.2 ± 26.8 | 215.4 ± 39.8 | −11.1 | ≤ .137 |
| Co. Th., μm | 83.7 ± 2.2 | 73.8 ± 2.1 | −11.8 | ≤ .001* |
| Co. Vol., mm3 | 0.29 ± 0.03 | 0.17 ± 0.02 | −41.1 | ≤ .001* |
| Morphological parameters . | WT mice . | Nude mice . | % change . | P . |
|---|---|---|---|---|
| TV, mm3 | 1.27 ± 0.15 | 1.28 ± 0.08 | 0.8 | ≤ .870 |
| BV, mm3 | 0.20 ± 0.04 | 0.17 ± 0.03 | −15.0 | ≤ .046* |
| BV/TV, % | 15.8 ± 1.18 | 13.1 ± 1.88 | −17.1 | ≤ .004* |
| Tb. Th., μm | 46.0 ± 2.11 | 41.1 ± 1.95 | −10.7 | ≤ .001* |
| Tb. Sp., μm | 192.1 ± 19.5 | 193.8 ± 11.5 | 0.9 | ≤ .835 |
| Tb. N./mm | 5.33 ± 0.46 | 5.24 ± 0.27 | −1.7 | ≤ .641 |
| SMI | 2.08 ± 0.13 | 2.3 ± 0.10 | 10.6 | ≤ .002* |
| Conn. D./mm3 | 242.2 ± 26.8 | 215.4 ± 39.8 | −11.1 | ≤ .137 |
| Co. Th., μm | 83.7 ± 2.2 | 73.8 ± 2.1 | −11.8 | ≤ .001* |
| Co. Vol., mm3 | 0.29 ± 0.03 | 0.17 ± 0.02 | −41.1 | ≤ .001* |
Trabecular indices, including TV, BV, BV/TV, Tb. Th., Tb. Sp., Tb. N., SMI, and Conn. D., and the cortical indices Co. Th. and Co. Vol. were computed from μCT scans. The data are presented as the mean ± SD of 8 mice per group. Percentage change between WT and nude mice is indicated as “% change.” Asterisks indicate significantly different results (P ≤ .05).
Serum CTx demonstrated a significantly enhanced rate of bone resorption in nude mice (Figure 3C).
Nude mice display lower total BM and B-cell specific OPG production
To investigate whether increased resorption in nude mice was related to an alteration in BM OPG levels, OPG production was quantitated in whole BM from nude and WT mice. Nude BM was found to produce significantly less OPG than an equivalent amount of WT BM (Figure 3D).
B cells were next immunomagnetically purified from the BM of WT and nude mice. WT B cells were found to secrete 3-fold more OPG than B cells isolated from nude mice (Figure 3E), suggesting that reduced B-cell OPG secretion is a consequence of T-cell deficiency.
To evaluate the mRNA levels of total OPG and RANKL in the BM of nude and WT mice, real-time RT-PCR was performed. The data show (Figure 3F) that enhanced bone resorption in nude mice is driven by an imbalance in the RANKL/OPG ratio, a consequence of reduced OPG, because RANKL levels were unchanged relative to WT mice.
CD40 KO and CD40L KO mice have decreased basal BMD and bone mass
Our data in Figure 2J show that activation of CD40 by CD40L stimulates OPG production in murine splenic B cells, consistent with published studies in human tonsil B cells.35 Consequently we next characterized the bone phenotype of CD40 and CD40L KO mice.
Our data revealed that CD40 KO (Figure 4A) and CD40L KO (Figure 4B) mice display significantly decreased BMD relative to their respective WT mice at the total body, spine, and femurs. Representative high-resolution (6 μm) cross-sectional μCT reconstructions of the distal femurs of CD40 (Figure 4C) and CD40L (Figure 4D) mice revealed a significant decrease in trabecular and cortical bone mass relative to their respective WT controls.
CD40 KO mice and CD40L KO mice display decreased baseline BMD and increased bone resorption, concurrent with decreased B-cell OPG production. Decreased BMD was observed by DXA in (A) CD40 KO mice and (B) CD40L KO mice relative to age-matched (24 weeks) WT controls of the same genetic background at multiple anatomic positions including total body, spine, and femurs. P < .01, n = 16 (WT), n = 14 (CD40 KO), n = 12 (WT and CD40 KO). μCT reconstructions showing reduced trabecular and cortical structure of representative femurs in (C) CD40 KO mice and in (D) CD40L KO mice relative to their respective WT controls. Increased biochemical indices of bone resorption (CTx) were observed in the serum of (E) CD40 KO mice and (F) CD40L KO mice. Decreased OPG production by IgM+ BM B cells derived from (G) CD40 KO mice and (H) CD40L KO mice compared with WT (ELISA). *P ≤ .001. Real-time RT-PCR quantitation of OPG and RANKL mRNA expression in (I) CD40 KO and (J) CD40L KO mice. Mean ± SD of 3 independent WT and CD40 KO mice. *P ≤ .01.
CD40 KO mice and CD40L KO mice display decreased baseline BMD and increased bone resorption, concurrent with decreased B-cell OPG production. Decreased BMD was observed by DXA in (A) CD40 KO mice and (B) CD40L KO mice relative to age-matched (24 weeks) WT controls of the same genetic background at multiple anatomic positions including total body, spine, and femurs. P < .01, n = 16 (WT), n = 14 (CD40 KO), n = 12 (WT and CD40 KO). μCT reconstructions showing reduced trabecular and cortical structure of representative femurs in (C) CD40 KO mice and in (D) CD40L KO mice relative to their respective WT controls. Increased biochemical indices of bone resorption (CTx) were observed in the serum of (E) CD40 KO mice and (F) CD40L KO mice. Decreased OPG production by IgM+ BM B cells derived from (G) CD40 KO mice and (H) CD40L KO mice compared with WT (ELISA). *P ≤ .001. Real-time RT-PCR quantitation of OPG and RANKL mRNA expression in (I) CD40 KO and (J) CD40L KO mice. Mean ± SD of 3 independent WT and CD40 KO mice. *P ≤ .01.
Morphological measurements were computed from μCT reconstructions of CD40 KO and CD40L KO femurs and WT controls (Tables 3–4). The data show a significant decrease in BV, BV/TV, and Tb. Th. in CD40 KO mice. No differences in Tb. Sp., Tb. N., or Conn. D. were detected, possibly due to the masking effect of a dramatically elevated SMI. A significant decrease in Co. Vol. was also observed although Co. Th. fell short of significance. By contrast, a large decrease in trabecular BV/TV was observed for CD40L KO mice (Table 4). This decrease resulted exclusively from a dramatic reduction in BV, because TV was unchanged. Decreased TV resulted from a significant decrease in Tb. Th., Tb. N., and a severely decreased Conn. D. As expected, a corresponding increase in Tb. Sp. was observed. Bone architecture denoted by the SMI was also significantly altered. Cortical indices (Co. Th. and Co. Vol.) revealed significantly reduced cortical bone mass.
Morphological analysis by μCT of femurs from CD40 mice compared with respective WT mice
| Morphological parameters . | WT mice . | CD40 KO mice . | % change . | P . |
|---|---|---|---|---|
| TV, mm3 | 1.29 ± 0.06 | 1.23 ± 0.13 | −4.7 | ≤ .158 |
| BV, mm3 | 0.32 ± 0.03 | 0.26 ± 0.03 | −18.8 | ≤ .001* |
| BV/TV, % | 24.47 ± 2.26 | 21.67 ± 2.55 | −11.4 | ≤ .008* |
| Tb. Th., μm | 73.07 ± 3.87 | 66.24 ± 5.83 | −9.3 | ≤ .002* |
| Tb. Sp., μm | 252.66 ± 15.66 | 252.94 ± 19.59 | 0.1 | ≤ .969 |
| Tb. N./mm | 4.13 ± 0.24 | 4.17 ± 0.15 | 1.0 | ≤ .619 |
| SMI | 0.47 ± 0.17 | 0.64 ± 0.15 | 36.2 | ≤ .014* |
| Conn. D./mm3 | 73.9 ± 14.5 | 85.3 ± 15.4 | 15.4 | ≤ .070 |
| Co. Th., μm | 106.3 ± 5.5 | 102.3 ± 5.1 | −3.8 | ≤ .072 |
| Co. Vol., μm | 0.56 ± 0.03 | 0.53 ± 0.03 | −5.4 | ≤ .020* |
| Morphological parameters . | WT mice . | CD40 KO mice . | % change . | P . |
|---|---|---|---|---|
| TV, mm3 | 1.29 ± 0.06 | 1.23 ± 0.13 | −4.7 | ≤ .158 |
| BV, mm3 | 0.32 ± 0.03 | 0.26 ± 0.03 | −18.8 | ≤ .001* |
| BV/TV, % | 24.47 ± 2.26 | 21.67 ± 2.55 | −11.4 | ≤ .008* |
| Tb. Th., μm | 73.07 ± 3.87 | 66.24 ± 5.83 | −9.3 | ≤ .002* |
| Tb. Sp., μm | 252.66 ± 15.66 | 252.94 ± 19.59 | 0.1 | ≤ .969 |
| Tb. N./mm | 4.13 ± 0.24 | 4.17 ± 0.15 | 1.0 | ≤ .619 |
| SMI | 0.47 ± 0.17 | 0.64 ± 0.15 | 36.2 | ≤ .014* |
| Conn. D./mm3 | 73.9 ± 14.5 | 85.3 ± 15.4 | 15.4 | ≤ .070 |
| Co. Th., μm | 106.3 ± 5.5 | 102.3 ± 5.1 | −3.8 | ≤ .072 |
| Co. Vol., μm | 0.56 ± 0.03 | 0.53 ± 0.03 | −5.4 | ≤ .020* |
Trabecular indices, including TV, BV, BV/TV, Tb. Th., Tb. Sp., Tb. N., SMI, and Conn. D., and the cortical indices Co. Th. and Co. Vol. were computed from μCT scans of CD40 KO mice and their respective WT controls. The data are presented as the mean ± SD. Percentage change between WT and KO mice is indicated as “% change.” Asterisks indicate significantly different results (P ≤ .05); n = 12 WT and 13 CD40 KO mice.
Morphological analysis by μCT of femurs from CD40L KO mice compared with respective WT mice
| Morphological parameters . | WT mice . | CD40L KO mice . | % change . | P . |
|---|---|---|---|---|
| TV, mm3 | 1.19 ± 0.05 | 1.17 ± 0.11 | −1.7 | ≤ .570 |
| BV, mm3 | 0.29 ± 0.02 | 0.19 ± 0.029 | −34.5 | ≤ .001* |
| BV/TV, % | 24.37 ± 1.66 | 16.24 ± 1.67 | −33.4 | ≤ .001* |
| Tb. Th., μm | 67.94 ± 2.99 | 63.57 ± 3.05 | −6.4 | ≤ .002* |
| Tb. Sp., μm | 230.14 ± 13.49 | 272.90 ± 18.39 | 18.6 | ≤ .001* |
| Tb. N./mm | 4.37 ± 0.19 | 3.91 ± 0.26 | −10.5 | ≤ .001* |
| SMI | 0.59 ± 0.11 | 1.11 ± 0.19 | 88.1 | ≤ .001* |
| Conn. D./mm3 | 90.10 ± 12.97 | 57.00 ± 8.94 | −36.7 | ≤ .001* |
| Co. Th., μm | 100.6 ± 4.5 | 94.3 ± 7.2 | −6.3 | ≤ .016* |
| Co. Vol., μm | 0.51 ± 0.03 | 0.48 ± 0.04 | −5.9 | ≤ .046* |
| Morphological parameters . | WT mice . | CD40L KO mice . | % change . | P . |
|---|---|---|---|---|
| TV, mm3 | 1.19 ± 0.05 | 1.17 ± 0.11 | −1.7 | ≤ .570 |
| BV, mm3 | 0.29 ± 0.02 | 0.19 ± 0.029 | −34.5 | ≤ .001* |
| BV/TV, % | 24.37 ± 1.66 | 16.24 ± 1.67 | −33.4 | ≤ .001* |
| Tb. Th., μm | 67.94 ± 2.99 | 63.57 ± 3.05 | −6.4 | ≤ .002* |
| Tb. Sp., μm | 230.14 ± 13.49 | 272.90 ± 18.39 | 18.6 | ≤ .001* |
| Tb. N./mm | 4.37 ± 0.19 | 3.91 ± 0.26 | −10.5 | ≤ .001* |
| SMI | 0.59 ± 0.11 | 1.11 ± 0.19 | 88.1 | ≤ .001* |
| Conn. D./mm3 | 90.10 ± 12.97 | 57.00 ± 8.94 | −36.7 | ≤ .001* |
| Co. Th., μm | 100.6 ± 4.5 | 94.3 ± 7.2 | −6.3 | ≤ .016* |
| Co. Vol., μm | 0.51 ± 0.03 | 0.48 ± 0.04 | −5.9 | ≤ .046* |
Trabecular indices, including TV, BV, BV/TV, Tb. Th., Tb. Sp., Tb. N., SMI, and Conn. D., and the cortical indices Co. Th. and Co. Vol. were computed from μCT scans of CD40 and CD40L KO mice and their respective WT controls. The data are presented as the mean ± SD. Percentage change between WT and KO mice is indicated as “% change.” Asterisks indicate significantly different results (P ≤ .05); n = 11 mice per group for CD40L KO and WT mice.
In both CD40 KO (Figure 4E) and CD40L KO mice (Figure 4F), decreased BMD and BV/TV was consistent with enhanced biochemical indices of bone resorption (CTx). B cells isolated from the BM of CD40 KO mice (Figure 4G) and CD40L KO mice (Figure 4H) were found to secrete significantly lower concentrations of OPG than their WT counterparts. Real-time RT-PCR confirmed that while RANKL mRNA was not significantly different, a decreased level of OPG transcript in CD40 KO BM (Figure 4I) and CD40L KO BM (Figure 4J) likely accounted for the increase in bone resorption and net decrease in BMD and BV/TV.
Taken together, our data suggest that B cells are critical regulators of physiological bone turnover in vivo by virtue of their capacity to secrete OPG and that T cells promote enhanced B-cell OPG secretion, in part via activation of the B-cell costimulatory molecule CD40, by T-cell–expressed CD40L.
Discussion
Our study defines a novel role for both T cells and B cells in the maintenance of basal peak bone mass in vivo and defines B cells as major producers of OPG. The loss of mature B-cell–derived OPG in our KO models likely leads to only a maximum 40% to 50% reduction in total BM OPG production. Nonetheless, we observed significant decreases in BMD and bone mass by 3 months of age in B-cell KO mice. B-cell reconstitution of B-cell KO mice at 4 weeks of age prevented the loss of bone mass and restored OPG production in the BM. These data are consistent with the OPG KO mouse study of Bucay et al, which revealed that the loss of 50% of OPG production in heterozygous OPG KO mice exhibited significant loss of total bone density by at least 6 months of age.4 Similarly, in the characterization of the independently generated OPG KO mouse by Mizuno et al, quantitative indices of BMD, dry weigh, bone mineral content, and bone stiffness all fell intermediately (typically midway) between those of WT and KO mice at 3 months of age in male mice, although the data fell short of statistical significance, likely a consequence of the small population size (6 to 7 mice per group).5
In contrast to pathological bone turnover in which cytokines such as RANKL, IL-1, IL-6, IL-7, and TNFα lead to disrupted bone homeostasis, under basal conditions of bone modeling and remodeling, current concepts hold that the RANKL/OPG ratio is the primary mechanism of OC regulation. In humans polymorphisms in the OPG gene are associated with low BMD45 and with increased incidence of fracture.46 Our study focused exclusively on the role of lymphocytes in OPG regulation. RANKL was not seen to be changed in the models investigated, suggesting that osteoporosis was driven exclusively by decreased OPG, leading to an effective higher concentration of active RANKL. However, our data do not preclude additional exacerbating or compensatory effects through other pro-osteoclastogenic or antiosteoclastogenic factors whose regulation may potentially be altered in these KO models and lead to exacerbated or reduced phenotypes.
B-cell–derived OPG may serve to buffer changes in the rate of bone resorption associated with excess levels of osteoblastic RANKL. Consistent with in vitro studies using human tissues,35 we show that B-cell OPG production is further augmented in response to CD40 activation. Because CD40L is expressed predominantly by activated T cells, the CD40/CD40L costimulatory system may serve to neutralize the potential threat to bone homeostasis posed by RANKL secreted by activated T cells by eliciting enhanced levels of B-cell OPG. It is now well established that even under basal physiological conditions, the presentation of antigens of self and foreign origin is always proceeding.47–49 Consequently, immune surveillance is always in place, and B-cell–T-cell interactions are always ongoing even in healthy humans and animals. This low-grade immune response contrasts with the unbridled pathological T-cell activation that occurs during estrogen deficiency11–13 and in inflammation,14 where expanded and strongly activated T-cell populations secrete high concentrations of RANKL, potently amplified by inflammatory levels of TNFα11 and other cytokines. Although B cells may provide some protection against bone loss in pathological conditions, the T-cell–derived cytokine load may ultimately overwhelm the capacity of B-cell–derived OPG to counteract the upswing in osteoclastogenesis and to prevent bone destruction. Furthermore, under several pathological conditions associated with osteoporosis such as estrogen deficiency in humans,50 multiple myeloma,51 and in animal models of periodontal disease,15 B cells themselves may constitute a source of high concentrations of RANKL that may overcome the concurrent production of OPG. It is also possible that B-cell OPG may be down-regulated under such conditions. In contrast to these pathological states, our data suggest that under basal conditions B cells are not a significant source of RANKL in the BM microenvironment. While OPG protein was readily quantifiable in conditioned media from purified B cells, RANKL fell below the level of detection. Furthermore, the levels of total BM RANKL mRNA were not found to be significantly altered relative to WT in B-cell KO mice. This was consistent with the fact that B-cell KO mice were observed to be osteoporotic and deficient in OPG, suggesting that the production of OPG by B cells outweighs the production of RANKL under basal conditions. These data are also consistent with a recent study demonstrating that only 20% of B cells and T cells make RANKL under basal conditions but that this level rises to 50% of B cells and 90% of T cells in diseased periodontal tissue.15 By contrast, a previous study has suggested that B cells are a major source of endogenous RANKL.20 The reason for this difference is not known but may relate to technical differences in experimentation. For example, in the study of Manabe et al,20 flow cytometry for RANKL was performed without B-cell fixation and permeabilization, suggesting that membrane-associated RANKL rather than soluble RANKL was being investigated. RANKL production by cells other than OBs is generally soluble. Consequently, it is possible that some membrane-associated RANKL is present on basal B cells but that this was not detected in our ELISA, which specifically detects secreted RANKL.
Our data show that peripheral B cells such as those of the spleen also contribute OPG under basal conditions. T cells have the capacity to secrete RANKL, and OC precursors circulate within the macrophage population.2 Nonetheless, adventitious OC formation does not occur in the spleen. The reason for this is not clear, but production of large concentrations of OPG by splenic B cells (about 50% of total nucleated cells) and other splenocytes could act to suppress inappropriate osteoclastogenesis from occurring. This source of OPG is also likely to impact OC formation in other organs and in BM by way of the peripheral circulation.
Our data showed that ex vivo CD40L stimulation of splenic B cells induced a 2-fold stimulation of OPG production. The fact that B cells isolated from nude mice show a dramatic 3-fold reduction in OPG production while B cells derived from CD40 and CD40L KO mice showed a more modest 40% to 45% reduction suggests that additional T-cell signals distinct from the CD40/CD40L axis may also be important for OPG production by B cells. Such signals may include the T-cell receptor (TCR) and/or CD28. In addition, our μCT data showed only a modest loss of bone mass in the CD40 KO relative to CD40L KO mice. This was unexpected because CD40 and CD40L are counterparts and ablation of either ligand or receptor might be expected to have a similar effect. The reason for this difference is not clear, but it is possible that molecules other than CD40 may be capable of compensating in part, perhaps by substituting for this receptor on B cells. Consistent with previous human studies,35 activation of the CD40 costimulatory molecule on murine B cells up-regulated B-cell OPG production. BCR crosslinking using anti-IgM antibody is an established mechanism for activating B cells in vitro44 but failed to induce an up-regulation in OPG secretion. This is likely due to the fact that CD40 and IgM induce BCR activation through different pathways.52 This selectivity of OPG regulation could potentially be exploited to artificially augment B-cell OPG production to maximize basal bone mass and density. Indeed, the CD40-CD40L interaction has recently been highlighted as a target for therapeutic intervention in several disease states including arthrosclerosis,53 Alzheimer disease,54 and renal transplant rejection.55 Osteoporosis may be an unintended consequence of such therapies should the murine data be applicable to humans. Furthermore, the pharmacologic manipulation of the CD40/CD40L pathway may represent a novel mechanism to maximize basal bone mass in individuals with low bone density or persons at high risk for bone fracture. Our studies may also be relevant for the osteoporosis or osteopenia associated with multiple conditions in which lymphocyte functions are perturbed, including rheumatoid arthritis, periodontitis, multiple myeloma, AIDS, and transplantation-induced bone destruction.
Authorship
Contribution: M.N.W designed the experiments, analyzed data, and wrote the paper; Y.L., G.T., A.L., X.Y., H.Z., and W.-P.Q. performed the research; and all authors checked the final version of the manuscript.
Y.L., G.T., and A.L. contributed equally to this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. Neale Weitzmann, Division of Endocrinology & Metabolism & Lipids, 101 Woodruff Circle, WMRB 1305, Emory University School of Medicine, Atlanta, GA 30322; e-mail: mweitzm@emory.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the National Osteoporosis Foundation (M.N.W.), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (DK067389) (M.N.W.), National Cancer Institute (NCI) (U54CA119338) (M.N.W.), University Research Committee of Emory University (M.N.W.), a T32 training award from the NIDDK (DK007298) (Y.L.), and partial financial support from Dr Dwight A. Towler (Washington University) (G.T.).
The authors thank Dr Roberto Pacifici for critical reading of the manuscript and Dr Michaela Robbie-Ryan for helpful discussions. We thank Dr Dwight A. Towler for generous access to the μCT instrument of the Division of Bone and Mineral Diseases at Washington University.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal